Effect of Cu2O Substrate on Photoinduced Hydrophilicity of TiO2 and ZnO Nanocoatings
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Surface Characterization
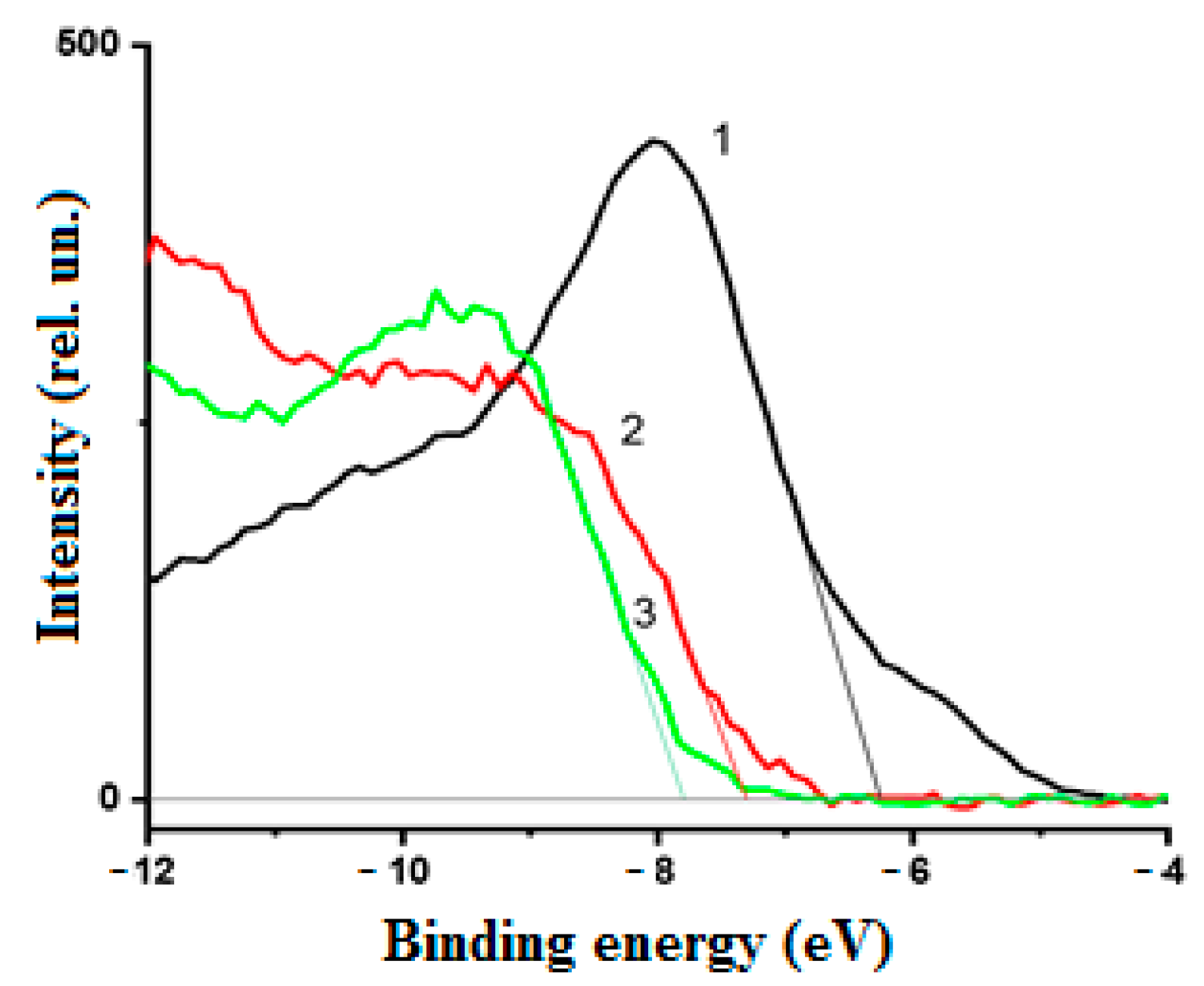
3.2. Electronic Properties of the Nanocoating Components
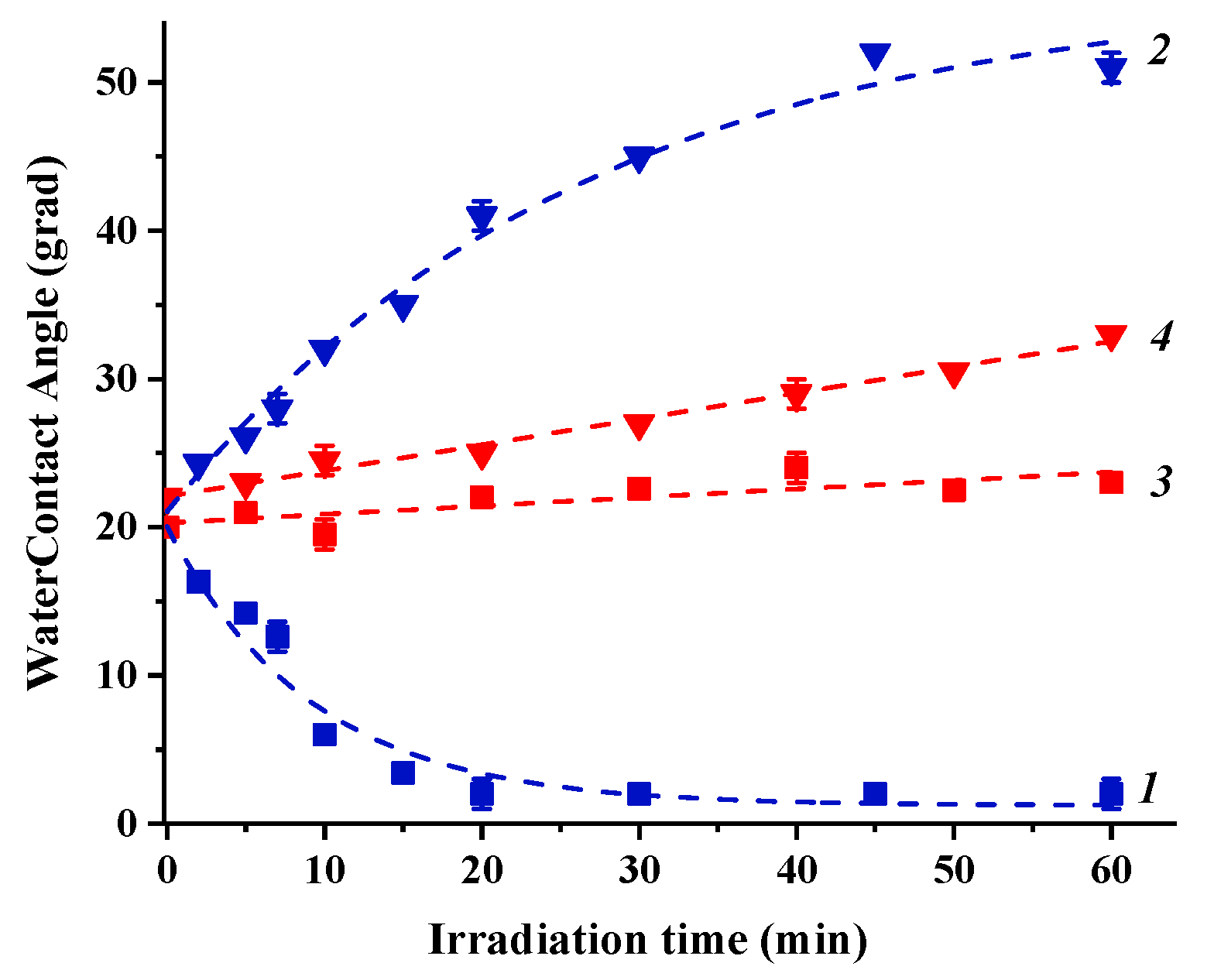
3.3. Effect of Light Irradiation on the Photoinduced Hydrophilicity of Heterostructured Nanocoating Surfaces
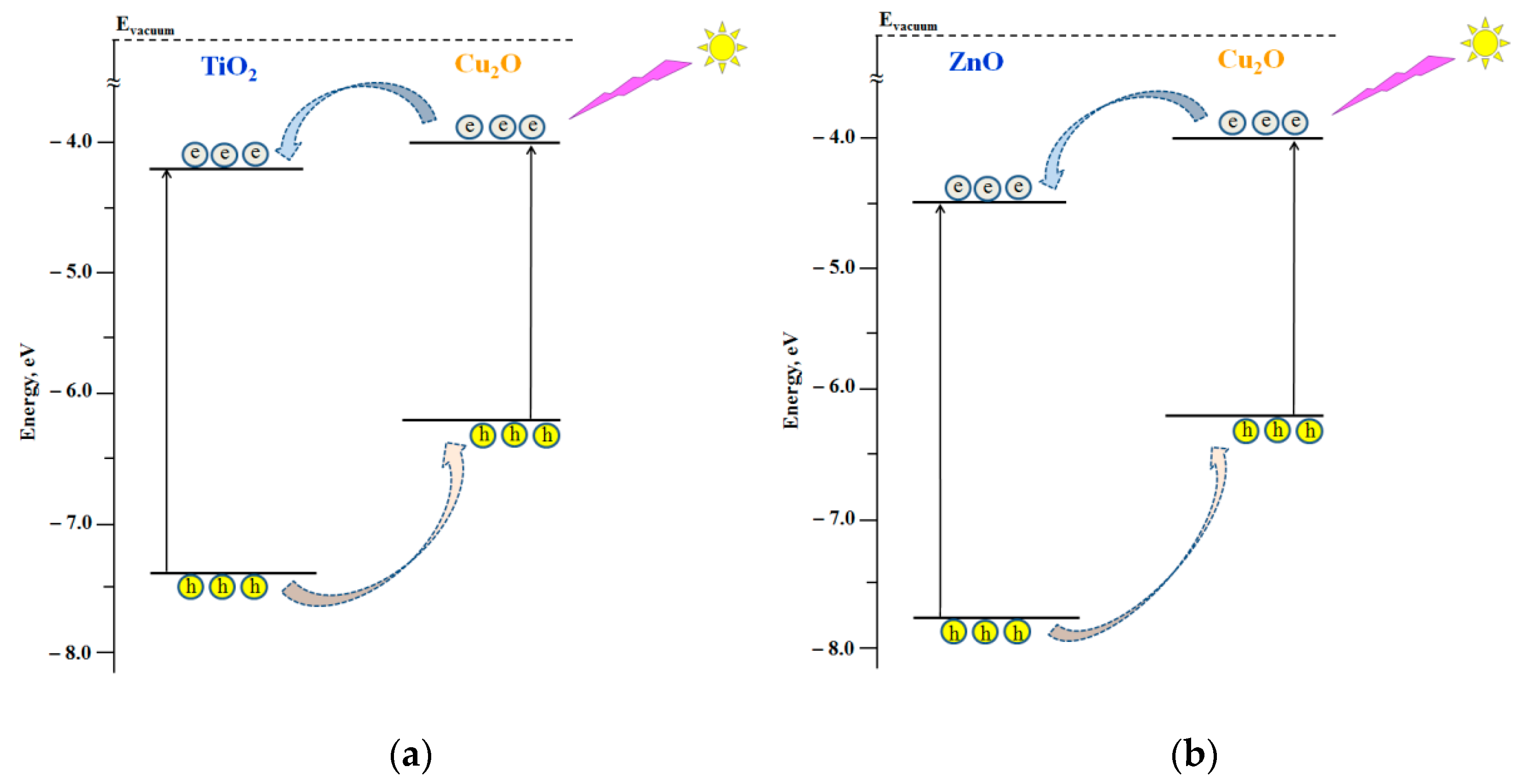
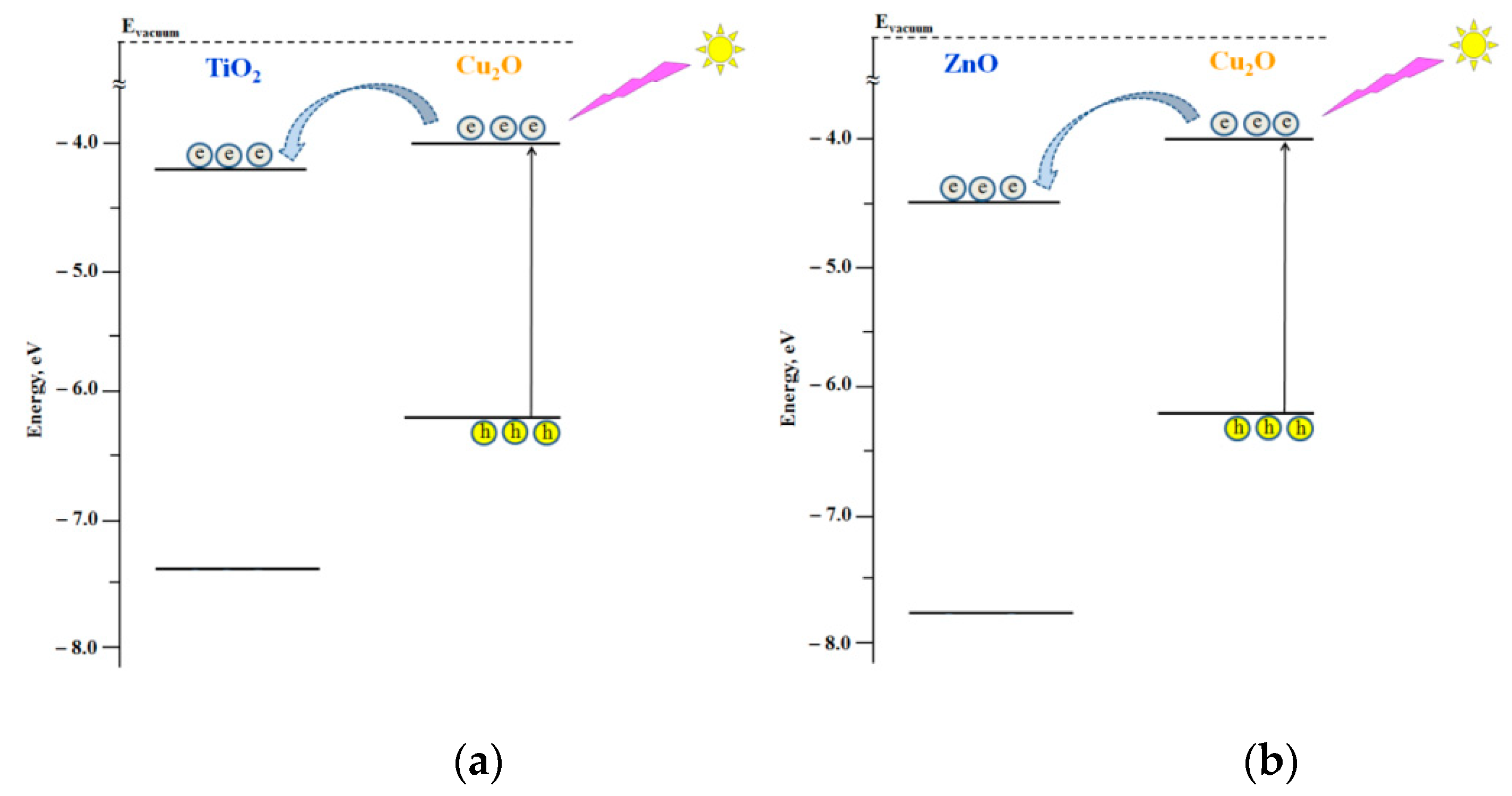
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hozumi, A.; Jiang, L.; Lee, H.; Shimomura, M. (Eds.) Stimuli-Responsive Dewetting/Wetting Smart Surfaces and Interfaces. In Bio-Logically-Inspired Systems (V. 11); Springer: Cham, Switzerland, 2018; p. 464. [Google Scholar] [CrossRef]
- He, J. Self-Cleaning Coatings: Structure, Fabrication and Application. In RSC Smart Materials (V. 21); RSC: London, UK, 2016; p. 327. [Google Scholar]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176-177, 396–428. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nat. Cell Biol. 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Emeline, A.V. Photoinduced Hydrophilicity of Surfaces of Thin Films. Colloid J. 2021, 83, 20–48. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Emeline, A.V.; Bahnemann, D.W. Effect of the TiO2–ZnO Heterostructure on the Photoinduced Hydrophilic Conversion of TiO2 and ZnO Surfaces. J. Phys. Chem. C 2019, 123, 8884–8891. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Oparicheva, U.G.; Grishina, A.E.; Maevskaya, M.; Emeline, A.V.; Bahnemann, D.W. Dependences of ZnO Photoinduced Hydrophilic Conversion on Light Intensity and Wavelengths. J. Phys. Chem. C 2015, 119, 9824–9828. [Google Scholar] [CrossRef]
- Emeline, A.V.; Rudakova, A.; Sakai, M.; Murakami, T.; Fujishima, A. Factors Affecting UV-Induced Superhydrophilic Conversion of a TiO2 Surface. J. Phys. Chem. C 2013, 117, 12086–12092. [Google Scholar] [CrossRef]
- Yan, X.; Abe, R.; Ohno, T.; Toyofuku, M.; Ohtani, B. Action spectrum analyses of photoinduced superhydrophilicity of titania thin films on glass plates. Thin Solid Films 2008, 516, 5872–5876. [Google Scholar] [CrossRef][Green Version]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Wang, S.; Feng, X.; Yao, J.; Jiang, L. Controlling Wettability and Photochromism in a Dual-Responsive Tungsten Oxide Film. Angew. Chem. Int. Ed. 2006, 45, 1264–1267. [Google Scholar] [CrossRef]
- Lim, H.S.; Kwak, D.; Lee, D.Y.; Lee, S.G.; Cho, K. UV-Driven Reversible Switching of a Roselike Vanadium Oxide Film between Superhydrophobicity and Superhydrophilicity. J. Am. Chem. Soc. 2007, 129, 4128–4129. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Maevskaya, M.; Emeline, A.; Bahnemann, D.W. Light-Controlled ZrO2 Surface Hydrophilicity. Sci. Rep. 2016, 6, srep34285. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Emeline, A.V.; Bulanin, K.; Chistyakova, L.; Maevskaya, M.; Bahnemann, D.W. Self-cleaning properties of zirconium dioxide thin films. J. Photochem. Photobiol. A Chem. 2018, 367, 397–405. [Google Scholar] [CrossRef]
- Zhang, L.; Dillert, R.; Bahnemann, D.W.; Vormoor, M. Photo-induced hydrophilicity and self-cleaning: Models and reality. Energy Environ. Sci. 2012, 5, 7491–7507. [Google Scholar] [CrossRef]
- Gaminian, H.; Montazer, M. Enhanced Self-Cleaning Properties on Polyester Fabric under Visible Light Through Single-Step Synthesis of Cuprous Oxide Doped Nano-TiO2. Photochem. Photobiol. 2015, 91, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, D.; Kumar, P.; Purkayastha, D.D. Superhydrophilicity of photocatalytic ZnO/SnO2 heterostructure for self-cleaning applications. J. Sol-Gel Sci. Technol. 2019, 92, 575–584. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A. Semiconductor Photocatalysis—Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, W.; Xu, Q.; Li, G.; Liu, S.; Jia, X.; Qin, Y.; Wang, Z.L. Type-II hetero-junction dual shell hollow spheres loaded with spatially separated cocatalyst for enhancing visible light hydrogen evolution. Nano Energy 2017, 38, 518–525. [Google Scholar] [CrossRef]
- Rudakova, A.V. How we can manipulate the photoinduced surface hydrophilicity. Book of Abstract. In Proceedings of the 7th International Conference on Semiconductor Photochemistry, SP7, Milan, Italy, 11–14 September 2019. [Google Scholar]
- Jiang, X.; Chen, X. Crystallization behavior and hydrophilic performances of V2O5–TiO2 films prepared by sol–gel dip-coating. J. Cryst. Growth 2004, 270, 547–552. [Google Scholar] [CrossRef]
- Miyauchi, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Photoinduced Hydrophilic Conversion of TiO2/WO3 Layered Thin Films. Chem. Mater. 2002, 14, 4714–4720. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. On the Origin of Enhanced Photocatalytic Activity of Copper-Modified Titania in the Oxidative Reaction Systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef]
- Huang, M.H.; Madasu, M. Facet-dependent and interfacial plane-related photocatalytic behaviors of semiconductor nanocrystals and heterostructures. Nano Today 2019, 28, 100768. [Google Scholar] [CrossRef]
- Xiong, L.; Yu, H.; Ba, X.; Zhang, W.; Yu, Y. Cu2O-based nanocomposites for environmental protection: Relationship between structure and photocatalytic activity, application, and mechanism. In Nanomaterials for Environmental Protection; Chapter: 3 (Part I: Remediation with Use of Metals, Metal Oxides, Complexes and Composites); Kharisov, B.I., Kharissova, O.V., Rasika Dias, H.V., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; p. 30. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Ma, L.-L.; Li, J.-L.; Yu, Y. In Situ Fenton Reagent Generated from TiO2/Cu2O Composite Film: A New Way to Utilize TiO2under Visible Light Irradiation. Environ. Sci. Technol. 2007, 41, 6264–6269. [Google Scholar] [CrossRef]
- Zhao, M.; Cao, L.; Sun, Y.; Lv, J.; Shang, F.; Mao, S.; Jiang, Y.; Xu, J.; Wang, F.; Zhou, Z.; et al. Microstructure, wettability and electrical properties of n-ZnO/ZnO-SL/p-Cu2O heterojunction. Appl. Phys. A 2015, 120, 335–340. [Google Scholar] [CrossRef]
- Xue, M.; Wang, W.; Wang, F.; Ou, J.; Li, W. Design and understanding of superhydrophobic ZnO nanorod arrays with controllable water adhesion. Surf. Coat. Technol. 2014, 258, 200–205. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Sibiński, M.; Rybak-Wilusz, E.; Cholewa, M.; Wisz, G.; Yavorskyi, R. Review of the development of copper oxides with titanium dioxide thin-film solar cells. AIP Adv. 2020, 10, 010701. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Chen, J.; Deng, Z.; Huang, Q.; Liu, Z.; Guo, W.; Cao, R. Highly Active Photocatalyst of Cu2O/TiO2 Octahedron for Hydrogen Generation. ACS Omega 2019, 4, 3392–3397. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, B.; Liu, S.; Duan, X.; Hu, Z. Synthesis and characterization of Cu2O/TiO2 photocatalysts for H2 evolution from aqueous solution with different scavengers. Appl. Surf. Sci. 2015, 324, 736–744. [Google Scholar] [CrossRef]
- Xiang, L.; Ya, J.; Hu, F.; Li, L.; Liu, Z. Fabrication of Cu2O/TiO2 nanotube arrays with enhanced visible-light photoelectrocatalytic activity. Appl. Phys. A 2017, 123, 160. [Google Scholar] [CrossRef]
- Liao, Y.; Deng, P.; Wang, X.; Zhang, D.; Li, F.; Yang, Q.; Zhang, H.; Zhong, Z. A Facile Method for Preparation of Cu2O-TiO2 NTA Heterojunction with Visible-Photocatalytic Activity. Nanoscale Res. Lett. 2018, 13, 221. [Google Scholar] [CrossRef]
- Lalitha, K.; Sadanandam, G.; Kumari, V.D.; Subrahmanyam, M.; Sreedhar, B.; Hebalkar, N.Y. Highly Stabilized and Finely Dispersed Cu2O/TiO2: A Promising Visible Sensitive Photocatalyst for Continuous Production of Hydrogen from Glycerol:Water Mixtures. J. Phys. Chem. C 2010, 114, 22181–22189. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Oral, A.; Menşur, E.; Aslan, M.; Başaran, E. The preparation of copper(II) oxide thin films and the study of their microstructures and optical properties. Mater. Chem. Phys. 2004, 83, 140–144. [Google Scholar] [CrossRef]
- Kaneva, N.V.; Dushkin, C.D.; Bojinova, A.S. ZnO thin films preparation on glass substrates by two different sol-gel meth-ods. Bulg. Chem. Commun. 2012, 44, 261–267. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]


| Sample | Crystalline Phase | Thickness, nm | Particle Diameter, nm | Smoothness, nm |
|---|---|---|---|---|
| TiO2 | Anatase | 55 | 50 | 3 |
| TiO2/Cu2O | Anatase/Cuprite | 45/85 | 40 | 3 |
| ZnO | Zincite | 100 | 15 | 4 |
| ZnO/Cu2O | Zincite /Cuprite | 120/80 | 15 | 5 |
| Sample | Ebg *, eV | EVB, eV | ECB, eV | EF, eV |
|---|---|---|---|---|
| TiO2 | 3.2 | −7.4 | −4.2 | −5.24 |
| ZnO | 3.3 | −7.8 | −4.5 | −5.14 |
| Cu2O | 2.2 | −6.2 | −4.0 | −5.04 |
| Coatings | WF, eV as Prepared | WF, eV after Wetting | WF, eV after UV-Irradiation | WF, eV after Vis Irradiation |
|---|---|---|---|---|
| Cu2O | 5.04 | 4.94 | 4.99 | 5.14 |
| TiO2 | 5.24 | 6.79 | 5.40 | 6.92 |
| TiO2/Cu2O | 5.17 | 5.64 | 4.59 | 4.98 |
| ZnO | 5.14 | 5.49 | 4.99 | 5.64 |
| ZnO/Cu2O | 5.09 | 5.35 | 5.06 | 5.36 |
| SFE, mJ/m2 | After Wetting | After UV Irradiation | After Vis Irradiation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| t | p | d | t | p | d | t | p | d | |
| TiO2 | 73.4 | 42.9 | 31.4 | 79.7 | 47.3 | 32.4 | 74.1 | 42.5 | 31.6 |
| TiO2/Cu2O | 74.4 | 41.6 | 32.8 | 56.3 | 30.3 | 26 | 67.8 | 42.9 | 24.9 |
| ZnO | 74.0 | 46.2 | 27.8 | 78.3 | 44.2 | 34.1 | 71.5 | 45.4 | 26.1 |
| ZnO/Cu2O | 75.3 | 44.2 | 31.1 | 73.7 | 47.2 | 26.5 | 71.4 | 39.6 | 31.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maevskaya, M.V.; Rudakova, A.V.; Emeline, A.V.; Bahnemann, D.W. Effect of Cu2O Substrate on Photoinduced Hydrophilicity of TiO2 and ZnO Nanocoatings. Nanomaterials 2021, 11, 1526. https://doi.org/10.3390/nano11061526
Maevskaya MV, Rudakova AV, Emeline AV, Bahnemann DW. Effect of Cu2O Substrate on Photoinduced Hydrophilicity of TiO2 and ZnO Nanocoatings. Nanomaterials. 2021; 11(6):1526. https://doi.org/10.3390/nano11061526
Chicago/Turabian StyleMaevskaya, Maria V., Aida V. Rudakova, Alexei V. Emeline, and Detlef W. Bahnemann. 2021. "Effect of Cu2O Substrate on Photoinduced Hydrophilicity of TiO2 and ZnO Nanocoatings" Nanomaterials 11, no. 6: 1526. https://doi.org/10.3390/nano11061526
APA StyleMaevskaya, M. V., Rudakova, A. V., Emeline, A. V., & Bahnemann, D. W. (2021). Effect of Cu2O Substrate on Photoinduced Hydrophilicity of TiO2 and ZnO Nanocoatings. Nanomaterials, 11(6), 1526. https://doi.org/10.3390/nano11061526









