Fabrication of Perovskite Film-Coated Hollow Capillary Fibers Using a Fast Solvent Exchange Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Micro-Fluid Assembly
2.2. Neat Perovskite Film Assembly with NdBr3 Additives
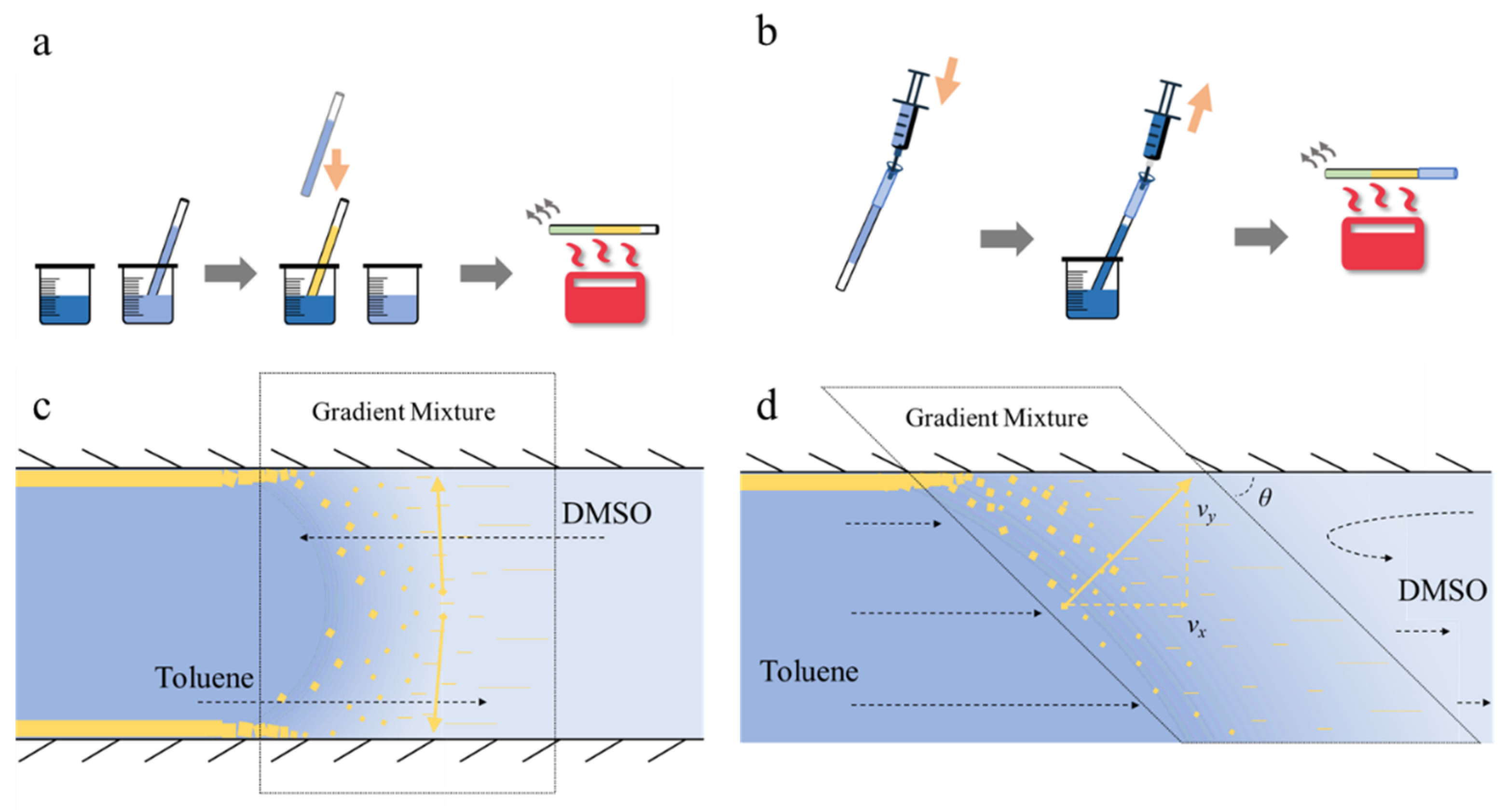
2.3. Solvent Exchange Perovskite Film Deposition Methods
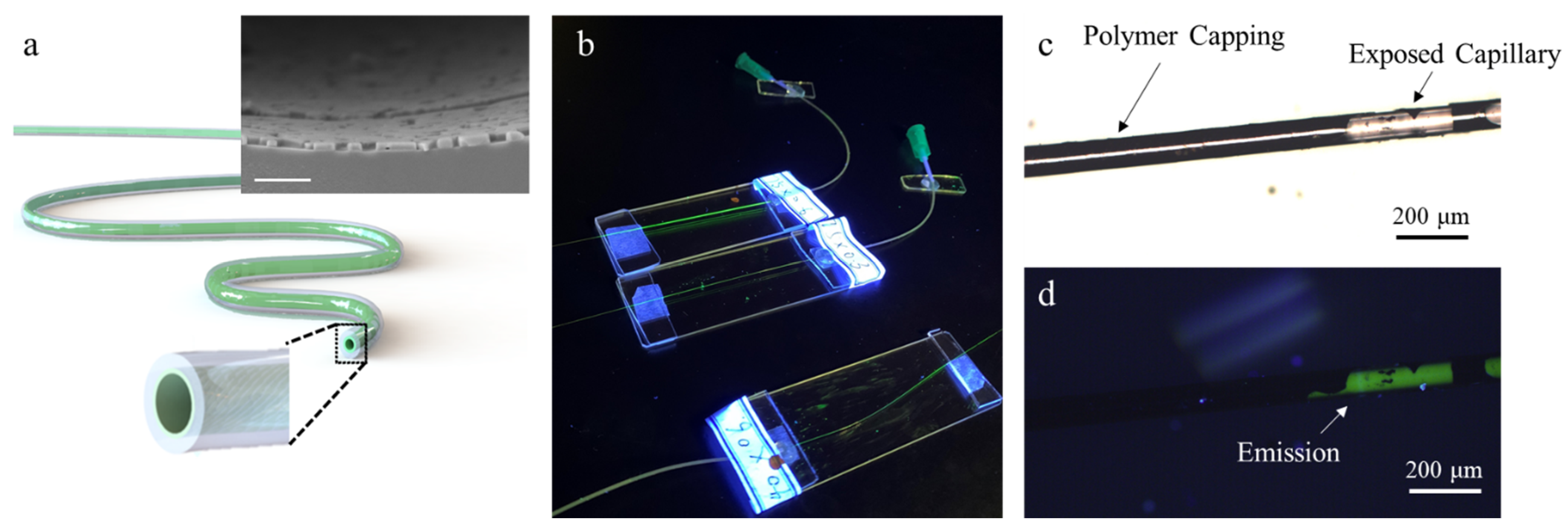
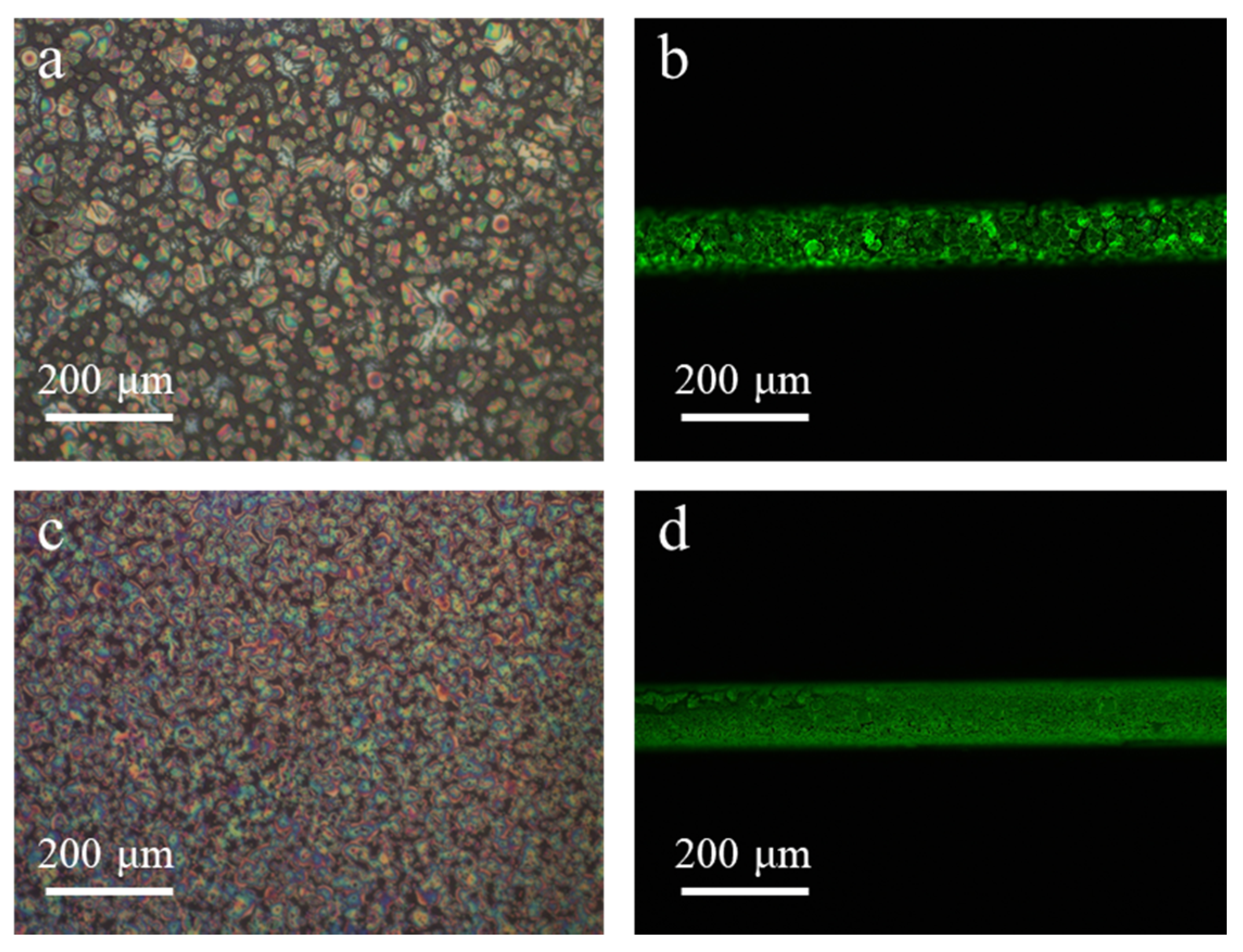
3. Results and Discussion
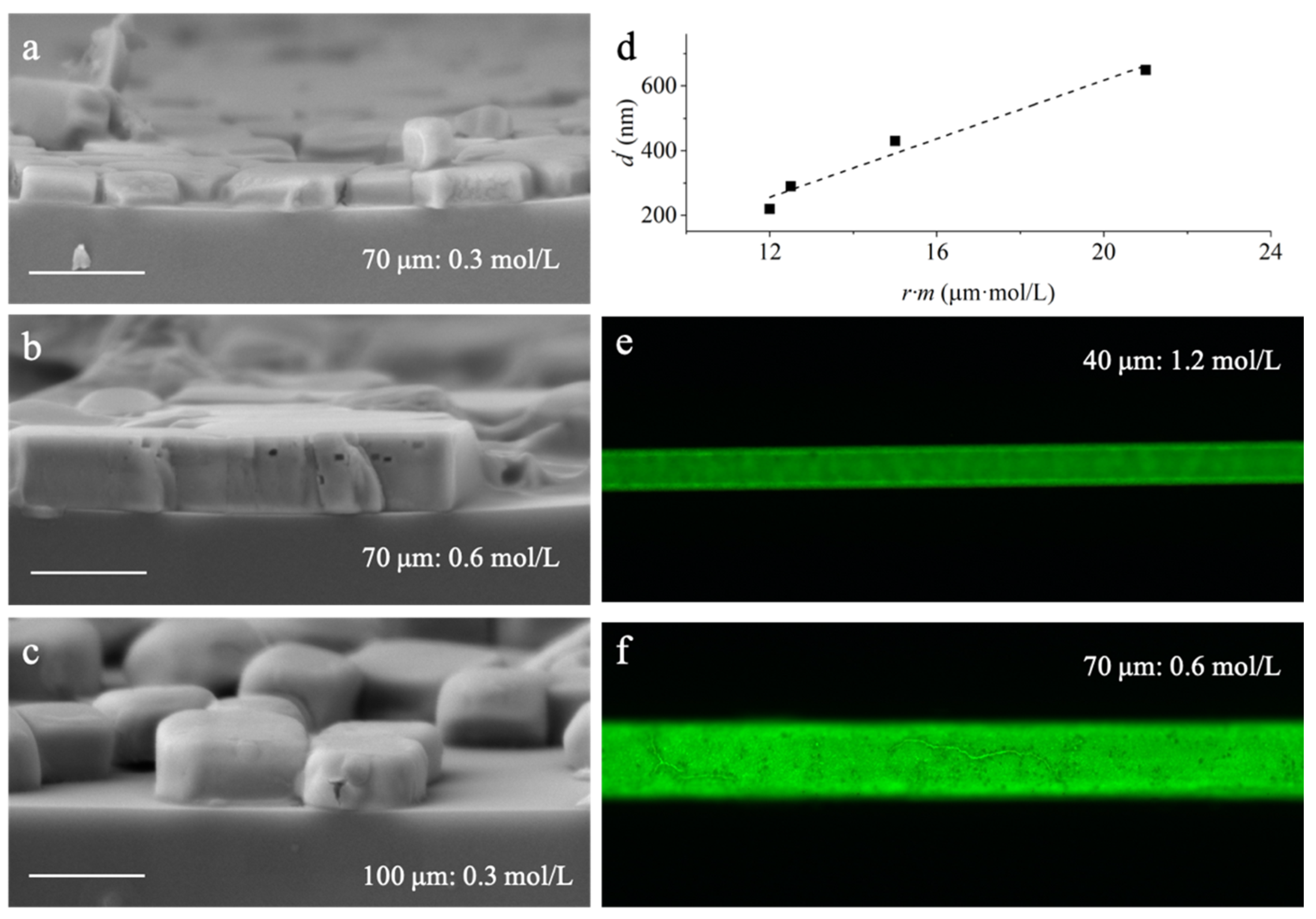
3.1. Diffusion Modulated Solvent Exchange
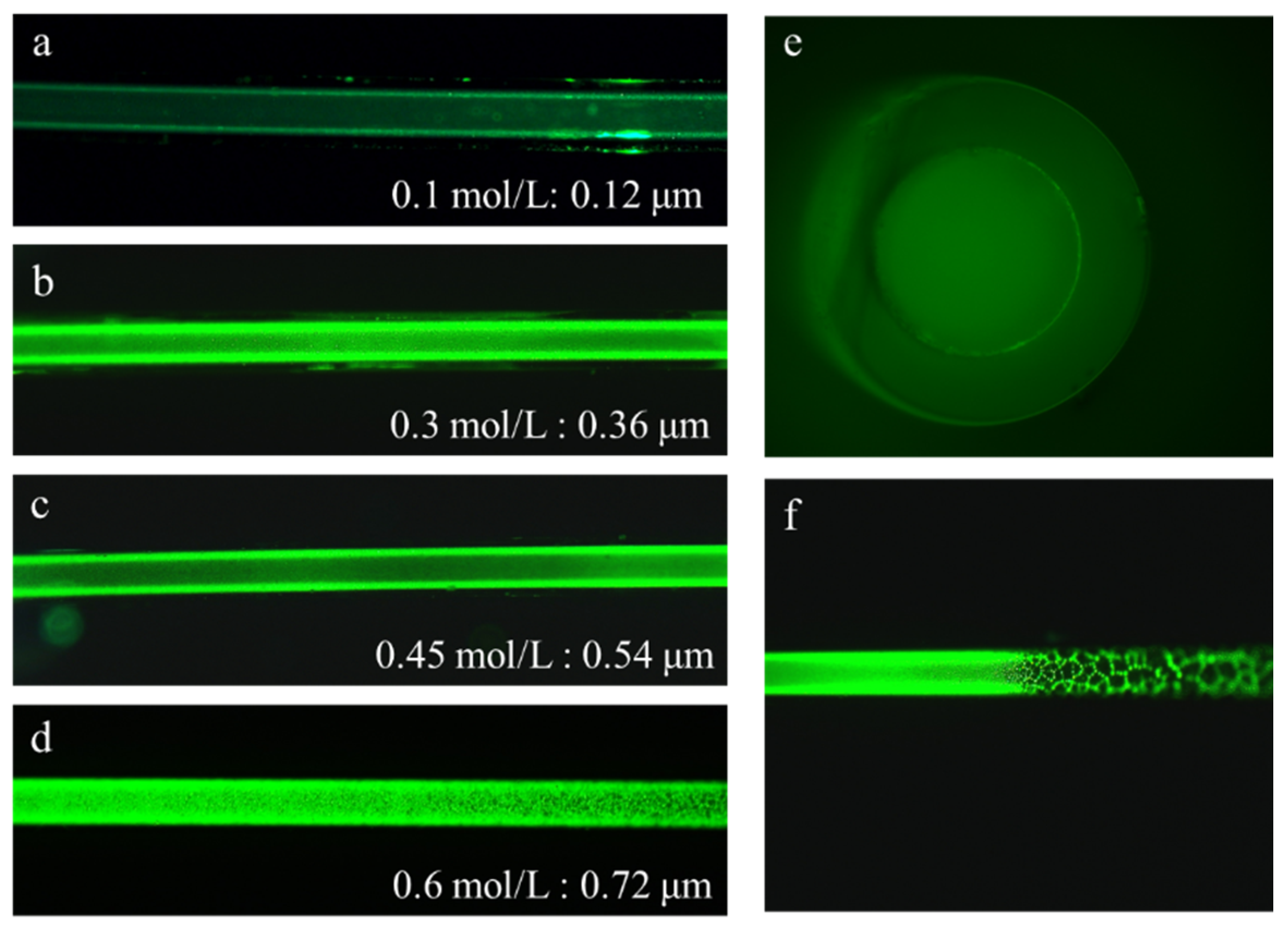
3.2. Flow Velocity Controlled Solvent Exchange
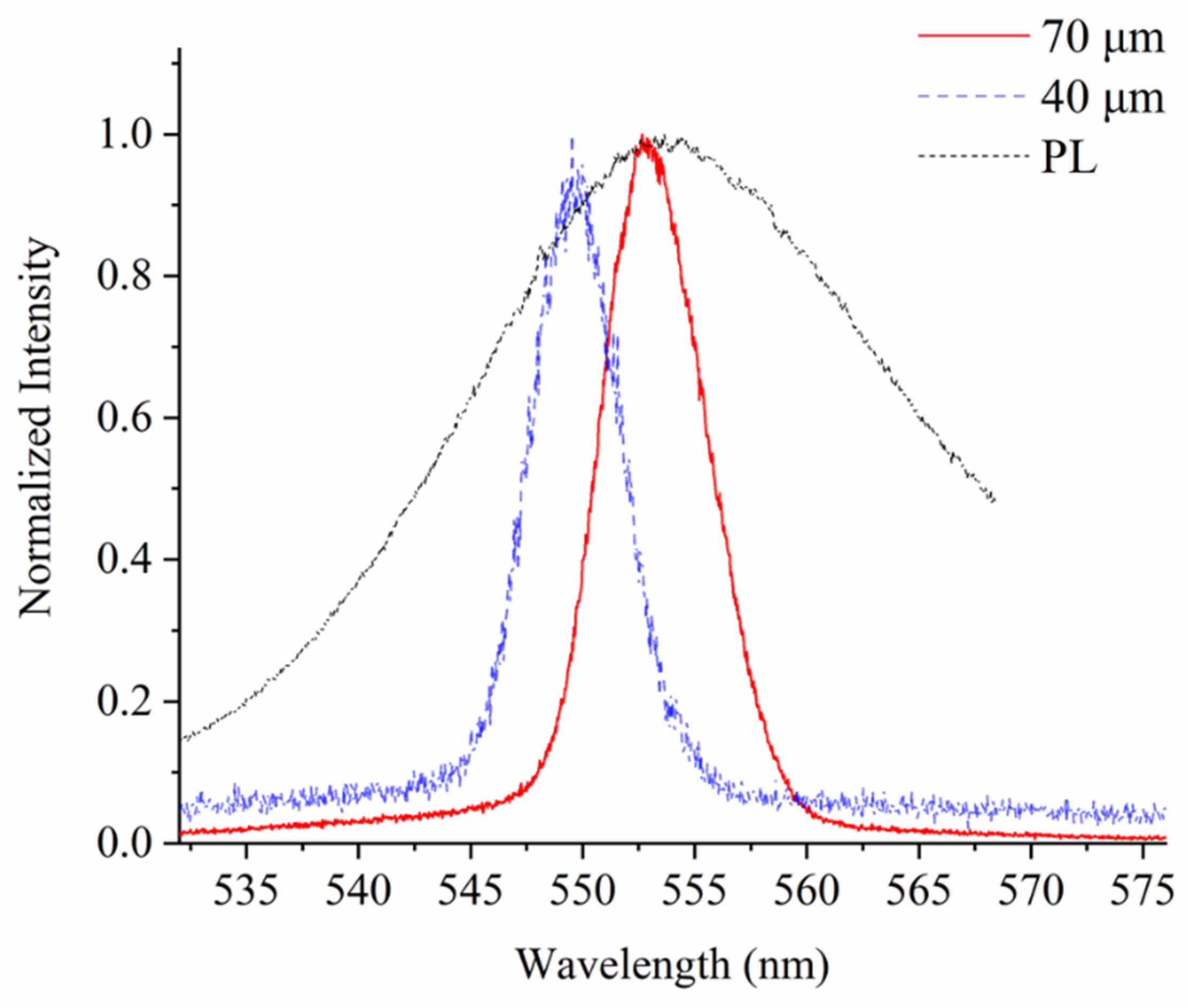
3.3. Structure Dependent Emission
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, Y.; Peng, B.; Bravi, I.; Yu, Y.; Dong, Y.; Liang, R.; Ou, Q.; Monserrat, B.; Zhang, S. Perovskite Nanocrystals: Highly Efficient Blue-Emitting CsPbBr3 Perovskite Nanocrystals through Neodymium Doping. Adv. Sci. 2020, 7, 2001698. [Google Scholar] [CrossRef]
- Kim, H.; Lee, C.; Im, J.; Lee, K.; Moehl, T.; Marchioro, A.; Moon, S.; Humphry-Baker, R.; Yum, J.; Moser, J. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.; Kakekhani, A.; Park, J.; Lee, T. Comprehensive defect suppression in perovskite nanocrystals for high-efficiency light-emitting diodes. Nat. Photonics 2021, 15, 148–155. [Google Scholar] [CrossRef]
- Won, Y.H.; Cho, O.; Kim, T.; Chung, D.Y.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef]
- Kim, T.; Kim, K.; Kim, S.; Choi, S.; Jang, H.; Seo, H.; Lee, H.; Chung, D.; Jang, E. Efficient and stable blue quantum dot light-emitting diode. Nature 2020, 586, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Wang, Y.; Liu, S.; Zeng, P.; Yang, X.; Liang, R.; Ou, Q.; Zhang, S. All-inorganic perovskite- based distributed feedback resonator. Opt. Express 2017, 25, A1154–A1161. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Andrew, P.; Jory, M. Relationship between photonic band structure and emission characteristics of a polymer distributed feedback laser. Phys. Rev. B 2013, 64, 125122. [Google Scholar] [CrossRef]
- Vannahme, C.; Klinkhammer, S.; Lemmer, U. Plastic lab-on-a-chip for fluorescence excitation with integrated organic semiconductor lasers. Opt. Express 2011, 19, 8179–8186. [Google Scholar] [CrossRef]
- Jia, Y.; Kerner, R.; Grede, A.; Rand, B.; Giebink, N. Continuous-wave lasing in an organic inorganic lead halide perovskite semiconductor. Nat. Photonics 2017, 11, 784–788. [Google Scholar] [CrossRef]
- Qin, C.; Sandanayaka, A.; Zhao, C. Stable room-temperature continuous-wave lasing in quasi-2D perovskite films. Nature 2020, 585, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Kerner, R.; Grede, A.; Rand, B.; Giebink, N. Factors that Limit Continuous-Wave Lasing in Hybrid Perovskite Semiconductors. Adv. Opt. Mater. 2019, 8, 1901514. [Google Scholar] [CrossRef]
- Nguyen, D.; Sun, J.; Lo, C.; Liu, J.; Tsai, W.; Li, M.; Yang, S.; Lin, C.; Tzeng, S.; Ma, Y.; et al. Ultralow-Threshold Continuous-Wave Room-Temperature Crystal-Fiber/Nanoperovskite Hybrid Lasers for All-Optical Photonic Integration. Adv. Mater. 2021, 33, 2006819. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yu, Y.; Gong, J.; Yang, C.; Zeng, P.; Dong, Y.; Yang, B.; Liang, R.; Ou, Q.; Zhang, S. Encapsulated room-temperature synthesized CsPbX3 perovskite quantum dots with high stability and wide color gamut for display. Opt. Mater. Express 2018, 8, 3494–3505. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, Z.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Shimabukuro, M.; Arai, H. Enhancement of Humidity-Sensitivity for Perovskite-Type Oxides Having Semi Conductivity. Chem. Lett. 2006, 7, 917–920. [Google Scholar]
- Yoshida, Y.; Nishimura, T.; Fujii, A. Dual ring laser emission of conducting polymers in microcapillary structures. Appl. Phys. Lett. 2005, 86, 539–543. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nuriman, J.; Huskens, W. Optical sensing systems for microfluidic devices: A review. Anal. Chim. Acta 2016, 601, 141–155. [Google Scholar] [CrossRef]
- Suter, J.; White, I.; Zhu, H. Label-free quantitative DNA detection using the liquid core optical ring resonator. Biosens. Bioelectron. 2008, 23, 1003–1009. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nishimura, T.; Fujii, A.; Ozaki, M.; Yoshino, K. Lasing of Poly(3-alkylthiophene) in Microcapillary Geometry. Jpn. J. Appl. Phys. 2005, 44, 1056–1058. [Google Scholar] [CrossRef]
- Zhang, S.; Zhai, T.; Cui, L. Tunable WGM Laser Based on the Polymer Thermo-Optic Effect. Polymers 2021, 13, 205. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, H.; Takeaki, R.; Tomita, S. Dye-doped polymer microring laser coupled with stimulated resonant Raman scattering. Appl. Phys. Lett. 2009, 95, 196. [Google Scholar] [CrossRef]
- Kurahashi, N.; Nguyen, V.; Sasaki, F.; Yanagi, H. Whispering gallery mode lasing in lead halide perovskite crystals grown in microcapillary. Appl. Phys. Lett. 2018, 113, 011107. [Google Scholar] [CrossRef]
- Dong, Y.; Zeng, P.; Yu, Y.; Xie, Y.; Yang, B.; Liang, R.; Ou, Q.; Zhang, S. Trivalent-Neodymium Additive Modulated MAPbBr3 Perovskite Nucleation and Growth: Ultrawide Processing Window for One-Step Fabrication of Efficient Light-Emitting Perovskites. Adv. Electron. Mater. 2020, 6, 1901162. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zeng, P.; Ou, Q.; Zhang, S. Fabrication of Perovskite Film-Coated Hollow Capillary Fibers Using a Fast Solvent Exchange Method. Nanomaterials 2021, 11, 1483. https://doi.org/10.3390/nano11061483
Li X, Zeng P, Ou Q, Zhang S. Fabrication of Perovskite Film-Coated Hollow Capillary Fibers Using a Fast Solvent Exchange Method. Nanomaterials. 2021; 11(6):1483. https://doi.org/10.3390/nano11061483
Chicago/Turabian StyleLi, Xuesong, Pan Zeng, Qiongrong Ou, and Shuyu Zhang. 2021. "Fabrication of Perovskite Film-Coated Hollow Capillary Fibers Using a Fast Solvent Exchange Method" Nanomaterials 11, no. 6: 1483. https://doi.org/10.3390/nano11061483
APA StyleLi, X., Zeng, P., Ou, Q., & Zhang, S. (2021). Fabrication of Perovskite Film-Coated Hollow Capillary Fibers Using a Fast Solvent Exchange Method. Nanomaterials, 11(6), 1483. https://doi.org/10.3390/nano11061483




