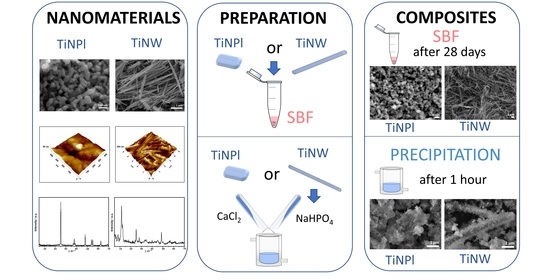
Precipitation at Room Temperature as a Fast and Versatile Method for Calcium Phosphate/TiO2 Nanocomposites Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 Nanomaterials
2.3. Mineralization of TiO2 Nanopalates (TiNPls) and Nanowires (TiNWs) in Simulated Body Fluid (SBF)
2.4. Mineralization of TiNPls and TiNWs by Spontaneous Precipitation of Calcium Phosphates
2.5. Characterization Methods
2.5.1. Powder X-ray Diffraction (PXRD)
2.5.2. Raman Spectroscopy (RS)
2.5.3. Brunauer–Emmett–Teller Surface Area Analysis (BET)
2.5.4. Dynamic Light Scattering (DLS) and Electrophoretic Light Scattering (ELS) Measurements
2.5.5. Thermogravimetric Analysis (TGA)
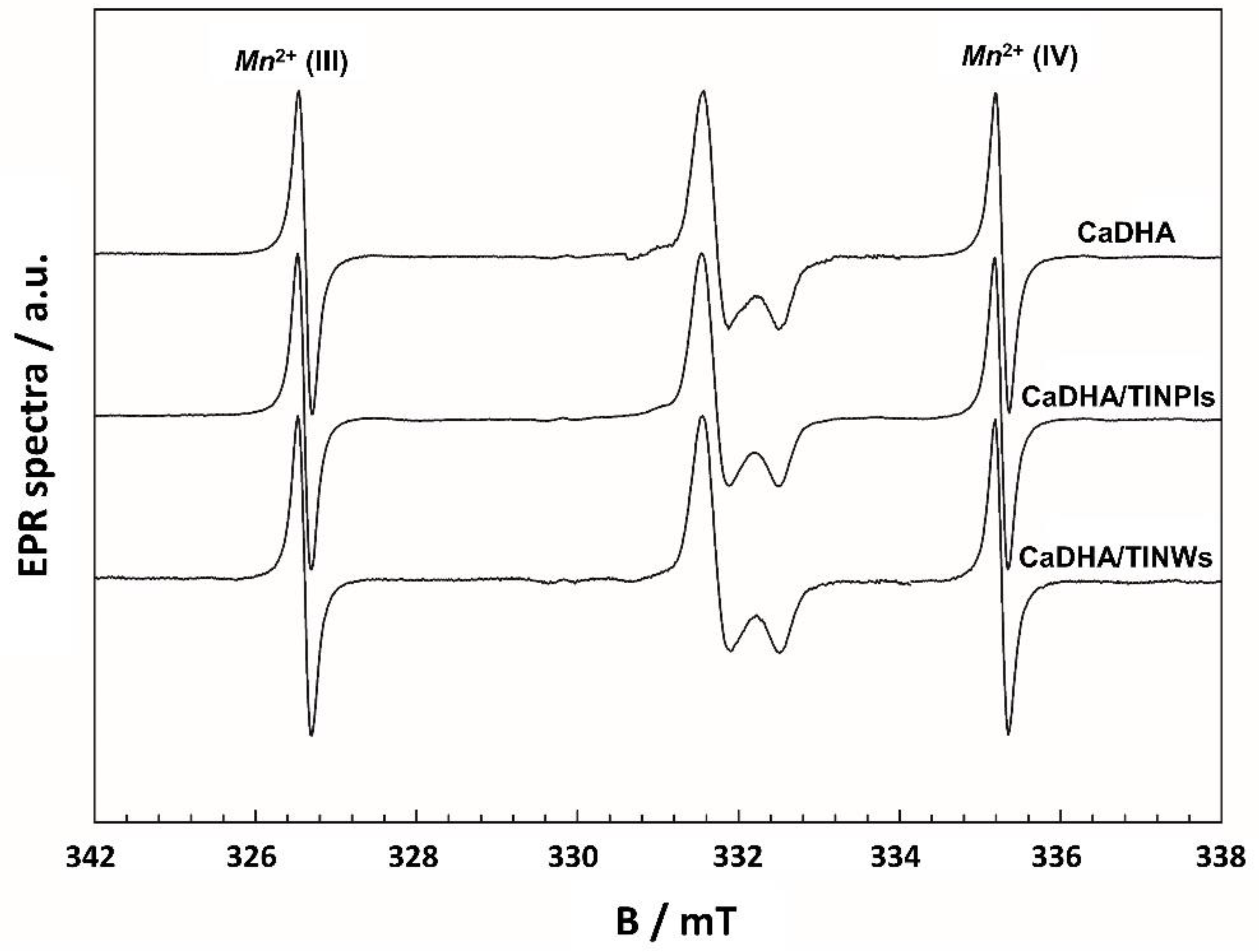
2.5.6. Electron Paramagnetic Resonance Spectroscopy (EPR)
2.5.7. Scanning Electron Microscopy (SEM)
2.5.8. Transmission Electron Microscopy and Selected Area Electron Diffraction (TEM/SAED)
2.5.9. Atomic Force Microscopy (AFM)
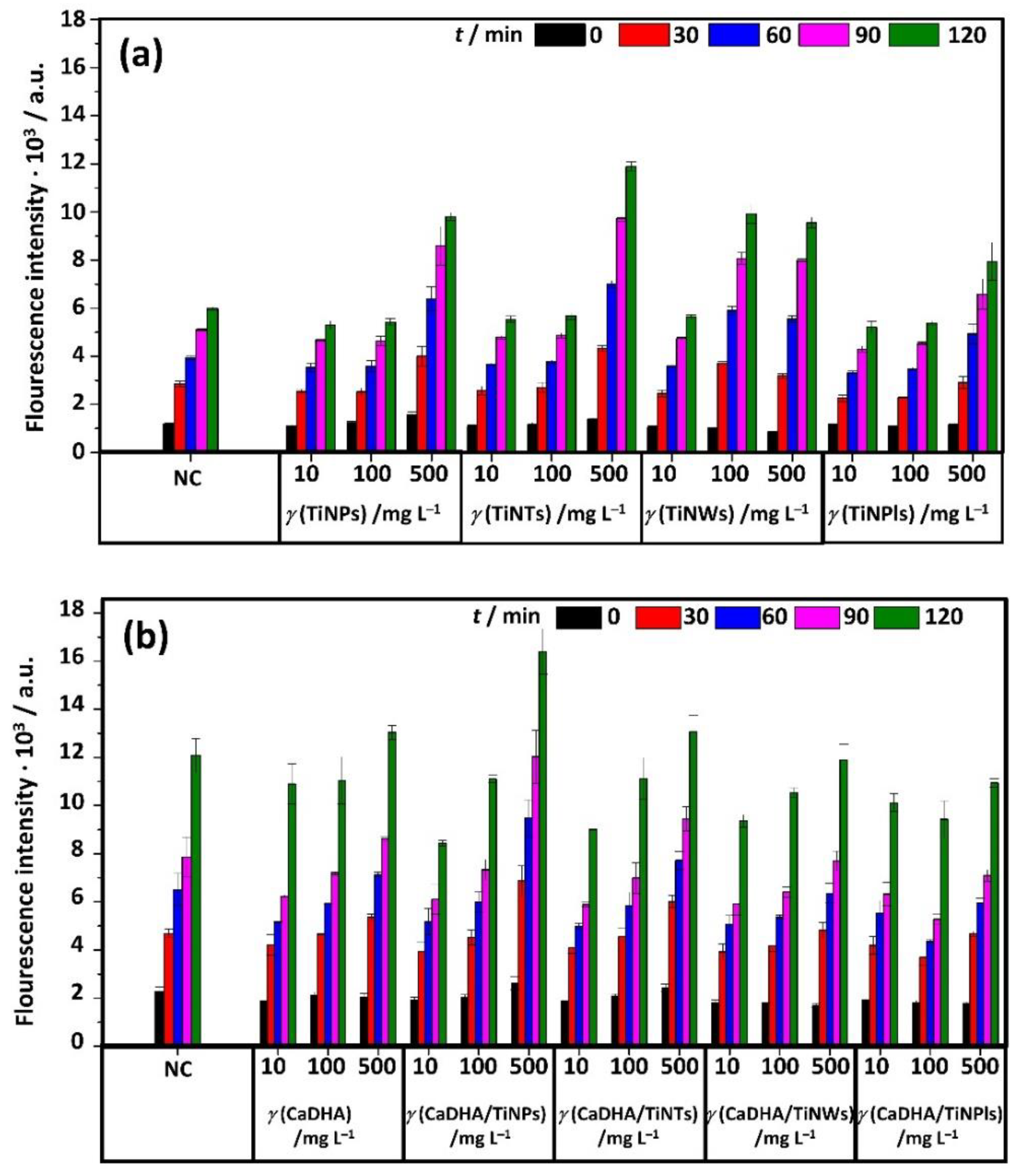
2.5.10. Acellular Reactive Oxygen Species (ROS) Generation
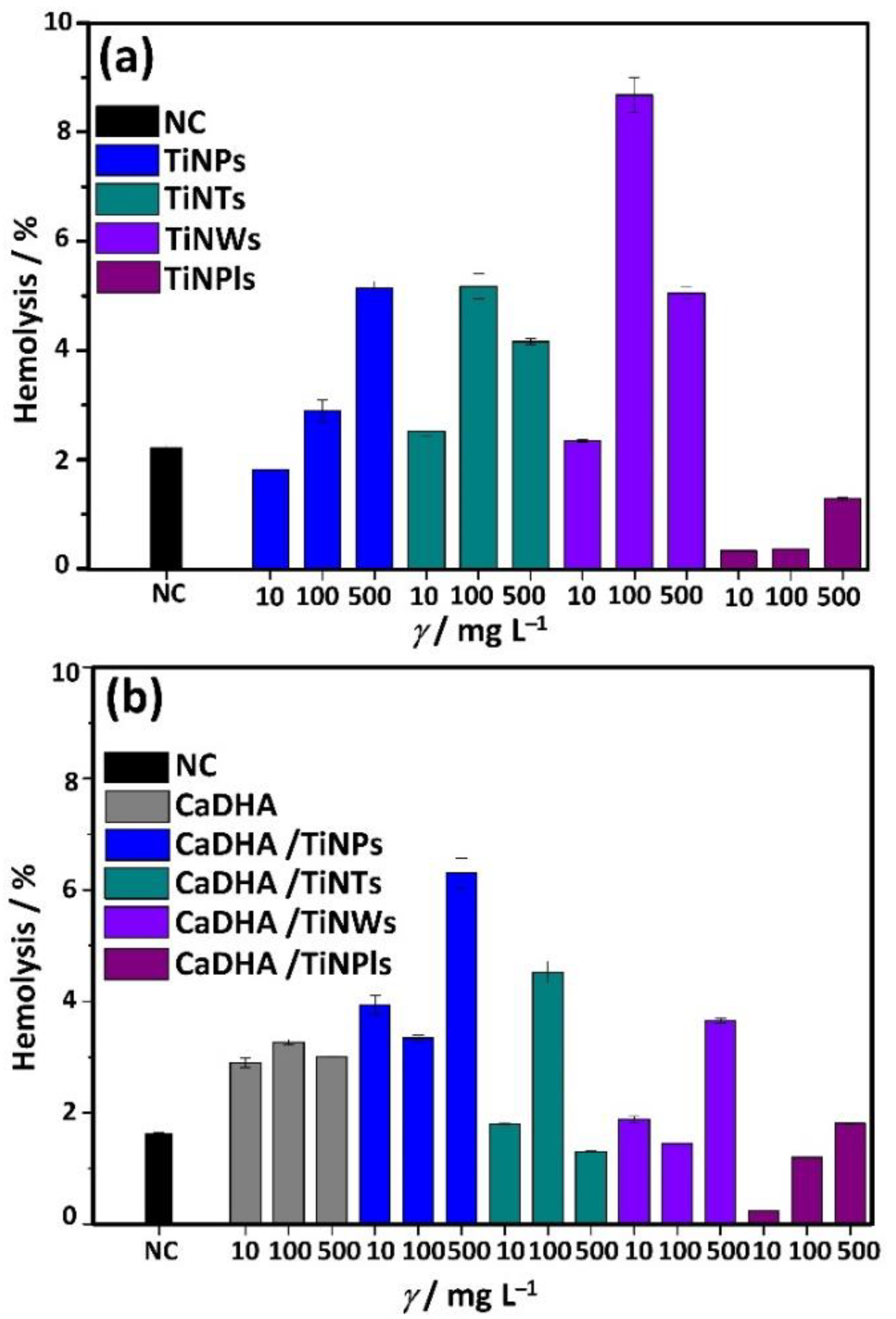
2.5.11. Hemocompatibility Assay
2.5.12. Data Analysis
3. Results
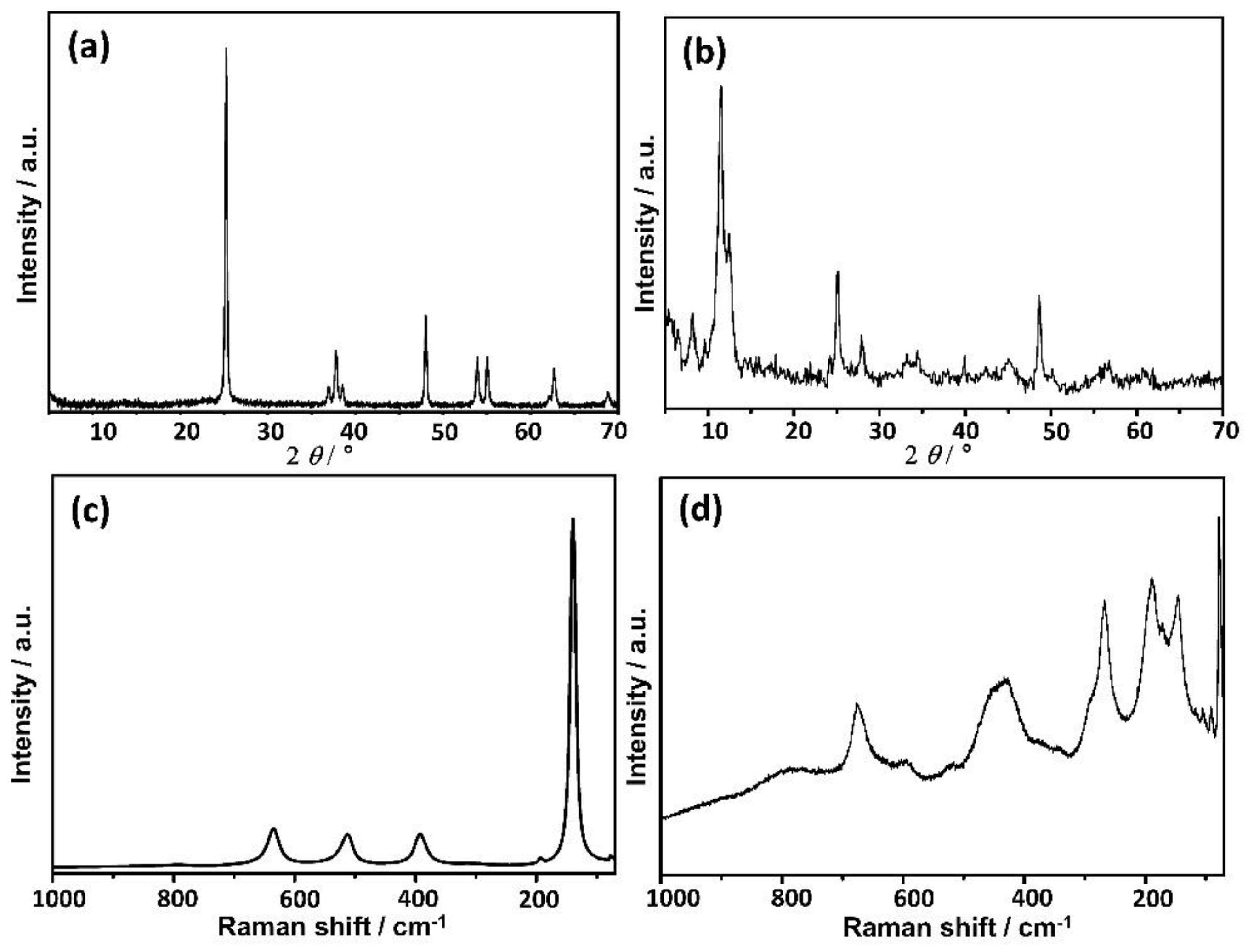
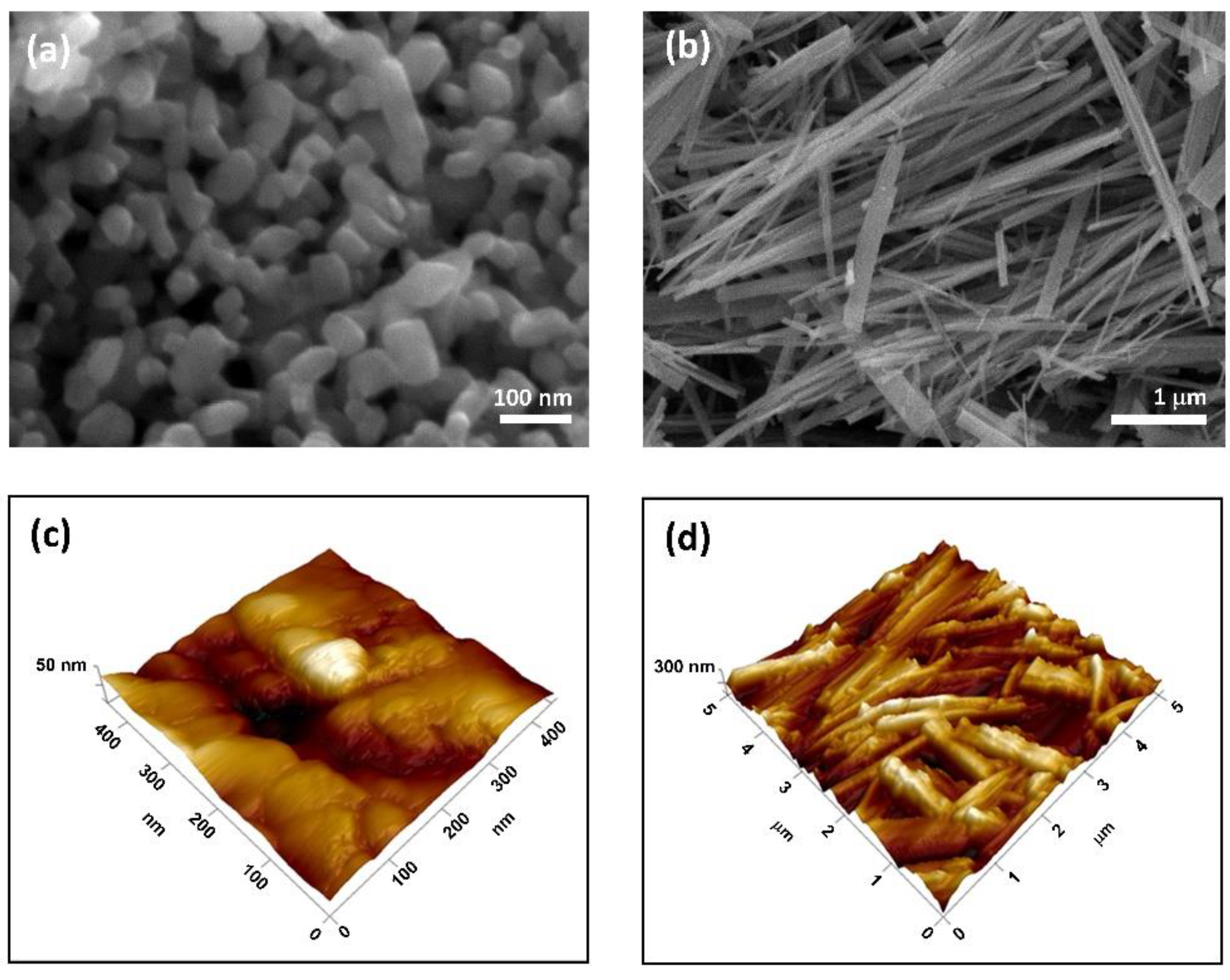
3.1. TiNPls and TiNWs Characterization
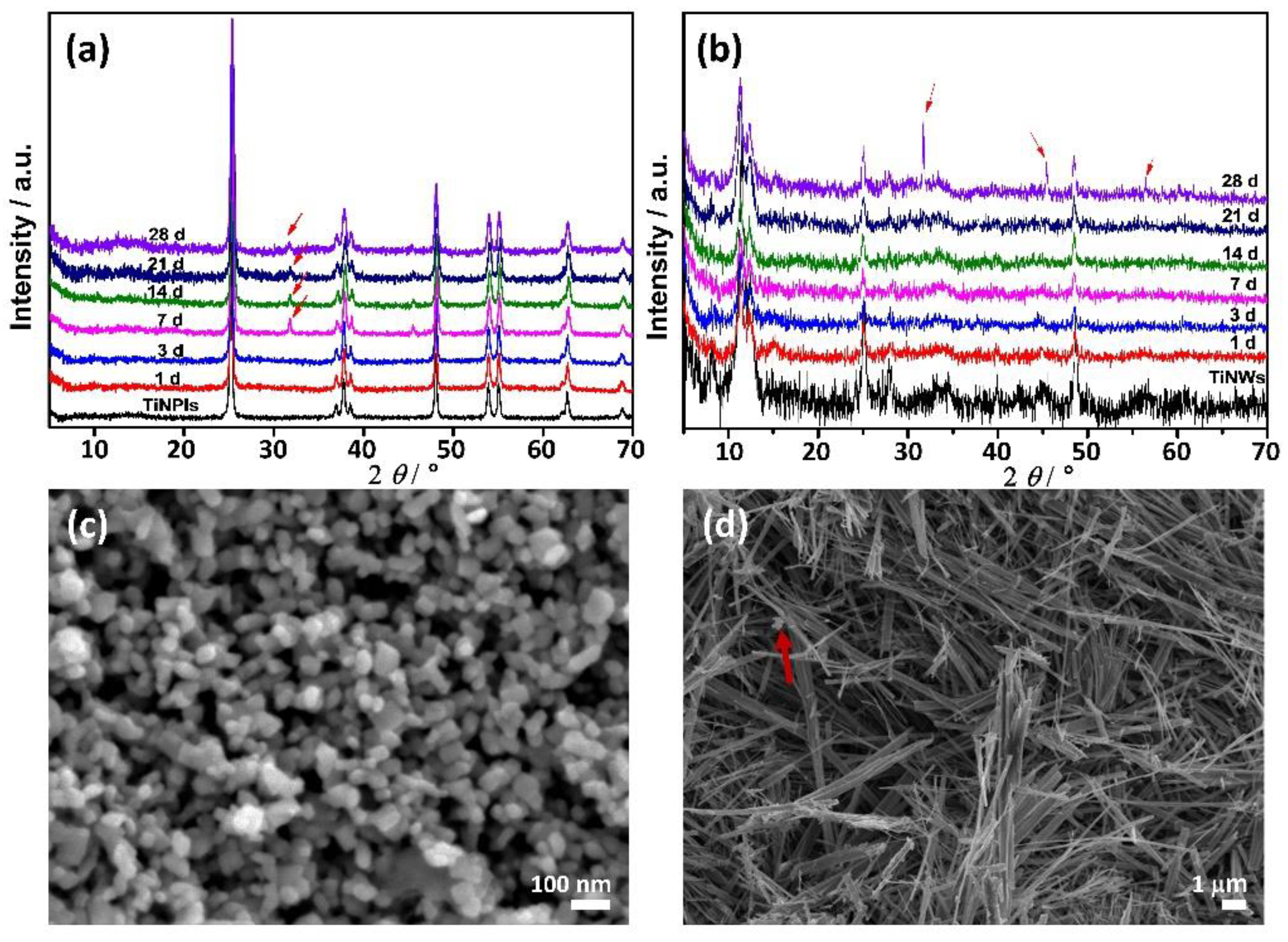
3.2. Mineralization of TiNPls and TiNWs in Corrected Simulated Body Fluid (c-SBF)
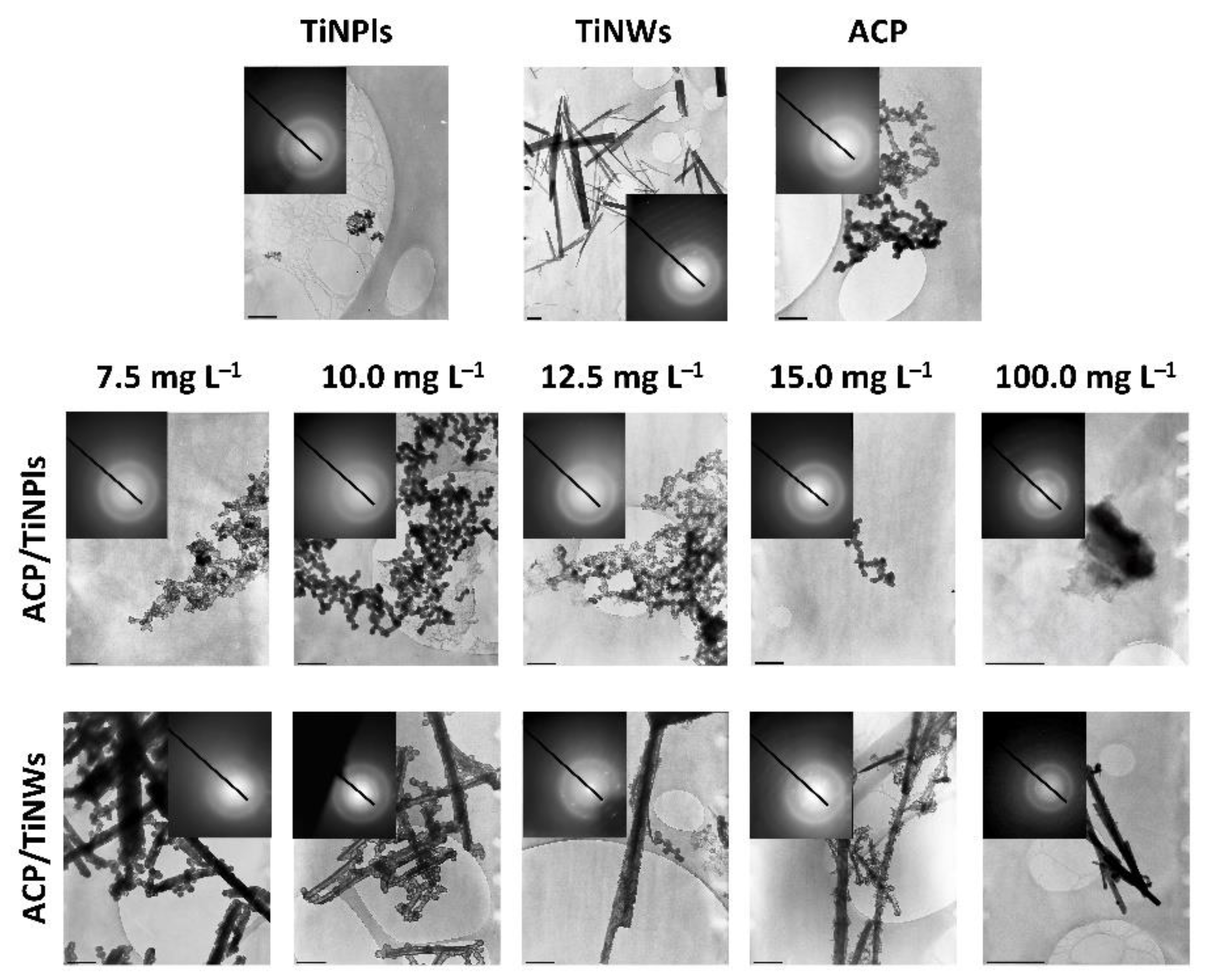
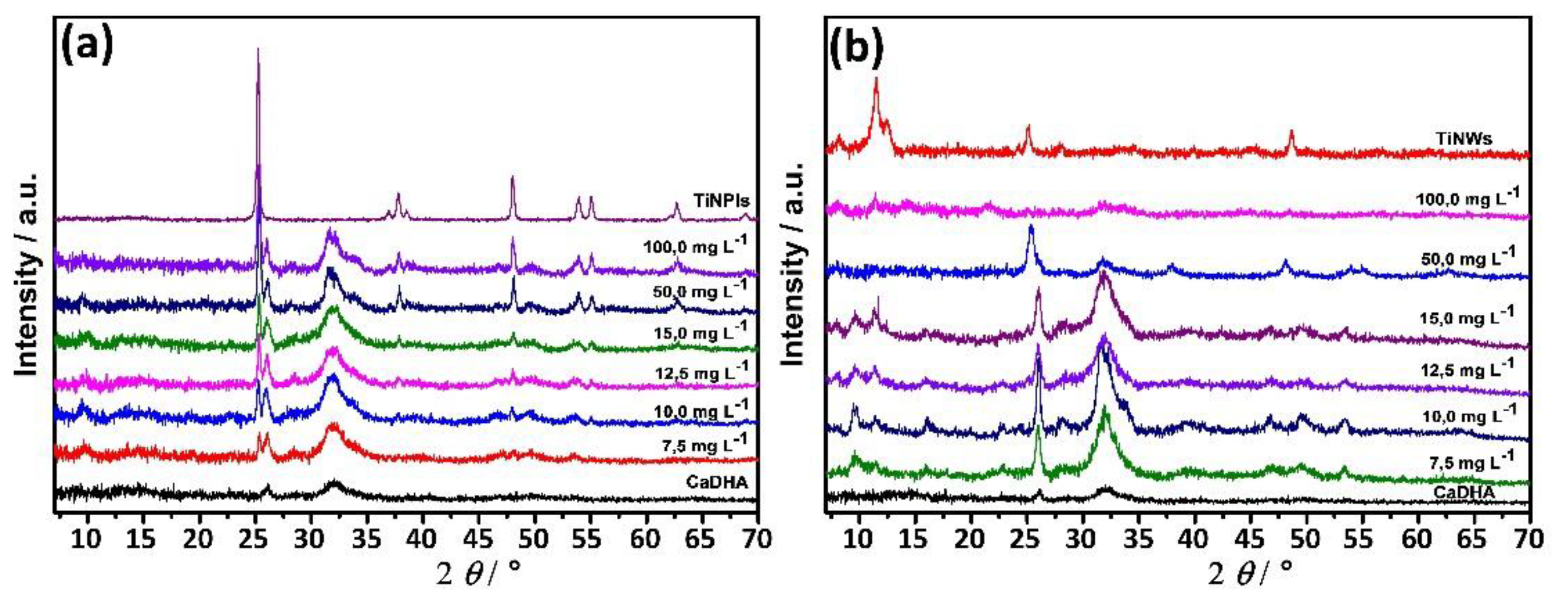
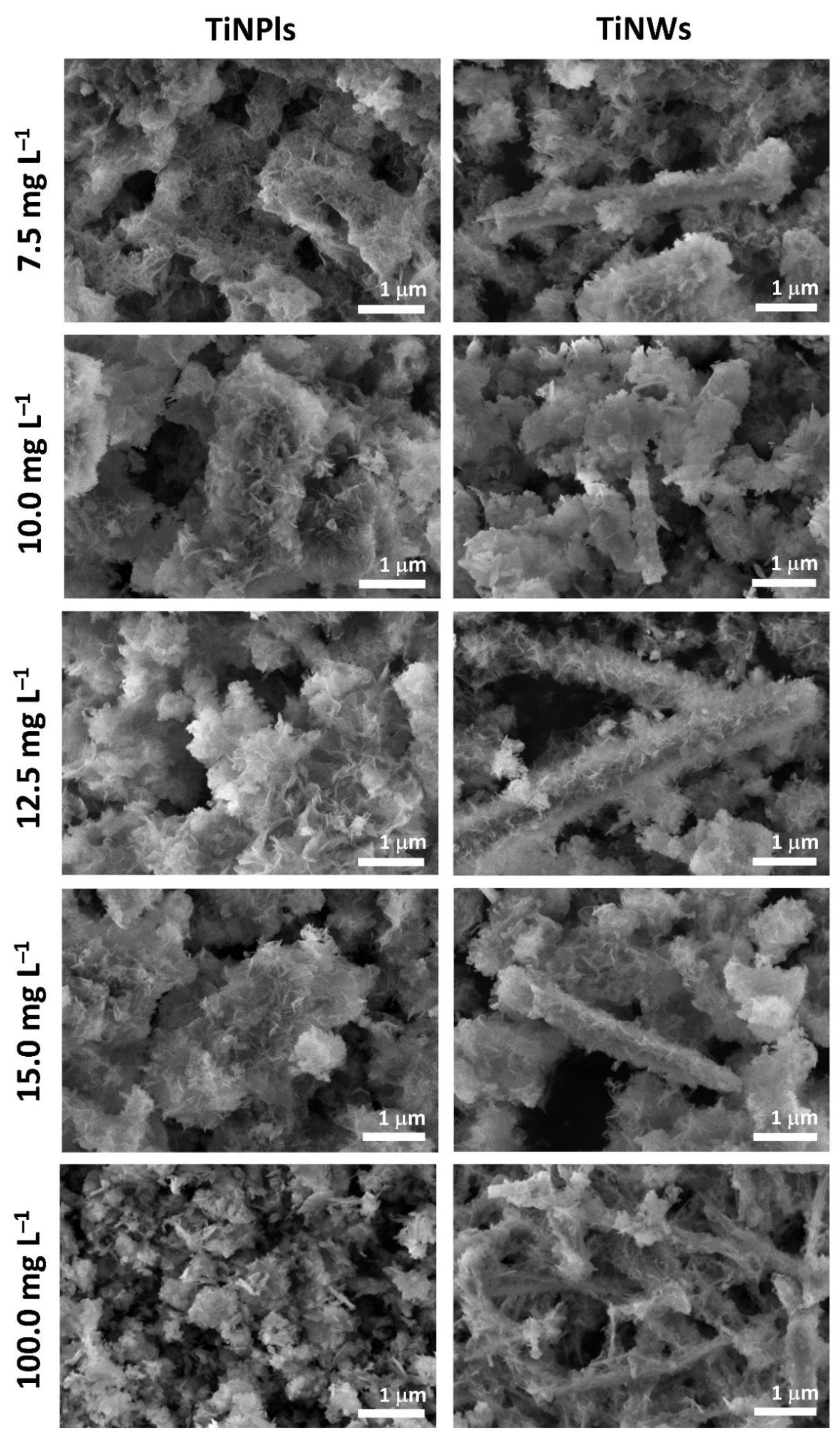
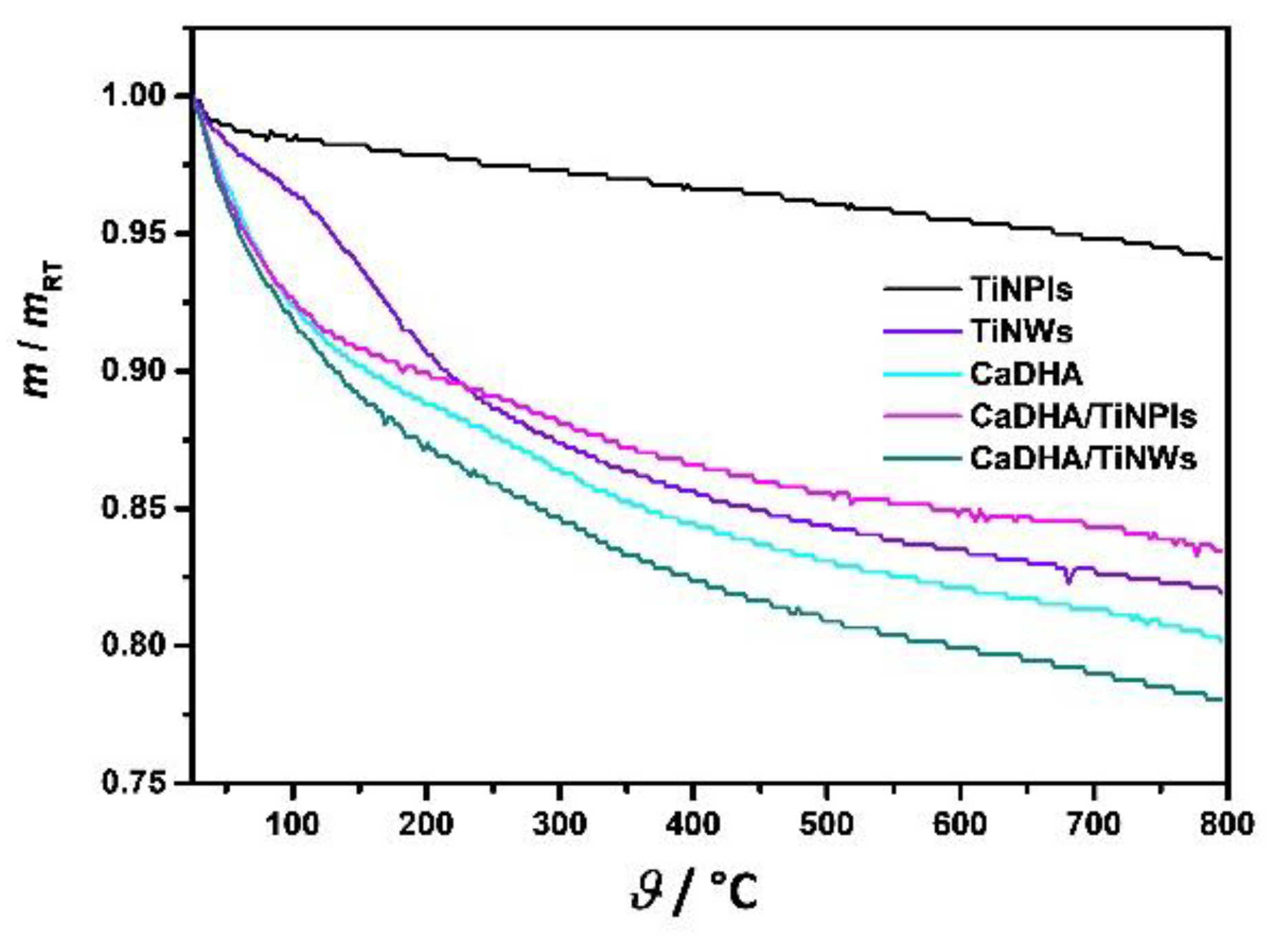
3.3. Mineralization of TiNPls and TiNWs by Spontaneous Precipitation
3.4. Acellular ROS Generation
3.5. Hemocompatibility of TiNMs and CaP/TiNMs Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cambron, J.; King, T. The bone and joint decade: 2000 to 2010. J. Manip. Physiol. Ther. 2006, 29, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Kargupta, R.; Bok, S.; Darr, C.M.; Crist, B.D.; Gangopadhyay, K.; Gangopadhyay, S.; Sengupta, S. Coatings and surface modifications imparting antimicrobial activity to orthopedic implants. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Nandakumar, A.; de Groot, F.B.; van Blitterswijk, C.A.; Habibovic, P. Plug and play: Combining materials and technologies to improve bone regenerative strategies: Plug and play for bone regeneration. J. Tissue Eng. Regen. Med. 2015, 9, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.H.; Ben-Nissan, B.; Matinlinna, J.P.; Conway, R.C. Current perspectives: Calcium phosphate nanocoatings and nanocomposite coatings in dentistry. J. Dent. Res. 2013, 92, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Tripathi, R.; Basu, B. Understanding phase stability, microstructure development and biocompatibility in calcium phosphate-titania composites, synthesized from hydroxyapatite and titanium powder mix. Mater. Sci. Eng. C 2009, 29, 97–107. [Google Scholar] [CrossRef]
- Oktar, F.N. Hydroxyapatite–TiO2 composites. Mater. Lett. 2006, 60, 2207–2210. [Google Scholar] [CrossRef]
- Ruso, J.M.; Verdinelli, V.; Hassan, N.; Pieroni, O.; Messina, P.V. Enhancing CaP biomimetic growth on TiO2 cuboids nanoparticles via highly reactive facets. Langmuir 2013, 29, 2350–2358. [Google Scholar] [CrossRef]
- Salarian, M.; Xu, W.Z.; Wang, Z.; Sham, T.-K.; Charpentier, P.A. Hydroxyapatite–TiO2-based nanocomposites synthesized in supercritical CO2 for bone tissue engineering: Physical and mechanical properties. ACS Appl. Mater. Interfaces 2014, 6, 16918–16931. [Google Scholar] [CrossRef]
- Sánchez-Hernández, A.K.; Martínez-Juárez, J.; Gervacio-Arciniega, J.J.; Silva-González, R.; Robles-Águila, M.J. Effect of ultrasound irradiation on the synthesis of hydroxyapatite/titanium oxide nanocomposites. Crystals 2020, 10, 959. [Google Scholar] [CrossRef]
- Vemulapalli, A.K.; Penmetsa, R.M.R.; Nallu, R.; Siriyala, R. HAp/TiO2 nanocomposites: Influence of TiO2 on microstructure and mechanical properties. J. Compos. Mater. 2020, 54, 765–772. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates: Applications in Nature, Biology, and Medicine; Pan Stanford: Singapore, 2012; ISBN 978-981-4316-62-0. [Google Scholar]
- Canal, C.; Ginebra, M.P. Fibre-reinforced calcium phosphate cements: A review. J. Mech. Behav. Biomed. Mater. 2011, 4, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Erceg, I.; Selmani, A.; Gajović, A.; Panžić, I.; Iveković, D.; Faraguna, F.; Šegota, S.; Ćurlin, M.; Strasser, V.; Kontrec, J.; et al. Calcium phosphate formation on TiO2 nanomaterials of different dimensionality. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124615. [Google Scholar] [CrossRef]
- Kunze, J.; Müller, L.; Macak, J.M.; Greil, P.; Schmuki, P.; Müller, F.A. Time-dependent growth of biomimetic apatite on anodic TiO2 nanotubes. Electrochim. Acta 2008, 53, 6995–7003. [Google Scholar] [CrossRef]
- Oh, S.; Jin, S. Titanium oxide nanotubes with controlled morphology for enhanced bone growth. Mater. Sci. Eng. C 2006, 26, 1301–1306. [Google Scholar] [CrossRef]
- Colas, A.; Curtis, J. Biomaterials Science—An Introduction to Materials in Medicine, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemans, J.E., Eds.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2013; ISBN 978-0-12-374626-9. [Google Scholar]
- Enayati-Jazi, M.; Solati-Hashjin, M.; Nemati, A.; Bakhshi, F. Synthesis and characterization of hydroxyapatite/titania nanocomposites using in situ precipitation technique. Superlattices Microstruct. 2012, 51, 877–885. [Google Scholar] [CrossRef]
- Sato, M.; Aslani, A.; Sambito, M.A.; Kalkhoran, N.M.; Slamovich, E.B.; Webster, T.J. Nanocrystalline hydroxyapatite/titania coatings on titanium improves osteoblast adhesion. J. Biomed. Mater. Res. 2008, 84A, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, N.; Anjusree, G.S.; Madhavan, A.A.; Priyanka, P.; Sankar, D.; Nisha, N.; Lakshmi, S.V.; Jayakumar, R.; Balakrishnan, A.; Biswas, R. Synthesis and anti-staphylococcal activity of TiO2 nanoparticles and nanowires in ex vivo porcine skin model. J Biomed. Nanotechnol. 2014, 10, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Joseph Nathanael, A.; Mangalaraj, D.; Chen, P.C.; Ponpandian, N. Mechanical and photocatalytic properties of hydroxyapatite/titania nanocomposites prepared by combined high gravity and hydrothermal process. Compos. Sci. Technol. 2010, 70, 419–426. [Google Scholar] [CrossRef]
- Murgolo, S.; Moreira, I.; Piccirillo, C.; Castro, P.; Ventrella, G.; Cocozza, C.; Mascolo, G. Photocatalytic degradation of diclofenac by hydroxyapatite–TiO2 composite material: Identification of transformation products and assessment of toxicity. Materials 2018, 11, 1779. [Google Scholar] [CrossRef]
- Pushpakanth, S.; Srinivasan, B.; Sreedhar, B.; Sastry, T.P. An in situ approach to prepare nanorods of titania–hydroxyapatite (TiO2–HAp) nanocomposite by microwave hydrothermal technique. Mater. Chem. Phys. 2008, 107, 492–498. [Google Scholar] [CrossRef]
- Que, W.; Khor, K.A.; Xu, J.L.; Yu, L.G. Hydroxyapatite/Titania nanocomposites derived by combining high-energy ball milling with spark plasma sintering processes. J. Eur. Ceram. Soc. 2008, 28, 3083–3090. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Todorova, N.; Romanos, G.; Vaimakis, T.; Dillert, R.; Bahnemann, D.; Trapalis, C. Composite hydroxyapatite/TiO2 materials for photocatalytic oxidation of NOx. Mater. Sci. Eng. B 2012, 177, 1046–1052. [Google Scholar] [CrossRef]
- Milella, E. Preparation and characterisation of titania/hydroxyapatite composite coatings obtained by sol–gel process. Biomaterials 2001, 22, 1425–1431. [Google Scholar] [CrossRef]
- Duan, K.; Wang, R. Surface modifications of bone implants through wet chemistry. J. Mater. Chem. 2006, 16, 2309. [Google Scholar] [CrossRef]
- Koju, N.; Sikder, P.; Ren, Y.; Zhou, H.; Bhaduri, S.B. Biomimetic coating technology for orthopedic implants. Curr. Opin. Chem. Eng. 2017, 15, 49–55. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Barrere, F.; Snel, M.M.E.; van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Nano-scale study of the nucleation and growth of calcium phosphate coating on titanium implants. Biomaterials 2004, 25, 2901–2910. [Google Scholar] [CrossRef] [PubMed]
- Bracci, B.; Panzavolta, S.; Bigi, A. A new simplified calcifying solution to synthesize calcium phosphate coatings. Surf. Coat. Technol. 2013, 232, 13–21. [Google Scholar] [CrossRef]
- Sikirić, M.D.; Gergely, C.; Elkaim, R.; Wachtel, E.; Cuisinier, F.J.G.; Füredi-Milhofer, H. Biomimetic organic-inorganic nanocomposite coatings for titanium implants. J. Biomed. Mater. Res. Part. A 2009, 89, 759–771. [Google Scholar] [CrossRef]
- Han, X.; Kuang, Q.; Jin, M.; Xie, Z.; Zheng, L. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef]
- Sofianou, M.-V.; Trapalis, C.; Psycharis, V.; Boukos, N.; Vaimakis, T.; Yu, J.; Wang, W. Study of TiO2 anatase nano and microstructures with dominant {001} facets for NO oxidation. Environ. Sci Pollut. Res. 2012, 19, 3719–3726. [Google Scholar] [CrossRef]
- Selmani, A.; Špadina, M.; Plodinec, M.; Delač Marion, I.; Willinger, M.G.; Lützenkirchen, J.; Gafney, H.D.; Redel, E. An experimental and theoretical approach to understanding the surface properties of one-dimensional TiO 2 nanomaterials. J. Phys. Chem. C 2015, 119, 19729–19742. [Google Scholar] [CrossRef]
- Majer, M.; Roguljić, M.; Knežević, Ž.; Starodumov, A.; Ferenček, D.; Brigljević, V.; Mihaljević, B. Dose mapping of the panoramic 60Co Gamma Irradiation Facility at the Ruđer Bošković Institute–geant4 simulation and measurements. Appl. Radiat. Isot. 2019, 154, 108824. [Google Scholar] [CrossRef] [PubMed]
- IAEA International Atomic Energy Agency. Guidelines for Industrial Radiation Sterilization of Disposable Medical Products (Cobalt-60 Gamma Irradiation); TECDOC Series: Vienna, Austria, 1989. [Google Scholar]
- Stoll, S.; Schweiger, A. Easy spin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Vinković Vrček, I.; Pavičić, I.; Crnković, T.; Jurašin, D.; Babič, M.; Horák, D.; Lovrić, M.; Ferhatović, L.; Ćurlin, M.; Gajović, S. Does surface coating of metallic nanoparticles modulate their interference with in vitro assays? RSC Adv. 2015, 5, 70787–70807. [Google Scholar] [CrossRef]
- Oh, S.-J.; Kim, H.; Liu, Y.; Han, H.-K.; Kwon, K.; Chang, K.-H.; Park, K.; Kim, Y.; Shim, K.; An, S.S.A.; et al. Incompatibility of silver nanoparticles with lactate dehydrogenase leakage assay for cellular viability test is attributed to protein binding and reactive oxygen species generation. Toxicol. Lett. 2014, 225, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.T.; Yao, G.; Cerruti, M. The importance of amino acid interactions in the crystallization of hydroxyapatite. J. R. Soc. Interface 2013, 10, 20120906. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, K.; Chandra Bose, A.; Rameshbabu, N. X-ray peak broadening studies of nanocrystalline hydroxyapatite by Williamson–Hall analysis. Phys. B Condens. Matter 2010, 405, 4256–4261. [Google Scholar] [CrossRef]
- Ma, W.; Lu, Z.; Zhang, M. Investigation of structural transformations in nanophase titanium dioxide by Raman spectroscopy. Appl. Phys. A Mater. Sci. Process. 1998, 66, 621–627. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated titanates and TiO2 nanostructured materials: Synthesis, properties, and applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Suzuki, Y.; Pavasupree, S.; Yoshikawa, S.; Kawahata, R. Natural rutile-derived titanate nanofibers prepared by direct hydrothermal processing. J. Mater. Res. 2005, 20, 1063–1070. [Google Scholar] [CrossRef]
- Li, J.; Wan, W.; Zhou, H.; Li, J.; Xu, D. Hydrothermal synthesis of TiO2(B) nanowires with ultrahigh surface area and their fast charging and discharging properties in Li-Ion batteries. Chem. Commun. 2011, 47, 3439. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.R.; Armstrong, G.; Canales, J.; Bruce, P.G. TiO2-B nanowires. Angew. Chem. Int. Ed. 2004, 43, 2286–2288. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Cui, Z.; Zhu, S.; Liang, Y.; Li, Z.; Yang, X. Fabrication, characterization, and photocatalytic properties of anatase TiO2 nanoplates with exposed {001} facets. J. Nanopart. Res. 2014, 16, 2191. [Google Scholar] [CrossRef]
- Sofianou, M.-V.; Psycharis, V.; Boukos, N.; Vaimakis, T.; Yu, J.; Dillert, R.; Bahnemann, D.; Trapalis, C. Tuning the photocatalytic selectivity of TiO2 anatase nanoplates by altering the exposed crystal facets content. Appl. Catal. B Environ. 2013, 142–143, 761–768. [Google Scholar] [CrossRef]
- Tas, A.C. The use of physiological solutions or media in calcium phosphate synthesis and processing. Acta Biomater. 2014, 10, 1771–1792. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.-M.; Kokubo, T.; Miyaji, F.; Nakamura, T. Bonelike apatite formation induced on zirconia gel in a simulated body fluid and its modified solutions. J. Am. Ceram. Soc. 2004, 84, 2041–2044. [Google Scholar] [CrossRef]
- Stigter, M.; de Groot, K.; Layrolle, P. Incorporation of tobramycin into biomimetic hydroxyapatite coating on titanium. Biomaterials 2002, 23, 4143–4153. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.-M.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Effect of water treatment on the apatite-forming ability of NaOH-treated titanium metal. J. Biomed. Mater. Res. 2002, 63, 522–530. [Google Scholar] [CrossRef]
- Habibovic, P.; Barrère, F.; Blitterswijk, C.A.; Groot, K.; Layrolle, P. Biomimetic hydroxyapatite coating on metal implants. J. Am. Ceram. Soc. 2004, 85, 517–522. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Liou, S.-C.; Chen, S.-Y.; Lee, H.-Y.; Bow, J.-S. Structural characterization of nano-sized calcium deficient apatite powders. Biomaterials 2004, 25, 189–196. [Google Scholar] [CrossRef]
- Kokubo, T. (Ed.) Bioceramics and Their Clinical Applications; Woodhead Publishing: Boca Raton, FL, USA, 2008; ISBN 978-1-84569-422-7. [Google Scholar]
- Uchida, M.; Kim, H.-M.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J. Biomed. Mater. Res. 2003, 64, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Sikirić, M.D.; Füredi-Milhofer, H. The influence of surface active molecules on the crystallization of biominerals in solution. Adv. Colloid Interface Sci. 2006, 128–130, 135–158. [Google Scholar] [CrossRef]
- Bar-Yosef Ofir, P.; Govrin-Lippman, R.; Garti, N.; Füredi-Milhofer, H. The influence of polyelectrolytes on the formation and phase transformation of amorphous calcium phosphate. Cryst. Growth Des. 2004, 4, 177–183. [Google Scholar] [CrossRef]
- Eans, E.D.; Gillessen, I.H.; Posner, A.S. Intermediate states in the precipitation of hydroxyapatite. Nature 1965, 208, 365–367. [Google Scholar] [CrossRef]
- Selmani, A.; Coha, I.; Magdić, K.; Čolović, B.; Jokanović, V.; Šegota, S.; Gajović, S.; Gajović, A.; Jurašin, D.; Dutour Sikirić, M. Multiscale study of the influence of cationic surfactants on amorphous calcium phosphate precipitation. CrystEngComm 2015, 17, 8529–8548. [Google Scholar] [CrossRef]
- Christoffersen, J.; Christoffersen, M.R.; Kibalczyc, W.; Andersen, F.A. A contribution to the understanding of the formation of calcium phosphates. J. Cryst. Growth 1989, 94, 767–777. [Google Scholar] [CrossRef]
- Buljan Meić, I.; Kontrec, J.; Domazet Jurašin, D.; Njegić Džakula, B.; Štajner, L.; Lyons, D.M.; Dutour Sikirić, M.; Kralj, D. Comparative study of calcium carbonates and calcium phosphates precipitation in model systems mimicking the inorganic environment for biomineralization. Cryst. Growth Des. 2017, 17, 1103–1117. [Google Scholar] [CrossRef]
- Tõnsuaadu, K.; Gross, K.A.; Plūduma, L.; Veiderma, M. A review on the thermal stability of calcium apatites. J. Therm. Anal. Calorim 2012, 110, 647–659. [Google Scholar] [CrossRef]
- Mir, M.; Anwar Siddiqi, S.; Hussain, T.; Anwar Chaudhry, A.; Ur Rehman, I.; Khan, A.S.; Abbas, G. Synthesis and characterization of calcium deficient apatite granules for drug eluting bone graft applications. Ceram. Int. 2014, 40, 10719–10725. [Google Scholar] [CrossRef]
- You, D.G.; Deepagan, V.G.; Um, W.; Jeon, S.; Son, S.; Chang, H.; Yoon, H.I.; Cho, Y.W.; Swierczewska, M.; Lee, S.; et al. ROS-generating TiO2 nanoparticles for non-invasive sonodynamic therapy of cancer. Sci. Rep. 2016, 6, 23200. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.; Hilt, J.Z. Magnetic nanoparticles: Reactive oxygen species generation and potential therapeutic applications. J. Nanopart. Res. 2017, 19, 253. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Iorio, E.L.; Corsi, M.M. Oxidative stress, free radicals and bone remodeling. Clin. Chem. Lab. Med. 2008, 46. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Ueno, T.; Ikeda, T.; Tsukimura, N.; Ishijima, M.; Minamikawa, H.; Sugita, Y.; Yamada, M.; Wakabayashi, N.; Ogawa, T. Novel antioxidant capability of titanium induced by UV light treatment. Biomaterials 2016, 108, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ROS-induced cells damage and improvement of osteogenesis. Biomaterials 2017, 114, 82–96. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 2020, 14, 53. [Google Scholar] [CrossRef]
- Gali, N.K.; Ning, Z.; Daoud, W.; Brimblecombe, P. Investigation on the mechanism of non-photocatalytically TiO2-induced reactive oxygen species and its significance on cell cycle and morphology: Mechanism of TiO2 -induced ROS participation in cell toxicity. J. Appl. Toxicol. 2016, 36, 1355–1363. [Google Scholar] [CrossRef]
- Kermani, F.; Mollazadeh, S.; Kargozar, S.; Vahdati Khakhi, J. Solution combustion synthesis (SCS) of theranostic ions doped biphasic calcium phosphates; kinetic of ions release in simulated body fluid (SBF) and reactive oxygen species (ROS) generation. Mater. Sci. Eng. C 2021, 118, 111533. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Kesari, K.K.; Rani, M.; Paulraj, R. Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human breast cancer cells (MCF-7). J. Nanopart. Res. 2012, 14, 712. [Google Scholar] [CrossRef]
- Guichard, Y.; Schmit, J.; Darne, C.; Gaté, L.; Goutet, M.; Rousset, D.; Rastoix, O.; Wrobel, R.; Witschger, O.; Martin, A.; et al. Cytotoxicity and genotoxicity of nanosized and microsized titanium dioxide and iron oxide particles in Syrian hamster embryo cells. Ann. Occup. Hyg. 2012, 56, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-H.; Sun, X.-Y.; Ouyang, J.-M. Shape-dependent toxicity and mineralization of hydroxyapatite nanoparticles in A7R5 aortic smooth muscle cells. Sci. Rep. 2019, 9, 18979. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, P.H.; Knudsen, K.B.; Štrancar, J.; Umek, P.; Koklič, T.; Garvas, M.; Vanhala, E.; Savukoski, S.; Ding, Y.; Madsen, A.M.; et al. Effects of physicochemical properties of TiO2 nanomaterials for pulmonary inflammation, acute phase response and alveolar proteinosis in intratracheally exposed mice. Toxicol. Appl. Pharmacol. 2020, 386, 114830. [Google Scholar] [CrossRef]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium anticancer properties and impact on cellular redox status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-contacting biomaterials: In vitro evaluation of the hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Pramanik, N.; Mohapatra, S.; Alam, S.; Pramanik, P. Synthesis of hydroxyapatite/poly(vinyl alcohol phosphate) nanocomposite and its characterization. Polym. Compos. 2008, 29, 429–436. [Google Scholar] [CrossRef]
- Puvvada, N.; Panigrahi, P.K.; Pathak, A. Room temperature synthesis of highly hemocompatible hydroxyapatite, study of their physical properties and spectroscopic correlation of particle size. Nanoscale 2010, 2, 2631. [Google Scholar] [CrossRef]
- Roy Chowdhury, S.K.; Mishra, A.; Pradhan, B.; Saha, D. Wear characteristic and biocompatibility of some polymer composite acetabular cups. Wear 2004, 256, 1026–1036. [Google Scholar] [CrossRef]
- Ungureanu, C.; Dumitriu, C.; Popescu, S.; Enculescu, M.; Tofan, V.; Popescu, M.; Pirvu, C. Enhancing antimicrobial activity of TiO2/Ti by torularhodin bioinspired surface modification. Bioelectrochemistry 2016, 107, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, X.; Dai, H.; Li, S. Nanosize and surface charge effects of hydroxyapatite nanoparticles on red blood cell suspensions. ACS Appl. Mater. Interfaces 2012, 4, 4616–4622. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.-H.; Ling, Y.P.; Abdullah, W.Z.; Mustafa, A.Z.; Pung, S.-Y.; Yeoh, F.-Y. Physicochemical evaluation and in vitro hemocompatibility study on nanoporous hydroxyapatite. J. Mater. Sci. Mater. Med. 2019, 30, 44. [Google Scholar] [CrossRef] [PubMed]
| TiNPls | TiNWs | |||||
|---|---|---|---|---|---|---|
| dh/nm | vol. % | ζ/mV | dh/nm | vol. % | ζ/mV | |
| c-SBF | 847.8 ± 32.0 | 92.0 ± 5.2 | −10.5 ± 0.1 | 1078.0 ± 56.3 | 100 | −8.94 ± 0.6 |
| 5357.8 ± 137.8 | 8.0 ± 5.2 | |||||
| Anionic Reactant Solution | 216.2 ± 3.5 | 57.0 ± 14.9 | −53.0 ± 2.5 | 235.5 ± 32.1 | 1.4 ± 2.1 | −55.0 ± 2.0 |
| 1076.1 ± 170.7 | 31.1 ± 14.6 | 874.8 ± 34.3 | 93.3 ± 2.4 | |||
| 4743.7 ± 343.4 | 11.9 ± 2.4 | 5496.0 ± 100.0 | 5.3 ± 3.6 | |||
| γ/mg L−1 | Crystallite Size/nm | |
|---|---|---|
| TiNPls | TiNWs | |
| 0 | 3.92 | |
| 7.5 | 3.97 | 4.01 |
| 10.0 | 3.71 | 4.89 |
| 12.5 | 3.74 | 3.85 |
| 15.0 | 3.48 | 3.72 |
| 50.0 | 5.28 | 4.36 |
| 100.0 | 4.89 | 1.94 |
| Material | SBET/m2 g−1 |
|---|---|
| TiNPls | 24.70 |
| TiNWs | 21.49 |
| CaDHA | 81.81 |
| CaDHA/TiNPls | 51.66 |
| CaDHA/TiNWs | 35.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erceg, I.; Selmani, A.; Gajović, A.; Radatović, B.; Šegota, S.; Ćurlin, M.; Strasser, V.; Kontrec, J.; Kralj, D.; Maltar-Strmečki, N.; et al. Precipitation at Room Temperature as a Fast and Versatile Method for Calcium Phosphate/TiO2 Nanocomposites Synthesis. Nanomaterials 2021, 11, 1523. https://doi.org/10.3390/nano11061523
Erceg I, Selmani A, Gajović A, Radatović B, Šegota S, Ćurlin M, Strasser V, Kontrec J, Kralj D, Maltar-Strmečki N, et al. Precipitation at Room Temperature as a Fast and Versatile Method for Calcium Phosphate/TiO2 Nanocomposites Synthesis. Nanomaterials. 2021; 11(6):1523. https://doi.org/10.3390/nano11061523
Chicago/Turabian StyleErceg, Ina, Atiđa Selmani, Andreja Gajović, Borna Radatović, Suzana Šegota, Marija Ćurlin, Vida Strasser, Jasminka Kontrec, Damir Kralj, Nadica Maltar-Strmečki, and et al. 2021. "Precipitation at Room Temperature as a Fast and Versatile Method for Calcium Phosphate/TiO2 Nanocomposites Synthesis" Nanomaterials 11, no. 6: 1523. https://doi.org/10.3390/nano11061523
APA StyleErceg, I., Selmani, A., Gajović, A., Radatović, B., Šegota, S., Ćurlin, M., Strasser, V., Kontrec, J., Kralj, D., Maltar-Strmečki, N., Barbir, R., Pem, B., Vinković Vrček, I., & Dutour Sikirić, M. (2021). Precipitation at Room Temperature as a Fast and Versatile Method for Calcium Phosphate/TiO2 Nanocomposites Synthesis. Nanomaterials, 11(6), 1523. https://doi.org/10.3390/nano11061523