Reducing Defects in Organic-Lead Halide Perovskite Film by Delayed Thermal Annealing Combined with KI/I2 for Efficient Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
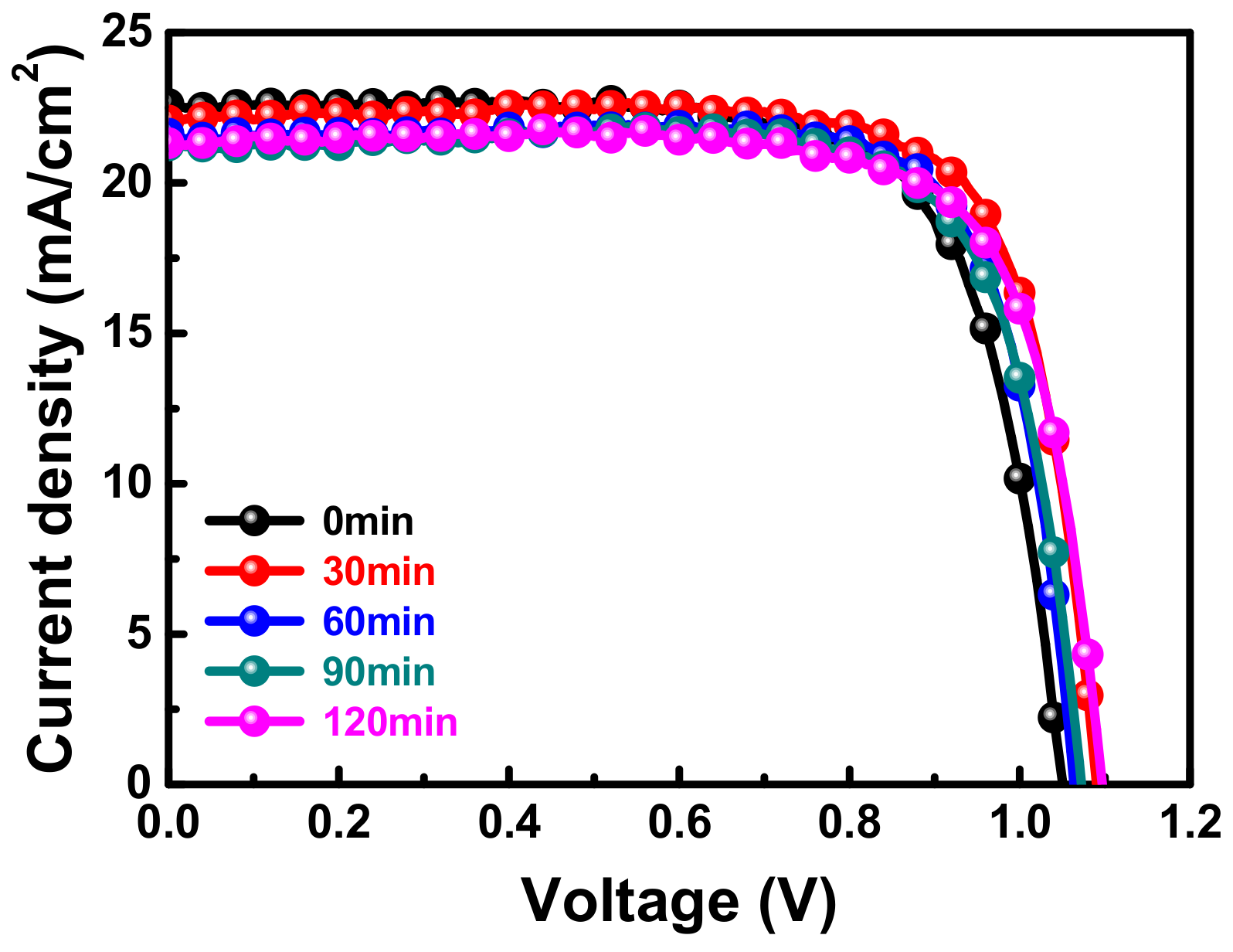
3.1. The Effect of Delayed Thermal Annealing on the Morphological Characteristics of the Surfaces of MAPbI3 Perovskite Films
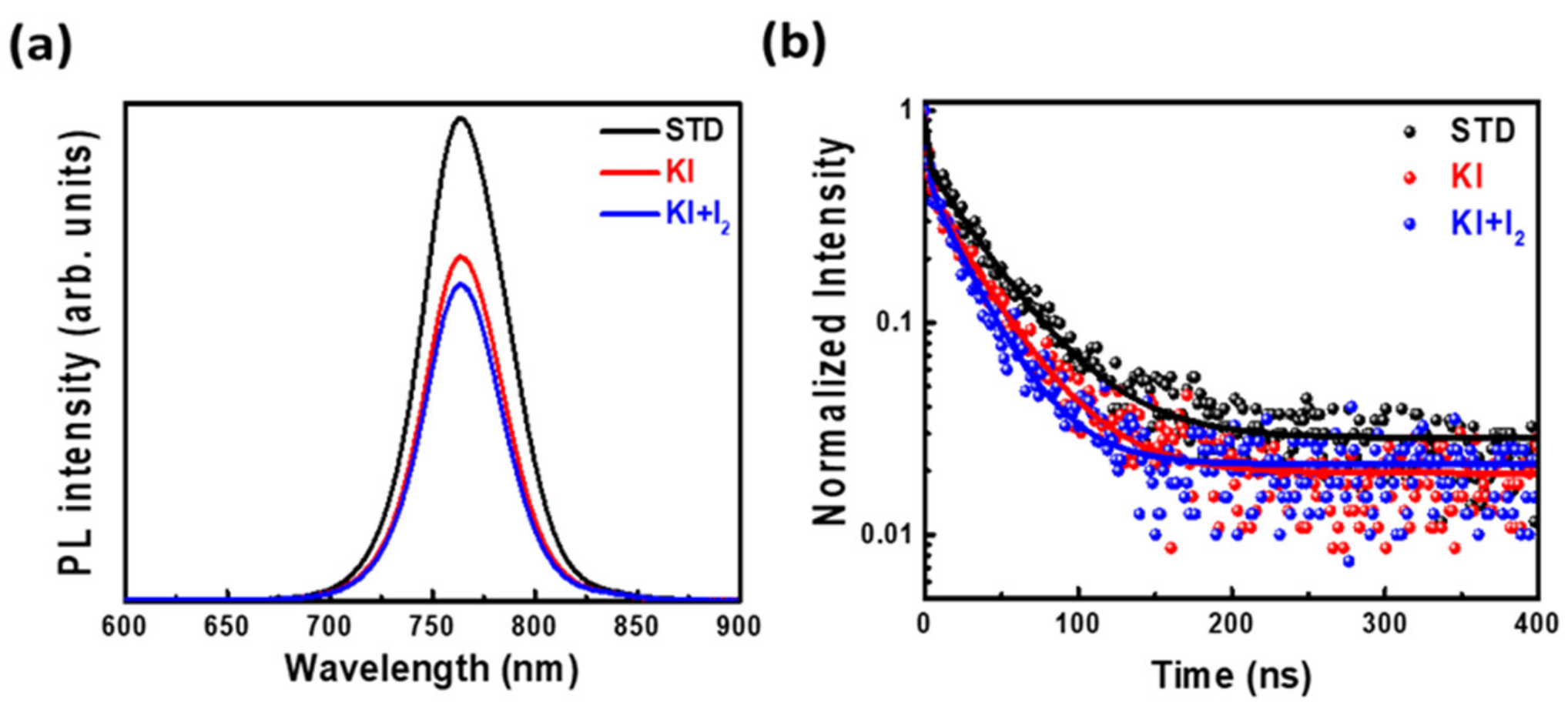
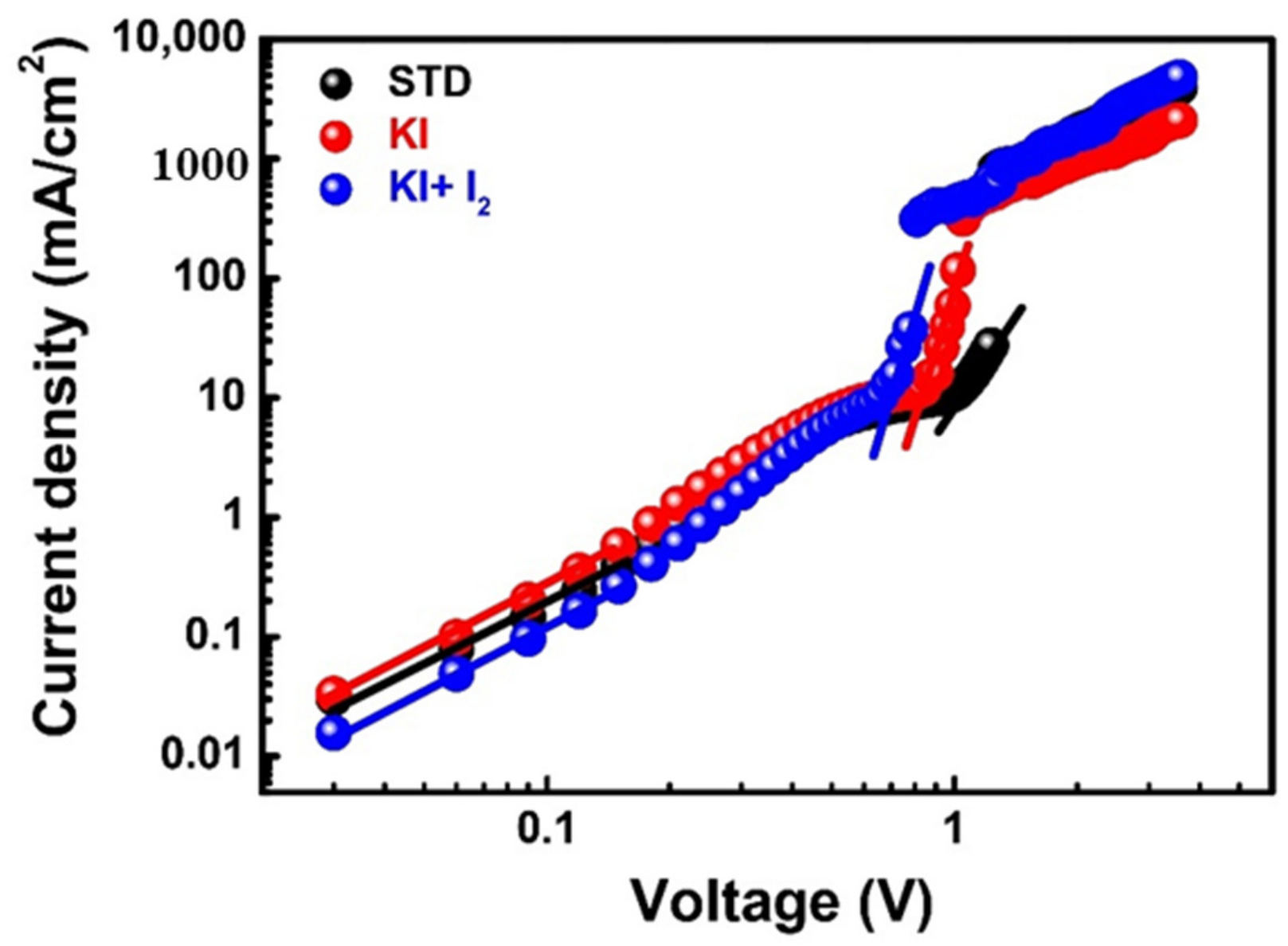
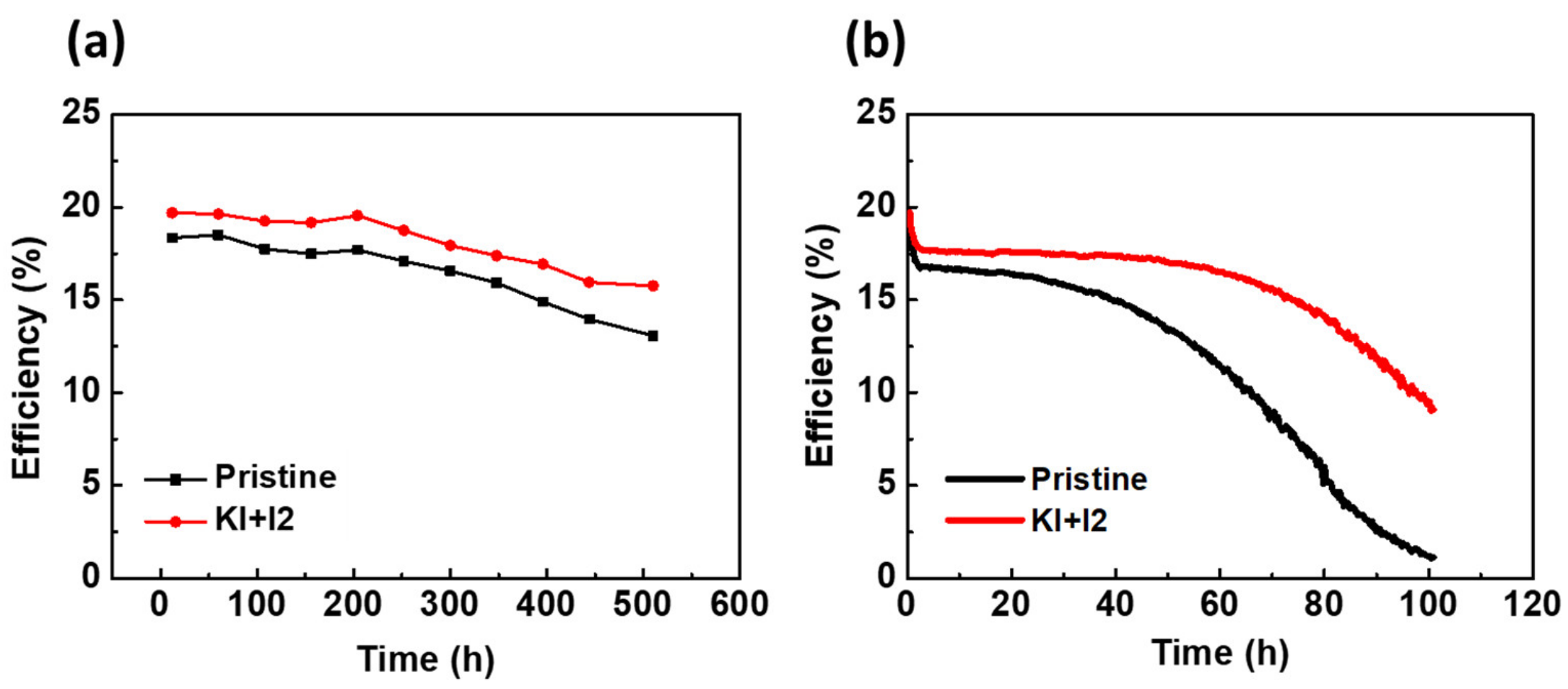
3.2. Reducing Defects in Perovskite Films by Doping with Potassium Iodide and Iodine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Park, N.-G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, K. Organic-inorganic hybrid lead halide perovskites for optoelectronic and electronic applications. Chem. Soc. Rev. 2016, 45, 655–689. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 15 June 2021).
- Saidaminov, M.I.; Adinolfi, V.; Comin, R.; Abdelhady, A.L.; Peng, W.; Dursun, I.; Yuan, M.; Hoogland, S.; Sargent, E.H.; Bakr, O.M. Planar-integrated single-crystalline perovskite photodetectors. Nat. Commun. 2015, 6, 8724. [Google Scholar] [CrossRef] [PubMed]
- Baloch, A.A.B.; Hossain, M.I.; Tabet, N.; Alharbi, F.H. Practical Efficiency Limit of Methylammonium Lead Iodide Perovskite (CH3NH3PbI3) Solar Cells. J. Phys. Chem. Lett. 2018, 9, 426–434. [Google Scholar] [CrossRef]
- Xiao, Z.; Yuan, Y.; Shao, Y.; Wang, Q.; Dong, Q.; Bi, C.; Sharma, P.; Gruverman, A.; Huang, J. Giant switchable photovoltaic effect in organometal trihalide perovskite devices. Nat. Mater. 2015, 14, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Wetzelaer, G.-J.A.H.; Scheepers, M.; Sempere, A.M.; Momblona, C.; Ávila, J.; Bolink, H.J. Trap-Assisted Non-Radiative Recombination in Organic–Inorganic Perovskite Solar Cells. Adv. Mater. 2015, 27, 1837–1841. [Google Scholar] [CrossRef]
- Conwell, E.; Weisskopf, V.F. Theory of Impurity Scattering in Semiconductors. Phys. Rev. 1950, 77, 388–390. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Barker, A.J.; Grancini, G.; Zhang, W.; Ball, J.M.; Kandada, A.R.S.; Snaith, H.J.; Petrozza, A. Carrier trapping and recombination: The role of defect physics in enhancing the open circuit voltage of metal halide perovskite solar cells. Energy Environ. Sci. 2016, 9, 3472–3481. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, J. Ion Migration in Organometal Trihalide Perovskite and Its Impact on Photovoltaic Efficiency and Stability. Acc. Chem. Res. 2016, 49, 286–293. [Google Scholar] [CrossRef]
- Pellet, N.; Gao, P.; Gregori, G.; Yang, T.Y.; Nazeeruddin, M.K.; Maier, J.; Grätzel, M. Mixed-organic-cation perovskite photovoltaics for enhanced solar-light harvesting. Angew. Chem. Int. Ed. Engl. 2014, 53, 3151–3157. [Google Scholar] [CrossRef]
- Ran, C.; Xu, J.; Gao, W.; Huang, C.; Dou, S. Defects in metal triiodide perovskite materials towards high-performance solar cells: Origin, impact, characterization, and engineering. Chem. Soc. Rev. 2018, 47, 4581–4610. [Google Scholar] [CrossRef]
- Tang, Z.; Bessho, T.; Awai, F.; Kinoshita, T.; Maitani, M.M.; Jono, R.; Murakami, T.N.; Wang, H.; Kubo, T.; Uchida, S.; et al. Hysteresis-free perovskite solar cells made of potassium-doped organometal halide perovskite. Sci. Rep. 2017, 7, 12183. [Google Scholar] [CrossRef]
- Zhao, P.; Yin, W.; Kim, M.; Han, M.; Song, Y.J.; Ahn, T.K.; Jung, H.S. Improved carriers injection capacity in perovskite solar cells by introducing A-site interstitial defects. J. Mater. Chem. A 2017, 5, 7905–7911. [Google Scholar] [CrossRef]
- Li, N.; Tao, S.; Chen, Y.; Niu, X.; Onwudinanti, C.K.; Hu, C.; Qiu, Z.; Xu, Z.; Zheng, G.; Wang, L.; et al. Cation and anion immobilization through chemical bonding enhancement with fluorides for stable halide perovskite solar cells. Nat. Energy 2019, 4, 408–415. [Google Scholar] [CrossRef]
- Buin, A.; Comin, R.; Xu, J.; Ip, A.H.; Sargent, E.H. Halide-Dependent Electronic Structure of Organolead Perovskite Materials. Chem. Mater. 2015, 27, 4405–4412. [Google Scholar] [CrossRef]
- Buin, A.; Pietsch, P.; Xu, J.; Voznyy, O.; Ip, A.H.; Comin, R.; Sargent, E.H. Materials processing routes to trap-free halide perovskites. Nano Lett. 2014, 14, 6281–6286. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yam, C. First-principles study of intrinsic defects in formamidinium lead triiodide perovskite solar cell absorbers. Phys. Chem. Chem. Phys. 2018, 20, 6800–6804. [Google Scholar] [CrossRef]
- Zhang, M.; Bing, J.; Cho, Y.; Li, Y.; Zheng, J.; Lau, C.F.J.; Green, M.A.; Huang, S.; Ho-Baillie, A.W.Y. Synergistic effect of potassium and iodine from potassium triiodide complex additive on gas-quenched perovskite solar cells. Nano Energy 2019, 63, 103853. [Google Scholar] [CrossRef]
- Ye, F.; Xie, F.; Yin, M.; He, J.; Wang, Y.; Tang, W.; Chen, H.; Yang, X.; Han, L. Effect of thermal-convection-induced defects on the performance of perovskite solar cells. Appl. Phys. Express 2017, 10, 075502. [Google Scholar] [CrossRef]
- Remeika, M.; Qi, Y. Scalable solution coating of the absorber for perovskite solar cells. J. Energy Chem. 2018, 27, 1101–1110. [Google Scholar] [CrossRef]
- Li, C.; Wang, A.; Xie, L.; Deng, X.; Liao, K.; Yang, J.-a.; Li, T.; Hao, F. Emerging alkali metal ion (Li+, Na+, K+ and Rb+) doped perovskite films for efficient solar cells: Recent advances and prospects. J. Mater. Chem. A 2019, 7, 24150–24163. [Google Scholar] [CrossRef]
- Correa-Baena, J.P.; Luo, Y.; Brenner, T.M.; Snaider, J.; Sun, S.; Li, X.; Jensen, M.A.; Hartono, N.T.P.; Nienhaus, L.; Wieghold, S.; et al. Homogenized halides and alkali cation segregation in alloyed organic-inorganic perovskites. Science 2019, 363, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Dong, Q.; Bi, C.; Yuan, Y.; Huang, J. Air-Stable, Efficient Mixed-Cation Perovskite Solar Cells with Cu Electrode by Scalable Fabrication of Active Layer. Adv. Energy Mater. 2016, 6, 1600372. [Google Scholar] [CrossRef]
- Ghosh, D.; Smith, A.R.; Walker, A.B.; Islam, M.S. Mixed A-Cation Perovskites for Solar Cells: Atomic-Scale Insights Into Structural Distortion, Hydrogen Bonding, and Electronic Properties. Chem. Mater. 2018, 30, 5194–5204. [Google Scholar] [CrossRef]
- Mali, S.S.; Patil, J.V.; Kim, H.; Kim, H.; Hong, C.K. A Dual-Retarded Reaction Processed Mixed-Cation Perovskite Layer for High-Efficiency Solar Cells. Adv. Funct. Mater. 2019, 29, 1807420. [Google Scholar] [CrossRef]
- Brennan, M.C.; Draguta, S.; Kamat, P.V.; Kuno, M. Light-Induced Anion Phase Segregation in Mixed Halide Perovskites. ACS Energy Lett. 2018, 3, 204–213. [Google Scholar] [CrossRef]
- Wu, S.; Li, Z.; Zhang, J.; Liu, T.; Zhu, Z.; Jen, A.K.Y. Efficient large guanidinium mixed perovskite solar cells with enhanced photovoltage and low energy losses. Chem. Commun. 2019, 55, 4315–4318. [Google Scholar] [CrossRef]
- Sun, Q.; Gong, X.; Li, H.; Liu, S.; Zhao, X.; Shen, Y.; Wang, M. Direct formation of I3-ions in organic cation solution for efficient perovskite solar cells. Sol. Energ. Mat. Sol. C 2018, 185, 111–116. [Google Scholar] [CrossRef]
- Seto, J.Y.W. The electrical properties of polycrystalline silicon films. J. Appl. Phys. 1975, 46, 5247–5254. [Google Scholar] [CrossRef]
- Khenkin, M.V.; Katz, E.A.; Abate, A.; Bardizza, G.; Berry, J.J.; Brabec, C.; Brunetti, F.; Bulović, V.; Burlingame, Q.; Di Carlo, A.; et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 2020, 5, 35–49. [Google Scholar] [CrossRef]
- Christians, J.A.; Schulz, P.; Tinkham, J.S.; Schloemer, T.H.; Harvey, S.P.; Tremolet de Villers, B.J.; Sellinger, A.; Berry, J.J.; Luther, J.M. Tailored interfaces of unencapsulated perovskite solar cells for >1000 hour operational stability. Nat. Energy 2018, 3, 68–74. [Google Scholar] [CrossRef]
- Domanski, K.; Roose, B.; Matsui, T.; Saliba, M.; Turren-Cruz, S.-H.; Correa-Baena, J.-P.; Carmona, C.R.; Richardson, G.; Foster, J.M.; De Angelis, F.; et al. Migration of cations induces reversible performance losses over day/night cycling in perovskite solar cells. Energy Environ. Sci. 2017, 10, 604–613. [Google Scholar] [CrossRef]
- Park, N.-G.; Grätzel, M.; Miyasaka, T.; Zhu, K.; Emery, K. Towards stable and commercially available perovskite solar cells. Nat. Energy 2016, 1, 16152. [Google Scholar] [CrossRef]
- Son, D.-Y.; Kim, S.-G.; Seo, J.-Y.; Lee, S.-H.; Shin, H.; Lee, D.; Park, N.-G. Universal Approach toward Hysteresis-Free Perovskite Solar Cell via Defect Engineering. J. Am. Chem. Soc. 2018, 140, 1358–1364. [Google Scholar] [CrossRef]
- Nie, W.; Blancon, J.-C.; Neukirch, A.J.; Appavoo, K.; Tsai, H.; Chhowalla, M.; Alam, M.A.; Sfeir, M.Y.; Katan, C.; Even, J.; et al. Light-activated photocurrent degradation and self-healing in perovskite solar cells. Nat. Commun. 2016, 7, 11574. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Luna, C.A.M.; Hildner, R.; Li, C.; Huettner, S. In situ investigation of light soaking in organolead halide perovskite films. APL Mater. 2019, 7, 041114. [Google Scholar] [CrossRef]
- Li, C.; Guerrero, A.; Huettner, S.; Bisquert, J. Unravelling the role of vacancies in lead halide perovskite through electrical switching of photoluminescence. Nat. Commun. 2018, 9, 5113. [Google Scholar] [CrossRef]


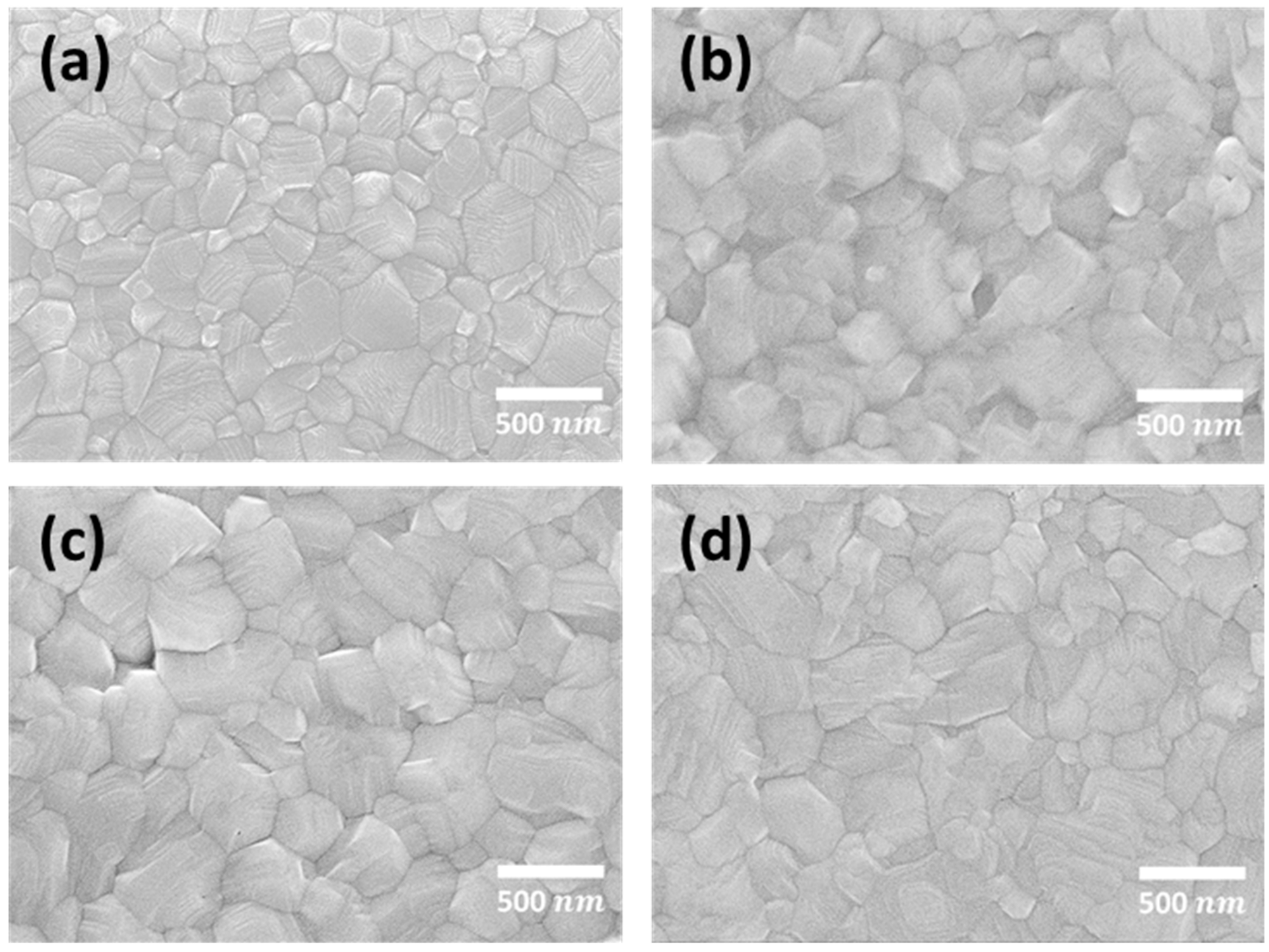

| Time (min) | 0 | 30 | 60 | 90 | 120 |
|---|---|---|---|---|---|
| Rmax (nm) | 398 | 268 | 154 | 137 | 241 |
| RMS (nm) | 56 | 33 | 19 | 21 | 32 |
| Time (min) | VOC (V) | JSC (mA cm−2) | FF (%) | PCE (%) | Rs (ohm) | |
|---|---|---|---|---|---|---|
| 0 | Maximum | 1.050 | 22.62 | 73.4 | 17.45 | 9.2 |
| Mean deviation | 1.046 ± 0.010 | 22.19 ± 0.45 | 73.3 ± 0.6 | 17.05 ± 0.61 | - | |
| 30 | Maximum | 1.075 | 22.28 | 77.0 | 18.44 | 6.6 |
| Mean deviation | 1.076 ± 0.003 | 22.06 ± 0.57 | 74.2 ± 0.4 | 17.61 ± 0.76 | - | |
| 60 | Maximum | 1.072 | 22.05 | 78.2 | 18.48 | 5.2 |
| Mean deviation | 1.074 ± 0.011 | 22.04 ± 0.47 | 76.9 ± 0.1 | 18.20 ± 0.31 | - | |
| 90 | Maximum | 1.072 | 21.24 | 76.9 | 17.53 | 6.7 |
| Mean deviation | 1.076 ± 0.005 | 20.71 ± 0.76 | 76.6 ± 0.3 | 17.10 ± 0.62 | - | |
| 120 | Maximum | 1.096 | 21.32 | 76.3 | 17.85 | 6.9 |
| Mean deviation | 1.082 ± 0.008 | 21.01 ± 0.36 | 75.7 ± 1.4 | 17.25 ± 0.60 | - |
| Concentration (mM) | VOC (V) | JSC (mA cm−2) | FF (%) | PCE (%) | |
|---|---|---|---|---|---|
| 1.5 | Maximum | 1.101 | 23.46 | 70.7 | 18.26 |
| Mean deviation | 1.090 ± 0.014 | 23.13 ± 0.27 | 71.0 ± 1.6 | 17.92 ± 0.32 | |
| 2.0 | Maximum | 1.102 | 22.99 | 73.5 | 18.60 |
| Mean deviation | 1.090 ± 0.008 | 23.15 ± 0.42 | 72.0 ± 1.4 | 18.17 ± 0.55 | |
| 3.0 | Maximum a | 1.091 | 22.83 | 77.7 | 19.36 |
| Mean deviation a | 1.083 ± 0.014 | 22.78 ± 0.61 | 76.3 ± 1.4 | 18.83 ± 0.47 | |
| Maximum b | 1.085 | 22.22 | 68.44 | 16.50 | |
| Mean deviation b | 1.079 ± 0.016 | 22.05 ± 0.72 | 67.2 ± 1.6 | 15.99 ± 0.66 | |
| 6.0 | Maximum | 1.074 | 22.18 | 72.7 | 17.33 |
| Mean deviation | 1.080 ± 0.021 | 21.89 ± 0.36 | 72.0 ± 2.4 | 17.10 ± 0.34 |
| Additive | A1 (%) | τ1 (ns) | A2 (%) | τ2 (ns) | τavg (ns) |
|---|---|---|---|---|---|
| STD | 40 | 1.3 | 60 | 38.4 | 23.5 |
| KI (30 mM) | 55 | 1.7 | 45 | 34.3 | 16.4 |
| KI + I2 (30 mM + 3 mM) | 73 | 1.9 | 27 | 44.1 | 13.3 |
| Condition | Electrical Conductivity (mS cm−1) | VTFL (V) | Trap Density (cm−3) | Mobility (cm2 V−1 s−1) |
|---|---|---|---|---|
| STD | 4.42 × 10−4 | 1.23 | 1.09 × 1016 | 8.40 × 10−2 |
| KI (30 mM) | 4.48 × 10−4 | 0.93 | 8.22 × 1015 | 3.52 × 10−2 |
| KI (30 mM)/I2 (3 mM) | 1.22 × 10−3 | 0.75 | 6.63 × 1015 | 1.26 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-M.; Chan, S.-H.; Chiu, W.-H.; Ahn, S.; Ting, C.-C.; Chang, Y.-H.; Suryanarayanan, V.; Wu, M.-C.; Liu, C.-Y. Reducing Defects in Organic-Lead Halide Perovskite Film by Delayed Thermal Annealing Combined with KI/I2 for Efficient Perovskite Solar Cells. Nanomaterials 2021, 11, 1607. https://doi.org/10.3390/nano11061607
Lee K-M, Chan S-H, Chiu W-H, Ahn S, Ting C-C, Chang Y-H, Suryanarayanan V, Wu M-C, Liu C-Y. Reducing Defects in Organic-Lead Halide Perovskite Film by Delayed Thermal Annealing Combined with KI/I2 for Efficient Perovskite Solar Cells. Nanomaterials. 2021; 11(6):1607. https://doi.org/10.3390/nano11061607
Chicago/Turabian StyleLee, Kun-Mu, Shun-Hsiang Chan, Wei-Hao Chiu, Seoungjun Ahn, Chang-Chieh Ting, Yin-Hsuan Chang, Vembu Suryanarayanan, Ming-Chung Wu, and Ching-Yuan Liu. 2021. "Reducing Defects in Organic-Lead Halide Perovskite Film by Delayed Thermal Annealing Combined with KI/I2 for Efficient Perovskite Solar Cells" Nanomaterials 11, no. 6: 1607. https://doi.org/10.3390/nano11061607
APA StyleLee, K.-M., Chan, S.-H., Chiu, W.-H., Ahn, S., Ting, C.-C., Chang, Y.-H., Suryanarayanan, V., Wu, M.-C., & Liu, C.-Y. (2021). Reducing Defects in Organic-Lead Halide Perovskite Film by Delayed Thermal Annealing Combined with KI/I2 for Efficient Perovskite Solar Cells. Nanomaterials, 11(6), 1607. https://doi.org/10.3390/nano11061607








