A Molecular Shape Recognitive HPLC Stationary Phase Based on a Highly Ordered Amphiphilic Glutamide Molecular Gel
Abstract
1. Introduction
2. Materials and Methods
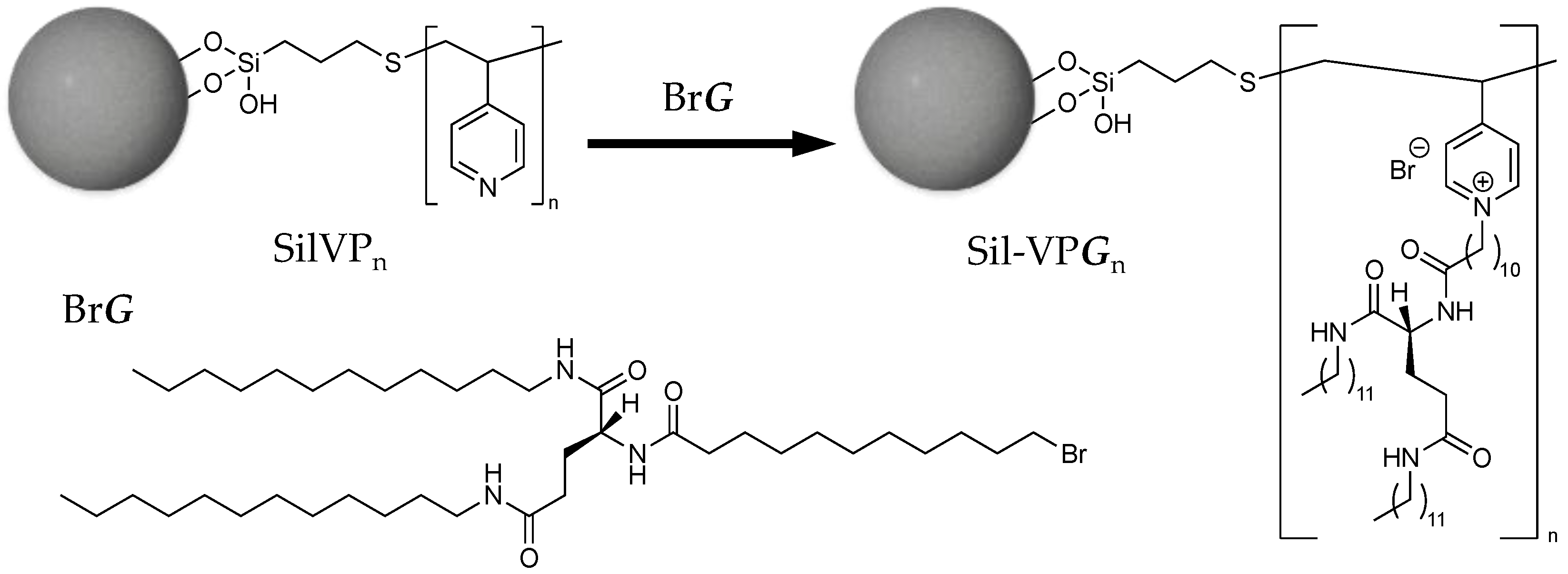
2.1. Stabilization of Glutamide-Derived Lipid-Branched Polymers on Porous Silica (Sil−VPG15)
2.1.1. Materials and Instruments
2.1.2. Synthesis of Glutamide-Derived Lipid
2.1.3. Preparation of Poly(4-Vinylpyridine)-Grafted Porous Silica
2.1.4. Quaternization of Pyridyl Side Chains with Glutamide-Derived Lipid
2.2. HPLC Performance of Sil−VPG15 as the Stationary Phase
2.2.1. Chemicals
2.2.2. Chromatographic Procedures
3. Results and Discussion
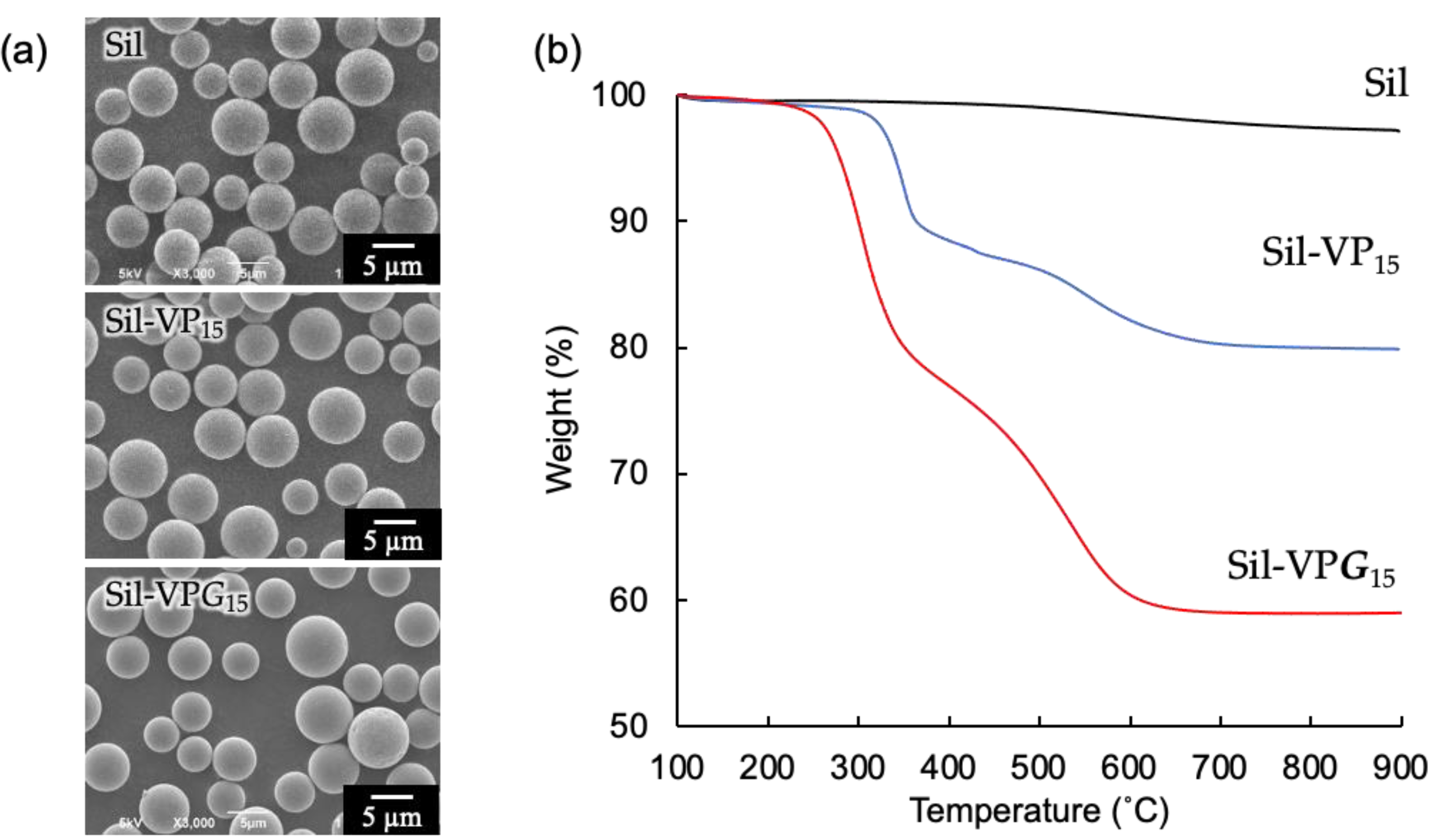
3.1. Characterization of Amphiphilic Glutamide-Based Molecular Gel on the Porous Silica Particle
3.1.1. Grafting of Amphiphilic Glutamide-Based Molecular Gel
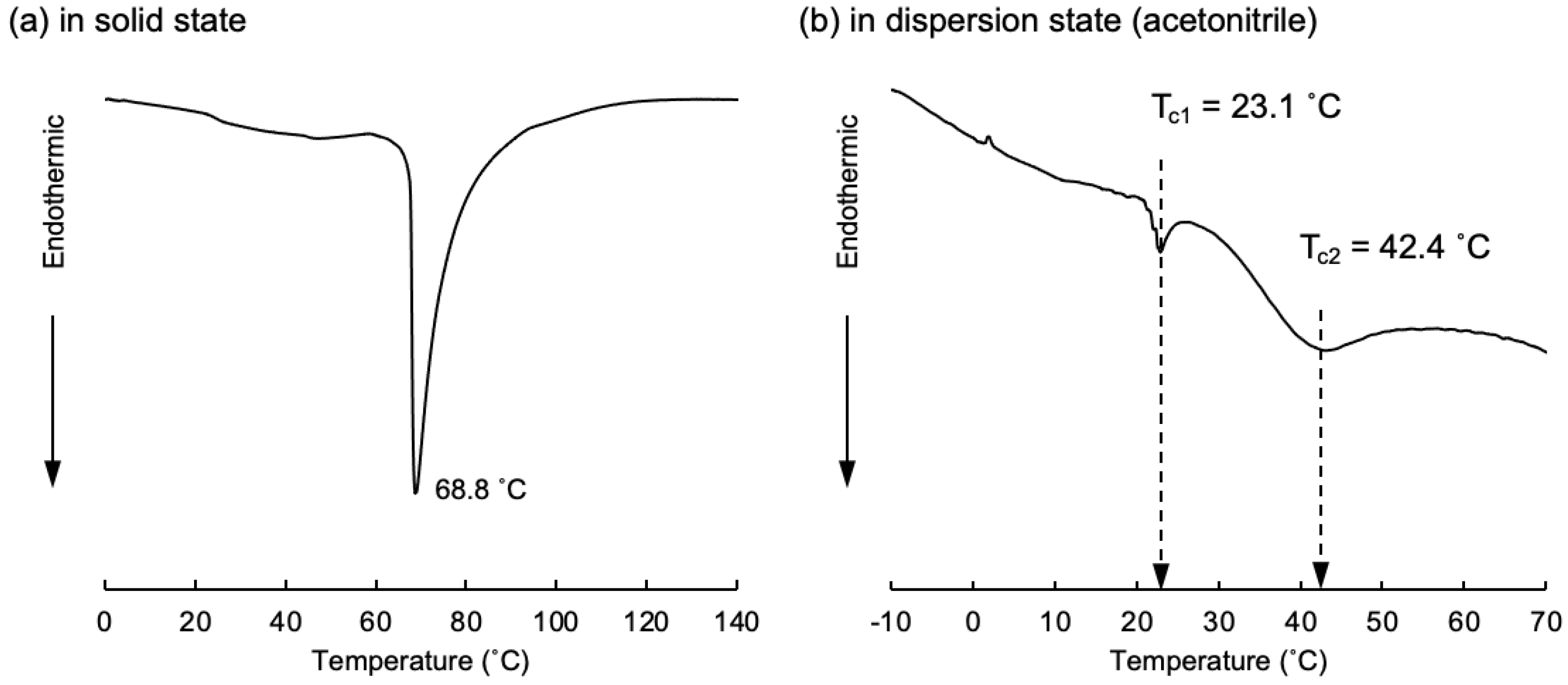
3.1.2. Evaluation of Amphiphilic Glutamide-Based Molecular Gels on the Silica Surface
3.2. HPLC Performance of Amphiphilic Glutamide-Based Molecular Gel
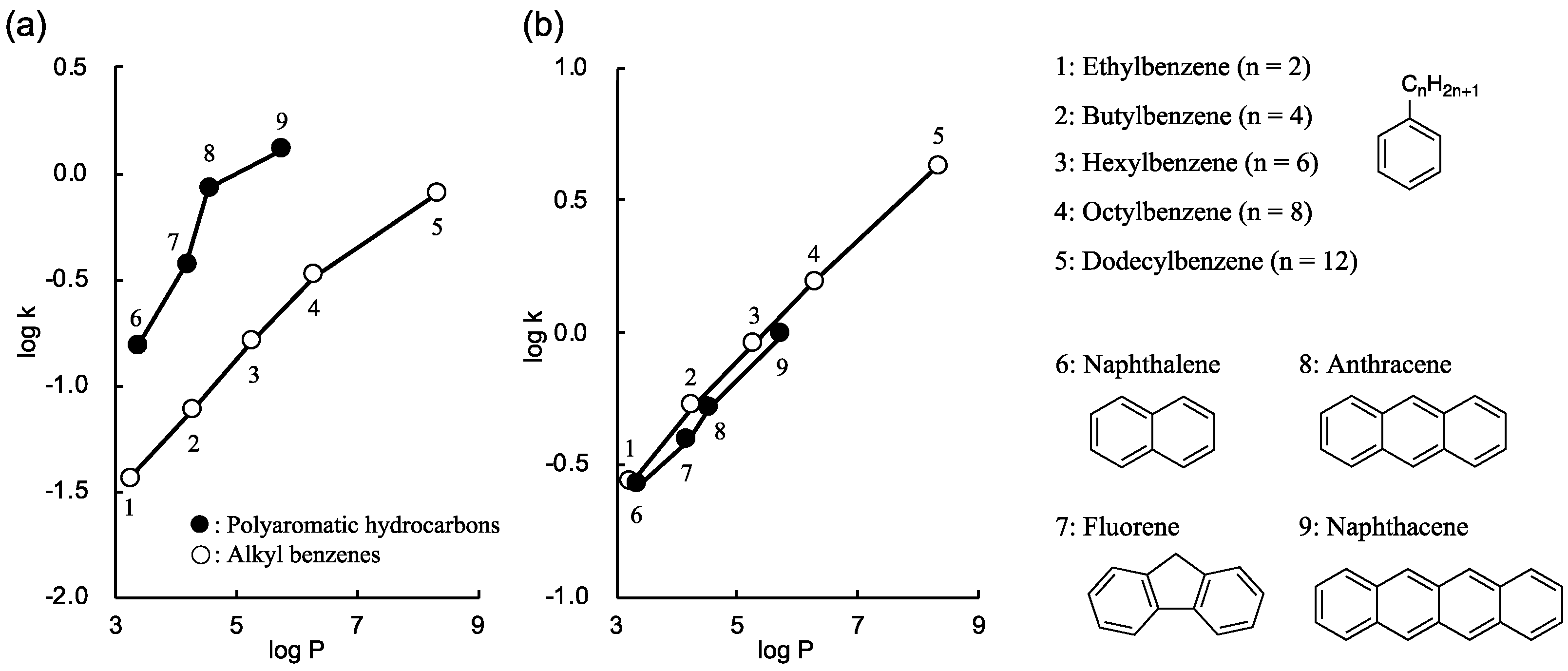
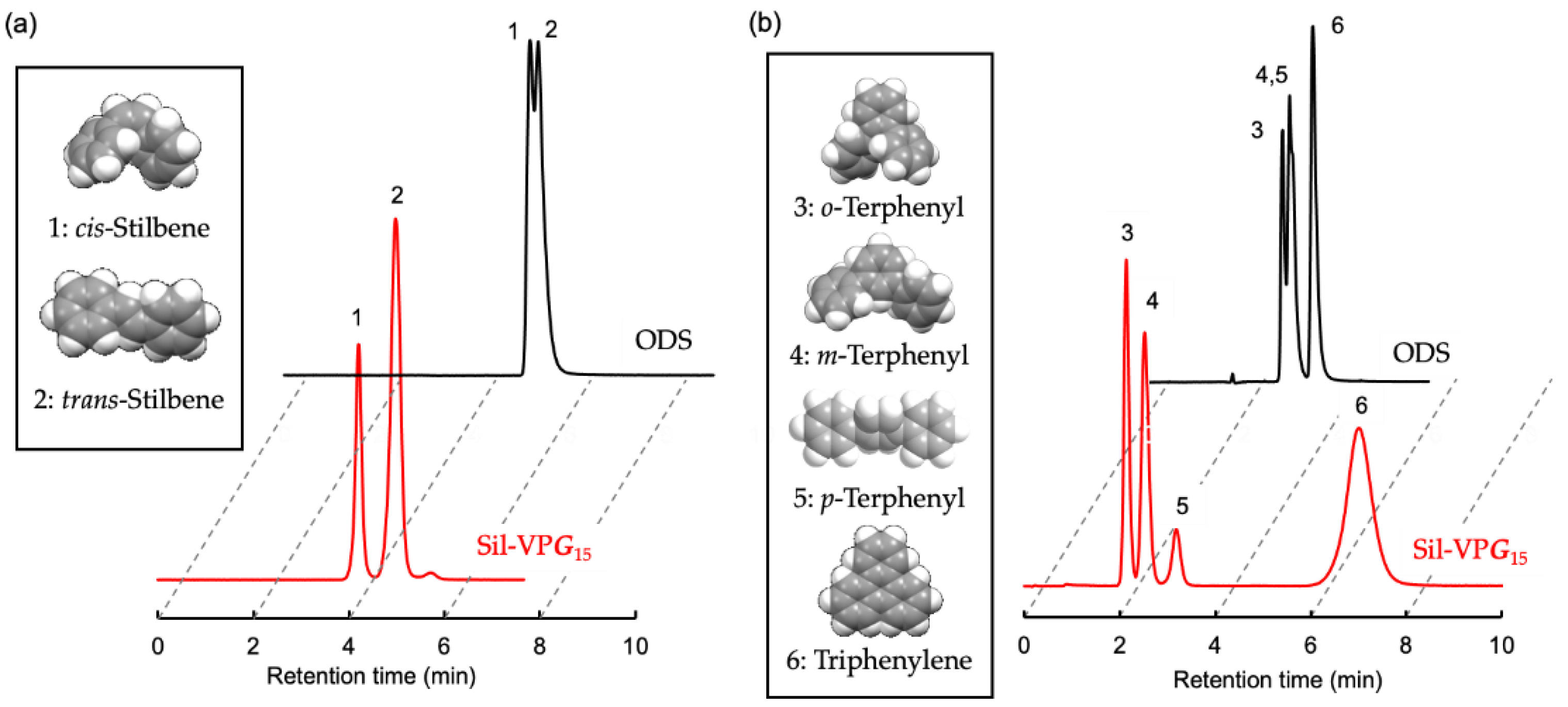
3.2.1. Evaluation of Sil−VPG15 as an HPLC Stationary Phase
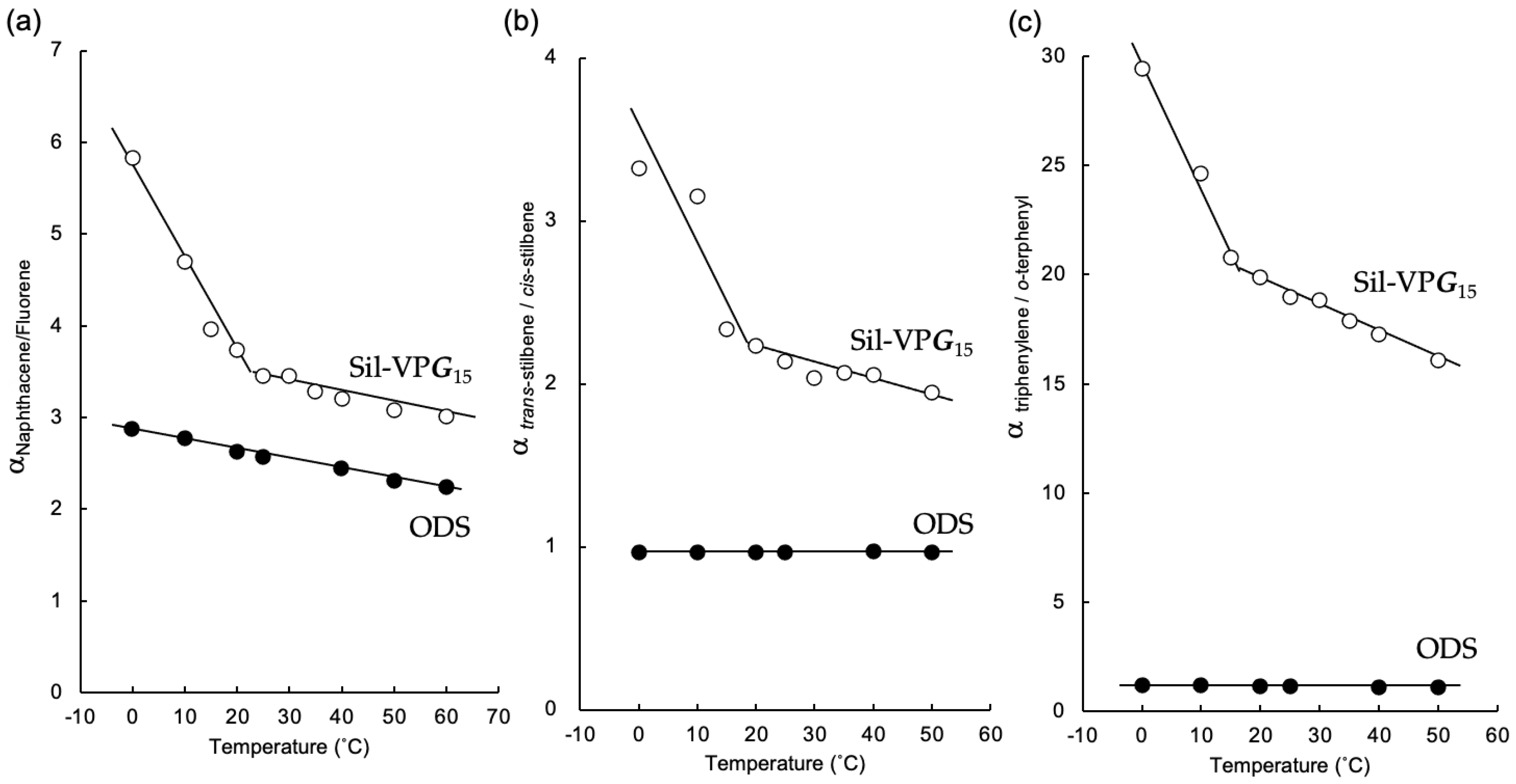
3.2.2. Temperature Dependence of the Separation Factors for PAH Isomers
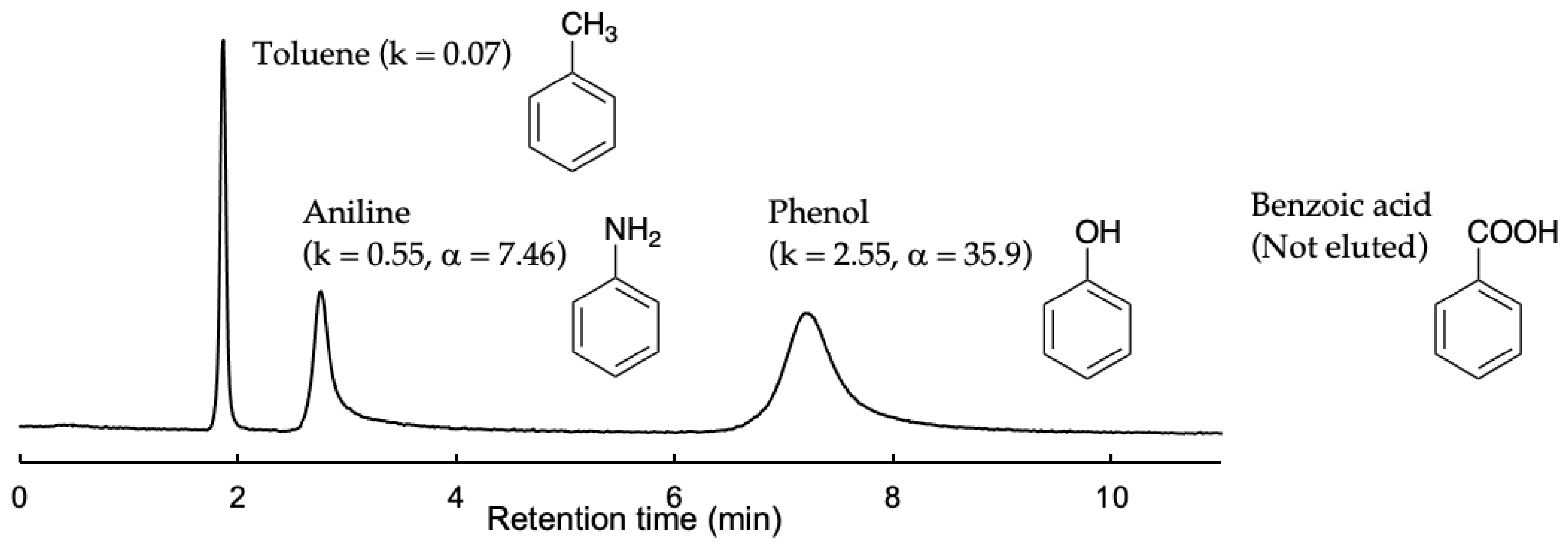
3.2.3. Selective Separations of Aromatic Compounds Having Hydrophilic Functional Groups
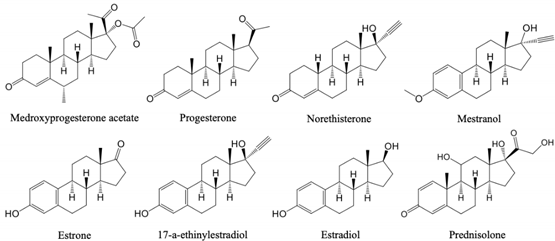
3.2.4. Application of Sil−VPG15 Column to Separations of Steroids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shi, X.; Qiao, L.; Xu, G. Recent development of ionic liquid stationary phases for liquid chromatography. J. Chromatogr. A 2015, 1420, 1–15. [Google Scholar] [CrossRef]
- Wang, Q.; Baker, G.A.; Bakerc, S.N.; Colón, L.A. Surface confined ionic liquid as a stationary phase for HPLC. Analyst 2006, 131, 1000–1005. [Google Scholar] [CrossRef]
- Sun, M.; Feng, J.; Chen, W.; Li, L.; Duan, H.; Luo, C. Improvement of the chromatographic separation performance of an imidazolium ionic liquid functionalized silica column by in situ anion-exchange with dodecyl sulfonate and dodecylbenzene sulfonate anions. J. Sep. Sci. 2014, 37, 1283–1288. [Google Scholar] [CrossRef]
- Qiao, L.; Dou, A.; Shi, X.; Li, H.; Shan, Y.; Lu, X.; Xu, G. Development and evaluation of new imidazolium-based zwitterionic stationary phases for hydrophilic interaction chromatography. J. Chromatogr. A 2013, 1286, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Liang, X.; Sun, M.; Jiang, S. Development of silica-based stationary phases for high-performance liquid chromatography. Anal. Bioanal. Chem. 2011, 399, 3307–3322. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cai, T.; Zhang, H.; Chen, J.; Li, Z.; Zhao, L.; Li, Z.; Qiu, H. Two copolymer-grafted silica stationary phases prepared by surface thiol-ene click reaction in deep eutectic solvents for hydrophilic interaction chromatography. J. Chromatogr. A 2020, 1609, 460446–460454. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cai, T.; Zhang, H.; Chen, J.; Li, Z.; Qiu, H. Poly(itaconic acid)-grafted silica stationary phase prepared in deep eutectic solvents and its unique performance in hydrophilic interaction chromatography. Talanta 2019, 191, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Skoczylas, M.; Bociana, S.; Buszewski, B. Dipeptide-bonded stationary phases for hydrophilic interaction liquid chromatography. RSC Adv. 2016, 6, 96389–96397. [Google Scholar] [CrossRef]
- Ray, S.; Takafuji, M.; Ihara, H. Chromatographic evaluation of a newly designed peptide-silica stationary phase in reverse phase liquid chromatography and hydrophilic interaction liquid chromatography: Mixed mode behavior. J. Chromatogr. A 2012, 1266, 43–52. [Google Scholar] [CrossRef]
- Ohyama, K.; Inoue, Y.; Kishikawa, N.; Kuroda, N. Preparation and characterization of surfactin-modified silica stationary phase for reversed-phase and hydrophilic interaction liquid chromatography. J. Chromatogr. A 2014, 1371, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Olesik, S.V. Homogeneous edge-plane carbon as stationary phase for reversed-phase liquid chromatography. Anal. Chem. 2015, 87, 3616–3622. [Google Scholar] [CrossRef]
- Noguchi, H.; Sultana, M.; Hano, N.; Kuwahara, Y.; Takafuji, M.; Nagaoka, S.; Qiu, H.; Ihara, H. Fabrication of carbon-like, π-conjugated organic layer on a nano-porous silica surface. Nanomaterials 2020, 10, 1882. [Google Scholar] [CrossRef]
- Candelaria1, L.; Frolova, L.V.; Kowalski, B.M.; Artyushkova, K.; Serov, A.; Kalugin, N.G. Surface-modified three-dimensional graphene nanosheets as a stationary phase for chromatographic separation of chiral drugs. Sci. Rep. 2018, 8, 14747. [Google Scholar] [CrossRef] [PubMed]
- Sander, L.C.; Wise, S.A. Synthesis and characterization of polymeric C18 stationary phases for liquid chromatography. Anal. Chem. 1984, 56, 504–510. [Google Scholar] [CrossRef]
- Jinno, K.; Nagoshi, T.; Tanaka, N.; Okamoto, M.; Fetzer, J.C.; Biggs, W.R. Eluation behaviour of planar and non-planar polycyclic aromatic hydrocarbons on various chemically bonded stationary phases in liquid chromatography. J. Chromatogr. A 1987, 392, 75–82. [Google Scholar] [CrossRef]
- Weiss, R.G.; Terech, P. (Eds.) Molecular Gels; Springer: Berlin, Germany, 2006. [Google Scholar]
- Ihara, H.; Takafuji, M.; Kuwahara, Y. Polymer functionalization by luminescent supramolecular gel. Polym. J. 2016, 48, 843–853. [Google Scholar] [CrossRef]
- Shimizu, T.; Masuda, M.; Minamikawa, H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 2005, 105, 1401–1444. [Google Scholar] [CrossRef]
- Ihara, H.; Takafuji, M.; Kuwahara, Y.; Okazaki, Y.; Ryu, N.; Sagawa, T.; Oda, R. Luminescent supramolecular gel for light management technology. In Molecular Technology; Yamamoto, H., Kato, T., Eds.; Wiley: Hoboken, NJ, USA, 2019; Volume 4, pp. 297–337. [Google Scholar]
- Weiss, R.G. (Ed.) Molecular Gels: Structure and Dynamics; Royal Society of Chemistry: Cambridge, UK, 2018. [Google Scholar]
- Escuder, B.; Miravet, J.F. (Eds.) Functional Molecular Gels; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Goto, T.; Okazaki, Y.; Ueki, M.; Kuwahara, Y.; Takafuji, M.; Oda, R.; Ihara, H. Induction of strong and tunable circularly polarized luminescence of non-chiral, non-metal, low-molecular-weight fluorophores using chiral nano-template. Angew. Chem. Int. Ed. 2017, 56, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, M.; Kawahara, T.; Sultana, N.; Ryu, N.; Yoshida, K.; Kuwahara, Y.; Oda, R.; Ihara, H. Extreme enhancement of secondary chirality through coordination-driven steric changes of terpyridyl ligand in glutamide-based molecular gels. RSC Adv. 2020, 10, 29627–29632. [Google Scholar] [CrossRef]
- Oishi, H.; Mashima, S.; Kuwahara, Y.; Takafuji, M.; Yoshida, K.; Oda, R.; Qiu, H.; Ihara, H. Polymer encapsulation and stabilization of molecular gel- based chiroptical information for strong, tunable circularly polarized luminescence film. J. Mater. Chem. C 2020, 8, 8732–8735. [Google Scholar] [CrossRef]
- Yoshida, K.; Kuwahara, Y.; Miyamoto, K.; Nakashima, S.; Jintoku, H.; Takafuji, M.; Ihara, H. A room-temperature phosphorescent polymer film containing a molecular web based on one-dimensional chiral stacking of a simple luminophore. Chem. Commun. 2017, 53, 5044–5047. [Google Scholar] [CrossRef]
- Ihara, H.; Takafuji, M.; Hirayama, C.; O’Brien, D.F. Effect of photopolymerization on the morphology of helical supramolecular assemblies. Langmuir 1992, 8, 1548–1553. [Google Scholar] [CrossRef]
- Kira, Y.; Okazaki, Y.; Sawada, T.; Takafuji, M.; Ihara, H. Amphiphilic molecular gels from omega-aminoalkylated L-glutamic acid derivatives with unique chiroptical property. Amino Acids 2010, 39, 587–697. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, M.; Kira, Y.; Tsuji, H.; Sawada, S.; Hachisako, H.; Ihara, H. Optical active polymer film tuned by chirally self-assembled molecular organogel. Tetrahedron 2007, 63, 7489–7494. [Google Scholar] [CrossRef]
- Rahman, M.M.; Takafuji, M.; Ansarian, H.R.; Derakhshan, M.; Ihara, H. Enhanced molecular shape selectivity through multiple carbonyl-p interaction with noble non-crystalline solid phase for RP-HPLC. Anal. Chem. 2005, 77, 6671–6681. [Google Scholar] [CrossRef]
- Takafuji, M.; Rahman, M.M.; Ansarian, H.R.; Derakhshan, M.; Sakurai, T.; Ihara, H. Dioctadecyl L-glutamide-derived lipid-grafted silica as a novel organic stationary phase for RP-HPLC. J. Chromatogr. A 2005, 1074, 223–228. [Google Scholar] [CrossRef]
- Noguchi, H.; Charoenraks, T.; Takafuji, M.; Ihara, H. Effects of substitution groups of glutamide-derived molecular gels on molecular shape recognition. J. Chromatogr. A 2015, 1392, 56–62. [Google Scholar] [CrossRef]
- Rahman, M.M.; Takafuji, M.; Ihara, H. Evaluation of selectivity for L-glutamide-derived highly ordered assemblies in reversed-phase high-performance liquid Chromatography. Talanta 2009, 77, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Gopal, V.; Prasad, T.K.; Rao, N.M.; Takafuji, M.; Rahman, M.M.; Ihara, H. Synthesis and in vitro evaluation of glutamide-containing cationic lipids for gene delivery. Bioconjugate Chem. 2006, 17, 1530–1536. [Google Scholar] [CrossRef]
- Ihara, H.; Nagaoka, S.; Tanaka, H.; Sakaki, S.; Hirayama, C. Lipid membrane analogue-immobilized silica gels for separation with molecular recognition. J. Liq. Chromatogr. 1996, 19, 2967–2984. [Google Scholar] [CrossRef]
- Biesalski, M.; Ruhe, J. Swelling of a polyelectrolyte brush in humid air. Langmuir 2000, 16, 1943–1950. [Google Scholar] [CrossRef]
- Takafuji, M.; Mimaki, T.; Xu, Z.; Ihara, H. Surface charge controlled magnetic nanoparticles with grafting of poly(4-vinylpyridine). J. Nanosci. Nanotechnol. 2005, 5, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, C.; Ihara, H.; Mukai, T. Lipid membrane analogues. Specific retention behavior in comb-shaped telomer-immobilized porous silica gels. Macromolecules 1992, 25, 6375–6376. [Google Scholar] [CrossRef]
- Ihara, H.; Goto, Y.; Sakurai, T.; Takafuji, M.; Sagawa, T.; Nagaoka, S. Enhanced molecular-shape selectivity for polyaromatic hydrocarbons through isotropic-to-crystalline phase transition of poly(octadecyl acrylate). Chem. Lett. 2001, 30, 1252–1253. [Google Scholar] [CrossRef]
- Ihara, H.; Fukui, M.; Mimaki, T.; Shundo, A.; Dong, W.; Derakhshan, M.; Sakurai, T.; Takafuji, M.; Nagaoka, S. Poly(4-vinylpyridine) as a novel organic end-capping reagent for silica and its specific selectivity for PAHs and dinitropyrenes in a reversed phase. Anal. Chim. Acta. 2005, 548, 51–57. [Google Scholar] [CrossRef]
- Rodriguez, E.L.; Poddar, S.; Iftekhar, S.; Suh, K.; Woolfork, A.G.; Ovbude, S.; Pekarek., A.; Walters, M.; Lott, S.; Hage, D.S. Affinity chromatography: A review of trends and developments over the past 50 years. J. Chromatogr. B 2020, 1157, 122332. [Google Scholar] [CrossRef]
- Kip, C.; Hamaloğlu, K.O.; Demir, C.; Tuncel., A. Recent trends in sorbents for bioaffinity chromatography. J. Sep. Sci. 2021, 44, 1273–1291. [Google Scholar] [CrossRef]
- Maekawa, Y.; Yamazaki, K.; Ihara, M.; Nagase, K.; Kanazawa, H. Simultaneous analysis of multiple oligonucleotides by temperature-responsive chromatography using a poly(N-isopropylacrylamide)-based stationary phase. Anal. Bioanal. Chem. 2020, 412, 5341–5351. [Google Scholar] [CrossRef] [PubMed]

| Stationary Phase | C% | H% | N% | C/N | Organic Phase (wt%) | |
|---|---|---|---|---|---|---|
| Sil−VP15 | Found | 13.4 | 1.73 | 2.18 | 6.13 | 17.2 |
| Calcd 1 | 15.7 | 1.37 | 2.56 | 6.13 | ||
| Sil−VPG15 | Found | 27.6 | 4.48 | 3.18 | 8.68 | 38.0 |
| Calcd 2 | 28.7 | 3.89 | 3.31 | 8.67 |
| Elutes | Sil−VPG15 | ODS | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 °C | 50 °C | 0 °C | 50 °C | |||||
| k | α | k | α | k | α | k | α | |
| cis-Stilbene | 0.21 | – | 0.05 | – | 0.61 | – | 0.26 | – |
| trans-Stilbene | 0.68 | 3.24 | 0.09 | 1.80 | 0.55 | 0.90 (1.11) | 0.22 | 0.85 (1.18) |
| o-Terphenyl | 0.28 | – | 0.09 | – | 0.70 | – | 0.31 | – |
| m-Terphenyl | 0.76 | 2.71 | 0.19 | 2.11 | 0.84 | 1.20 | 0.35 | 1.13 |
| p-Terphenyl | 1.74 | 6.21 | 0.34 | 3.78 | 0.94 | 1.34 | 0.37 | 1.19 |
| Triphenylene | 8.36 | 29.86 | 1.39 | 15.44 | 1.36 | 1.94 | 0.51 | 1.65 |
| Elutes | Sil−VPG15 | ||
|---|---|---|---|
| k | α | α (vs. Phenol) | |
| Phenol | 2.55 | – | |
| 2-Methyl phenol (o-isomer) | 2.37 | – | 0.93 |
| 3-Methyl phenol (m-isomer) | 2.18 | 0.92 | 0.86 |
| 4-Methyl phenol (p-isomer) | 2.66 | 1.22 | 1.04 |
| 1,2-Dihydroxy benzene (o-isomer) | 24.2 | – | 9.49 |
| 1,3-Dihydroxy benzene (m-isomer) | 27.4 | 1.13 | 10.75 |
| 1,4-Dihydroxy benzene (p-isomer) | 34.4 | 1.42 | 13.49 |
| 2-Aminophenol (o-isomer) | 12.4 | – | 4.86 |
| 3-Aminophenol (m-isomer) | 16.8 | 1.35 | 6.59 |
| 4-Aminophenol (p-isomer) | 24.2 | 1.95 | 9.49 |
| Elutes 1 | Sil−VPG15 | ODS | ||
|---|---|---|---|---|
| k | α | k | α | |
| Medroxyprogesterone acetate | 0.07 | 0.27 | 0.48 | 0.71 (1.42) |
| Progesterone | 0.26 | 1.00 | 0.68 | 1.00 |
| Norethisterone | 0.81 | 3.11 | 0.26 | 0.38 (2.62) |
| Mestranol | 1.16 | 4.48 | 0.39 | 0.57 (1.74) |
| Estrone | 4.06 | 15.7 | 0.22 | 0.32 (3.09) |
| 17-α-Ethinylestradiol | 12.3 | 47.6 | 0.14 | 0.21 (4.86) |
| Estradiol | 14.2 | 54.7 | 0.29 | 0.43 (2.34) |
| Prednisolone | 22.8 | 87.7 | 0.12 | 0.18 (5.67) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamoto, N.; Hu, Y.; Kuwahara, Y.; Ihara, H.; Takafuji, M. A Molecular Shape Recognitive HPLC Stationary Phase Based on a Highly Ordered Amphiphilic Glutamide Molecular Gel. Nanomaterials 2021, 11, 1574. https://doi.org/10.3390/nano11061574
Kawamoto N, Hu Y, Kuwahara Y, Ihara H, Takafuji M. A Molecular Shape Recognitive HPLC Stationary Phase Based on a Highly Ordered Amphiphilic Glutamide Molecular Gel. Nanomaterials. 2021; 11(6):1574. https://doi.org/10.3390/nano11061574
Chicago/Turabian StyleKawamoto, Naoki, Yongxing Hu, Yutaka Kuwahara, Hirotaka Ihara, and Makoto Takafuji. 2021. "A Molecular Shape Recognitive HPLC Stationary Phase Based on a Highly Ordered Amphiphilic Glutamide Molecular Gel" Nanomaterials 11, no. 6: 1574. https://doi.org/10.3390/nano11061574
APA StyleKawamoto, N., Hu, Y., Kuwahara, Y., Ihara, H., & Takafuji, M. (2021). A Molecular Shape Recognitive HPLC Stationary Phase Based on a Highly Ordered Amphiphilic Glutamide Molecular Gel. Nanomaterials, 11(6), 1574. https://doi.org/10.3390/nano11061574








