Assembly of Copolymer and Metal−Organic Framework HKUST-1 to Form Cu2−xS/CNFs Intertwining Network for Efficient Electrocatalytic Hydrogen Evolution
Abstract
1. Introduction
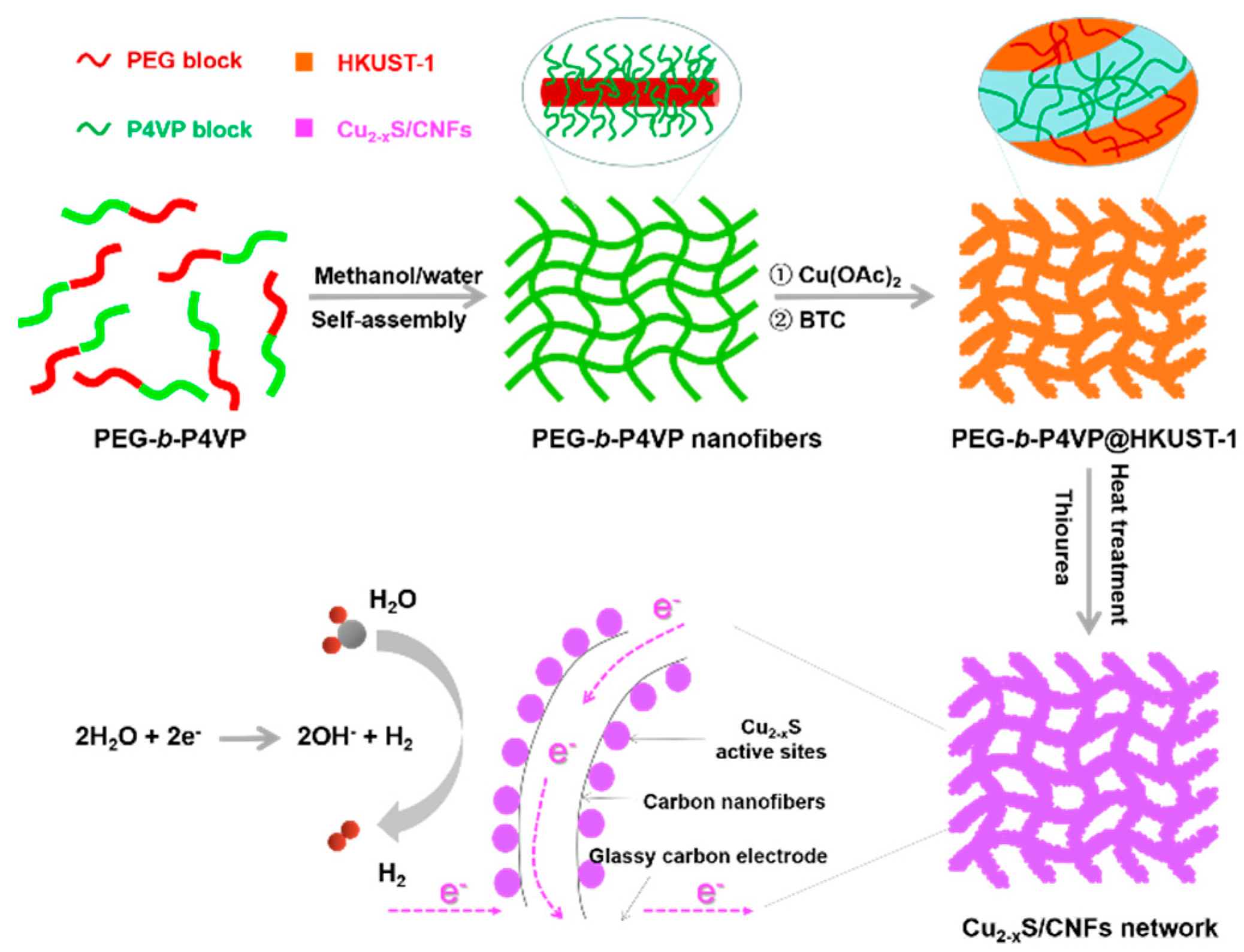
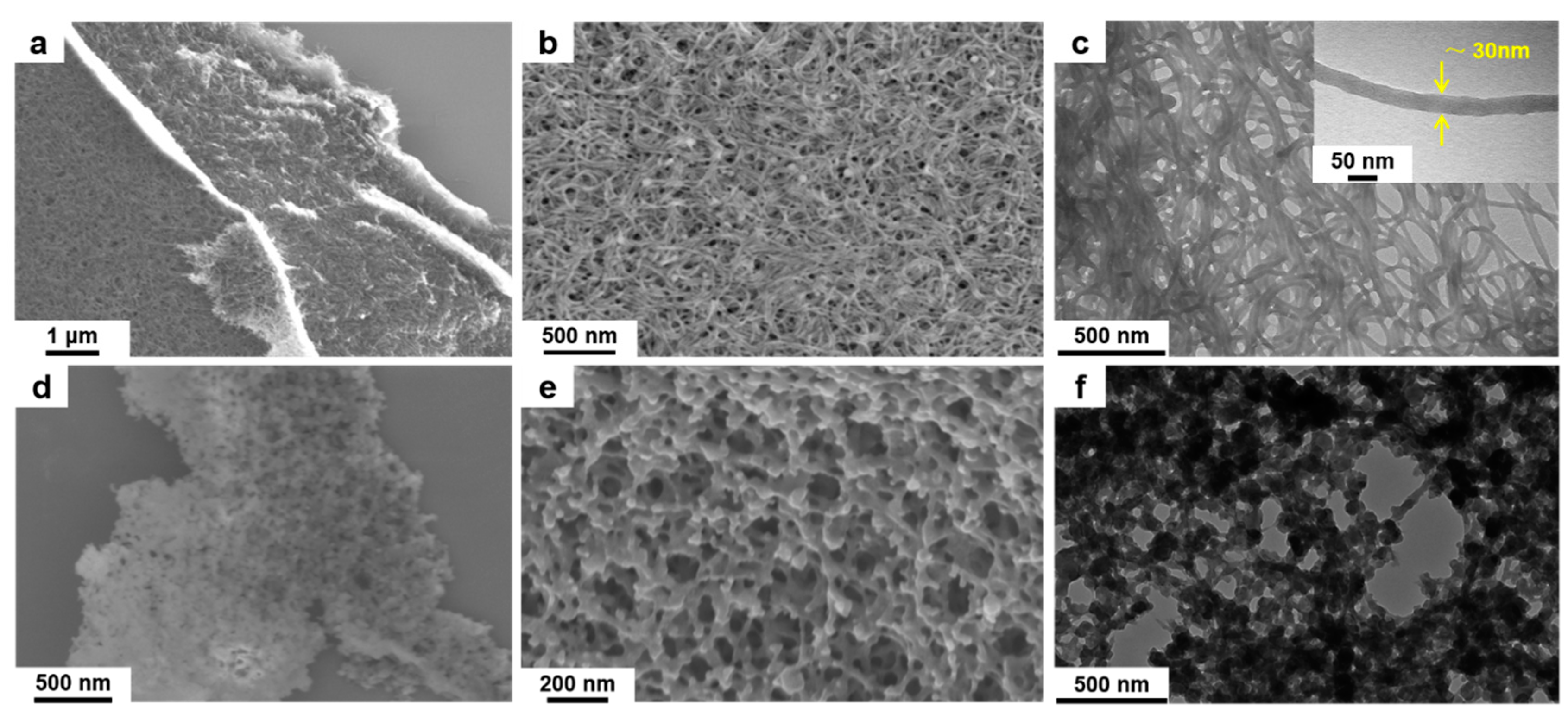
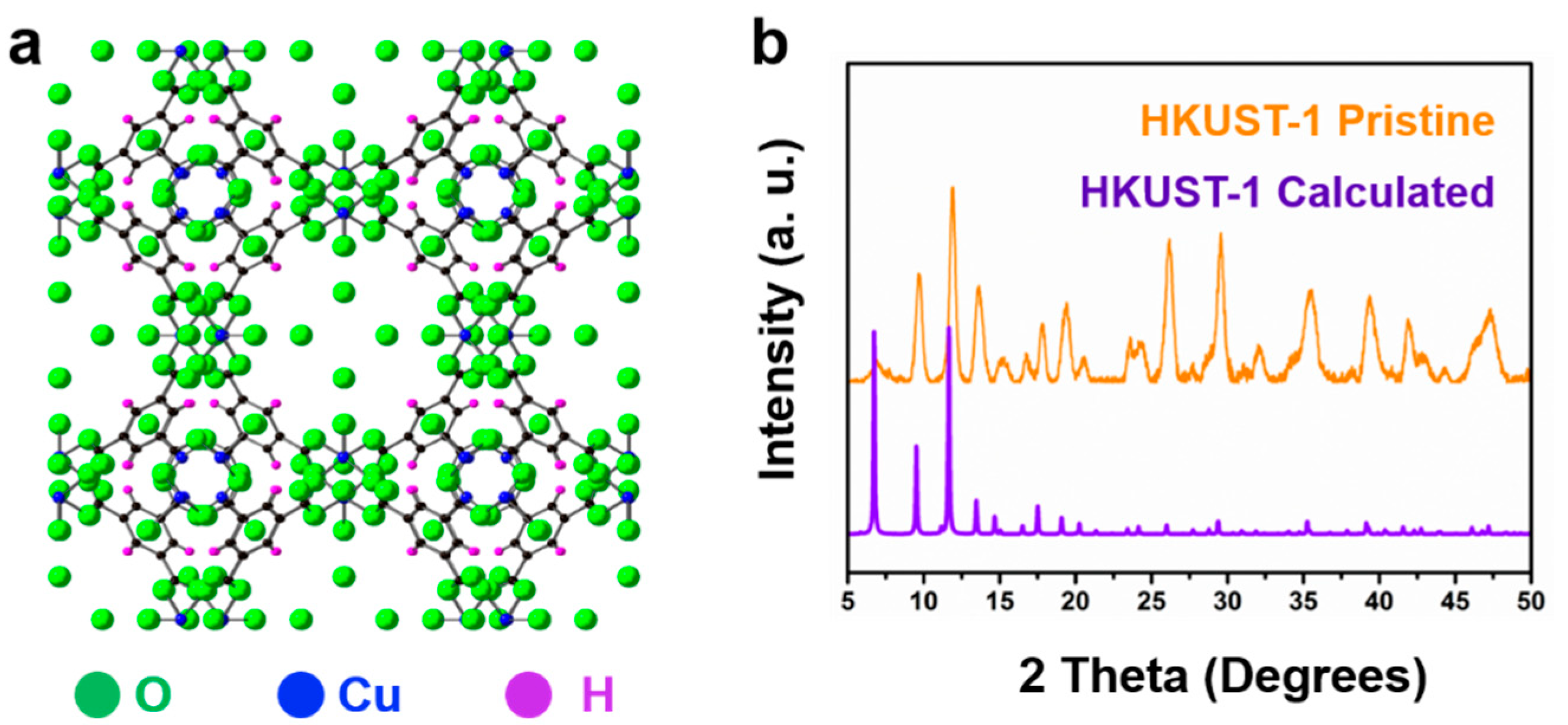
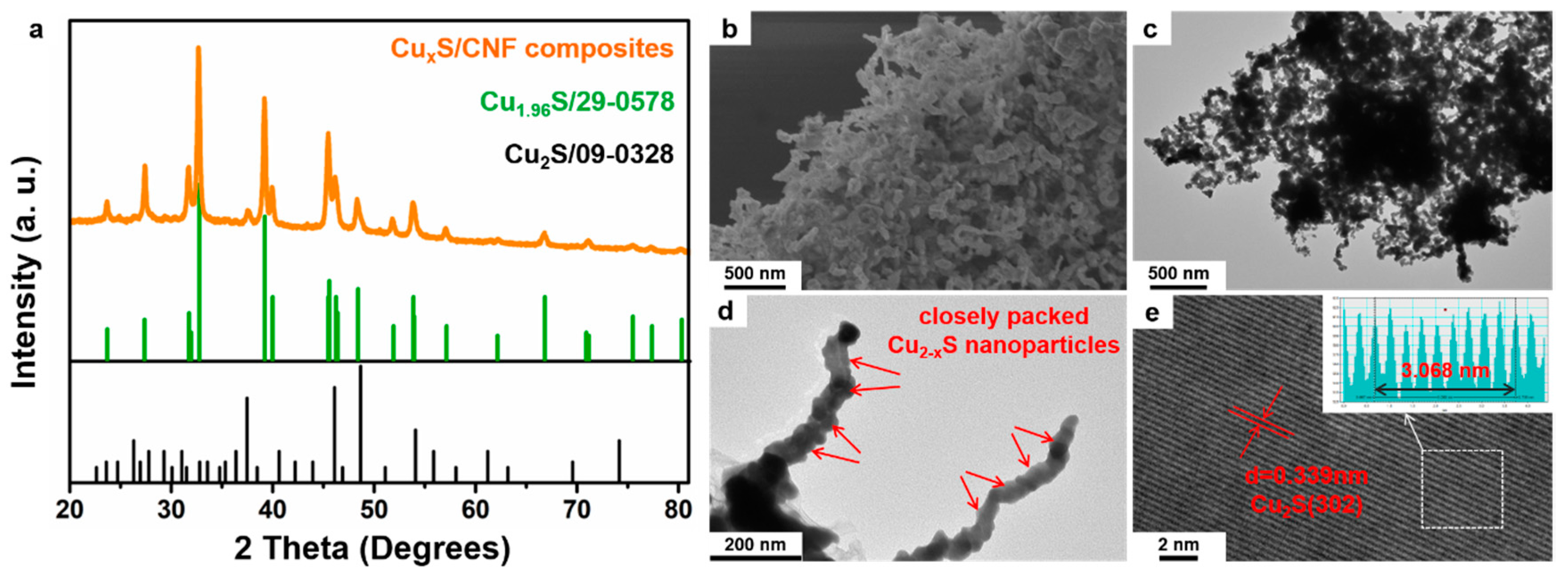
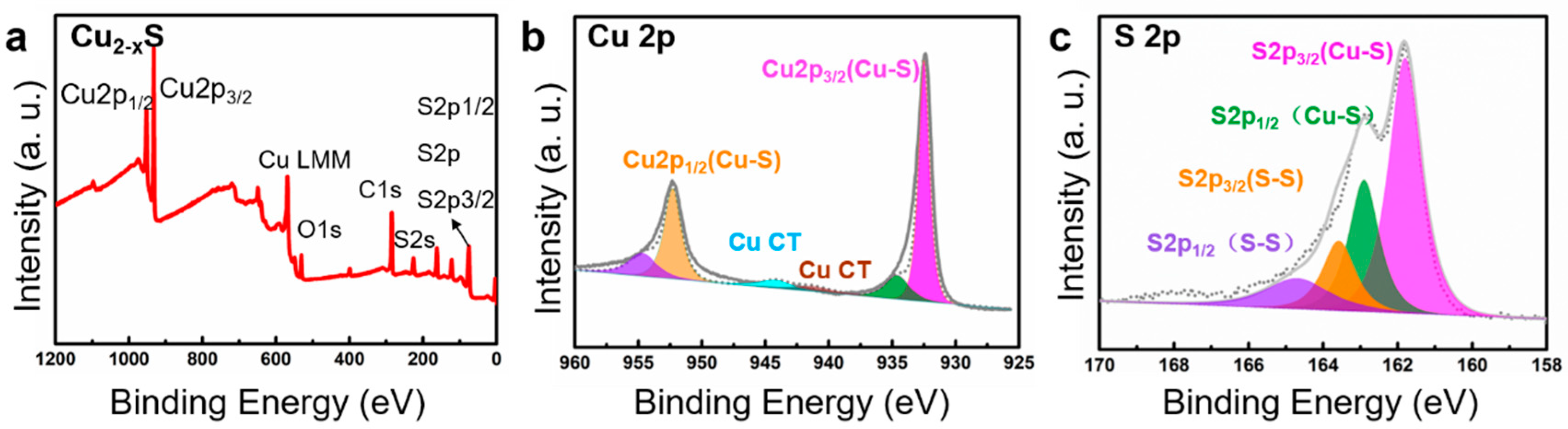
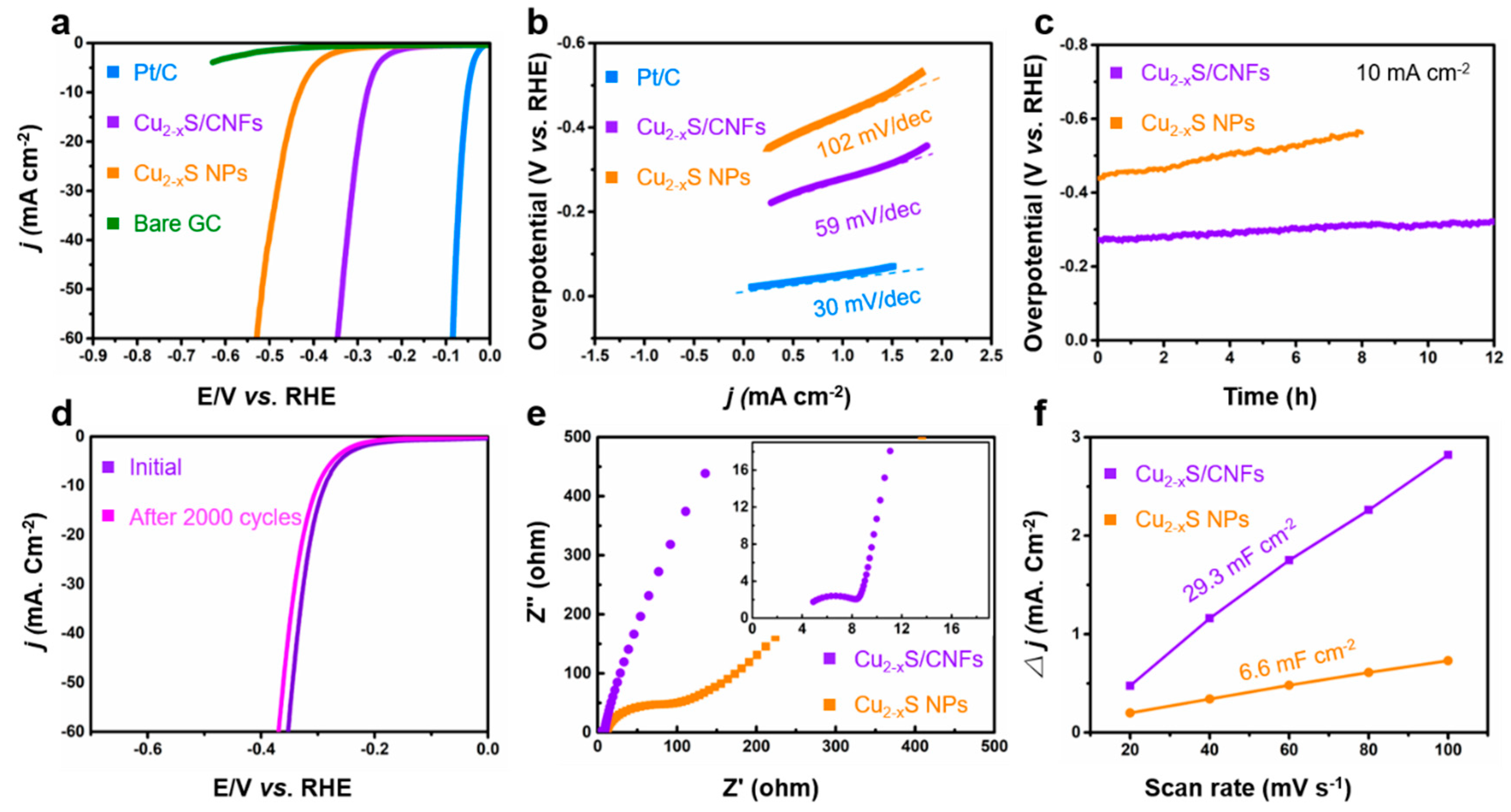
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durovic, M.; Hnat, J.; Bouzek, K. Electrocatalysts for the hydrogen evolution reaction in alkaline and neutral media. A comparative review. J. Power Sources 2021, 493, 229708. [Google Scholar] [CrossRef]
- Peng, L.S.; Wei, Z.D. Design and product engineering of high-performance electrode catalytic materials for water electrolysis. Prog. Chem. 2018, 30, 14–28. [Google Scholar]
- Bai, Y.J.; Fang, L.; Xu, H.T.; Gu, X.; Zhang, H.J.; Wang, Y. Strengthened synergistic effect of metallic MxPy(M = Co, Ni, and Cu) and carbon layer via peapod like architecture for both hydrogen and oxygen evolution reactions. Small 2017, 13, 1603718. [Google Scholar] [CrossRef] [PubMed]
- Menezes, P.W.; Indra, A.; Das, C.; Walter, C.; Gõbel, C.; Gutkin, V.; Schmeisser, D.; Driess, M. Uncovering the Nature of Active Species of Nickel Phosphide Catalysts in High-Performance Electrochemical Overall Water Splitting. ACS Catal. 2017, 7, 103–109. [Google Scholar] [CrossRef]
- Wen, L.L.; Yu, J.; Xing, C.C.; Liu, D.L.; Lyu, X.J.; Cai, W.P.; Li, X.Y. Flexible vanadium-doped Ni2P nanosheet arrays grown on carbon cloth for an efficient hydrogen evolution reaction. Nanoscale 2019, 11, 4198–4203. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Shi, K.; Chen, W.Z.; Gao, R.; Liu, Z.L.; Hao, H.G.; Wang, Y.Q. Enhanced electrocatalytic nitrogen reduction reaction performance by interfacial engineering of MOF-based sulfides FeNi2S4/NiS hetero-interface. Appl. Catal. B Environ. 2021, 287, 119956. [Google Scholar] [CrossRef]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; Royal Society of Chemistry: London, UK, 2009. [Google Scholar]
- MacGillivray, L.R.; Lukehart, C.M. Metal-Organic Framework Materials; John Wiley & Sons: Chichester, UK, 2014. [Google Scholar]
- Tuttle, R.R.; Folkman, S.J.; Rubin, H.N.; Finke, R.G.; Reynolds, M.M. Copper Metal–Organic Framework Surface Catalysis: Catalyst Poisoning, IR Spectroscopic, and Kinetic Evidence Addressing the Nature and Number of the Catalytically Active Sites En Route to Improved Applications. ACS Appl. Mater. Interfaces 2020, 12, 39043. [Google Scholar] [CrossRef] [PubMed]
- Nasani, R.; Saha, M.; Mobin, S.M.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; Kirillov, A.M.; Mukhopadhyay, S. Copper–organic frameworks assembled from in situ generated 5-(4-pyridyl) tetrazole building blocks: Synthesis, structural features, topological analysis and catalytic oxidation of alcohols. Dalton Trans. 2014, 43, 9944–9954. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.Z.; Wen, M.; Cai, Y.; Shi, Z.F.; Arol, A.S.; Kirillova, M.V.; Kirillov, A.M. Metal–Organic Architectures Assembled from Multifunctional Polycarboxylates: Hydrothermal Self-Assembly, Structures, and Catalytic Activity in Alkane Oxidation. Inorg. Chem. 2019, 58, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Chui, S.S.-Y.; Lo, M.-F.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Ge, L.; Wang, L.; Rudoiph, V.; Zhu, Z.H. Hierarchically structured metal–organic framework/ vertically-aligned carbon nanotubes hybrids for CO2 capture. RSC Adv. 2013, 3, 25360–25366. [Google Scholar] [CrossRef]
- Zhan, G.W.; Fan, L.L.; Zhou, S.F.; Yang, X. Fabrication of Integrated Cu2O@HKUST-1@Au Nanocatalysts via Galvanic Replacements toward Alcohols Oxidation Application. ACS Appl. Mater. Interfaces 2018, 10, 35234–35243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Y.; Guan, L.; Yu, L.; Lou, X.W. Formation of double-shelled zinc–cobalt sulfide dodecahedral cages from bimetallic zeolitic imidazolate frameworks for hybrid supercapacitors. Angew. Chem. Int. Ed. 2017, 56, 7141–7145. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.Y.; Gong, Y.; Lin, J.H. Low-temperature synthesis of NiS/MoS2/C nanowires/nanoflakes as electrocatalyst for hydrogen evolution reaction in alkaline medium via calcining/sulfurizing metal-organic frameworks. Electrochim. Acta 2018, 274, 74–83. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, Z.Y.; Huang, X.W.; Qin, Y.J.; Yang, H.P.; Ren, X.Z.; Zhang, Q.L.; Liu, J.H.; He, C.X. A unique space confined strategy to construct defective metal oxides within porous nanofibers for electrocatalysis. Energy Environ. Sci. 2020, 13, 5097–5103. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Gui, M.X.; Asif, M.; Yu, Y.; Dong, S.; Wang, H.T.; Wang, W.; Wang, F.; Xiao, F.; Liu, H.F. A facile modular approach to the 2D oriented assembly MOF electrode for non-enzymatic sweat biosensors. Nanoscale 2018, 10, 6629–6638. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Qiao, S.Z. Direct Growth of Well-Aligned MOF Arrays onto Various Substrates. Chem 2017, 2, 751–752. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Z.Y.; Jiang, H.L.; Yu, S.H. Nanowire-Directed Templating Synthesis of Metal−Organic Framework Nanofibers and Their Derived Porous Doped Carbon Nanofibers for Enhanced Electrocatalysis. J. Am. Chem. Soc. 2014, 136, 14385–14388. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.Y.; Li, H.Y.; Liu, J.; Song, J.P.; Fusaro, L.; Hu, Z.Y.; Chen, Y.X.; Li, Y.; Su, B.L. Weaving 3-D highly conductive hierarchically interconnected nanoporous web by threading MOF crystals onto multi walled carbon nanotubes for high performance Li–Se batter. J. Energy Chem. 2021, 59, 396–404. [Google Scholar] [CrossRef]
- Li, J.; Jiao, C.M.; Zhu, J.H.; Zhong, L.B.; Kang, T.; Aslam, S.; Wang, J.Y.; Zhao, S.F.; Qiu, Y.J. Hybrid co-based MOF nanoboxes/CNFs interlayer as microreactors for polysulfides-trapping in lithium-sulfur batteries. J. Energy Chem. 2021, 57, 469–476. [Google Scholar] [CrossRef]
- Han, W.G.; Dong, F.; Han, W.L.; Tang, Z.C. A strategy to construct uniform MOFs/PAN nanowire derived bead-like Co3O4 for VOC catalytic combustion. Chem. Commun. 2020, 56, 14307–14310. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Apostolopoulou-Kalkavoura, V.; da Costa, M.V.T.; Bergstrom, L.; Stromme, M.; Xu, C. Elastic Aerogels of Cellulose Nanofibers@Metal–Organic Frameworks for Thermal Insulation and Fire Retardancy. Nano Micro Lett. 2020, 12, 9. [Google Scholar] [CrossRef]
- Liu, P.Y.; Zhao, J.J.; Dong, Z.P.; Liu, Z.L.; Wang, Y.Q. Interwoving polyaniline and a metal-organic framework grown in situ for enhanced supercapacitor behavior. J. Alloys Compd. 2021, 854, 157181. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Ren, L.T.; Piao, Q.H.; Zhong, J.Q.; Wang, Y.B.; Li, H.W.; Chen, Y.F.; Wang, B. Flexible solid-state supercapacitor based on a metal-organic framework interwoven by electrochemically-deposited PANI. J. Am. Chem. Soc. 2015, 137, 4920–4923. [Google Scholar] [CrossRef] [PubMed]
- Centrone, A.; Yang, Y.; Speakman, S.; Bromberg, L.; Rutledge, G.C.; Hatton, T.A. Growth of metal-organic frameworks on polymer surfaces. J. Am. Chem. Soc. 2010, 132, 15687–15691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.K.; Jiang, M.; Chen, D.Y. DNA/Polymeric micelle self-assembly mimicking chromatin compaction. Angew. Chem. Int. Ed. 2012, 51, 8744–8747. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.Q.; Li, H.D.; Jiang, L.; Zhang, K.K.; Chen, D.Y. Solution-based fabrication of a highly catalytically active 3-D network constructed from 1-D metal-organic framework-coated polymeric worm-like micelles. Chem. Commun. 2015, 51, 10162–10165. [Google Scholar] [CrossRef]
- Cheng, Q.H.; Yang, C.; Tao, K.; Han, L. Inlaying ZIF-derived Co3S4 hollow nanocages on intertwined polypyrrole tubes conductive networks for high-performance supercapacitors. Electrochim. Acta 2020, 341, 136042. [Google Scholar] [CrossRef]
- Yu, Q.; Lv, J.S.; Liu, Z.H.; Xu, M.; Yang, W.; Owusu, K.A.; Mai, L.Q.; Zhao, D.Y.; Zhou, L. Macroscopic synthesis of ultrafine N-doped carbon nanofibers for superior capacitive energy storage. Sci. Bull. 2019, 64, 1617–1624. [Google Scholar] [CrossRef]
- Liu, I.P.; Teng, H.; Lee, Y.L. Highly electrocatalytic carbon black/copper sulfide composite counter electrodes fabricated by a facile method for quantum-dot-sensitized solar cells. J. Mater. Chem. A 2017, 5, 23146–23157. [Google Scholar] [CrossRef]
- Yilmaz, G.; Yam, K.M.; Zhang, C.; Fan, H.J.; Ho, G.W. In Situ Transformation of MOFs into Layered Double Hydroxide Embedded Metal Sulfides for Improved Electrocatalytic and Supercapacitive Performance. Adv. Mater. 2017, 29, 1606814. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Lee, Y.; Kim, I.; Hyun, S.; Lee, T.H.; Yun, S.; Yoon, W.S.; Moon, Y.; Lee, J.; Kim, S.; et al. Highly efficient nanocarbon coating layer on the nanostructured copper sulfide-metal organic framework derived carbon for advanced sodium-ion battery anode. Materials 2019, 12, 1324. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.J.; Zhang, H.J.; Xiao, L.; Liu, L.; Xu, H.T.; Qiu, H.J.; Wang, Y. Novel peapod-like Ni2P nanoparticles with improved electrochemical properties for hydrogen evolution and lithium storage. Nanoscale 2015, 7, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Li, Y.; Liu, G.; Hu, J. Assembly of Copolymer and Metal−Organic Framework HKUST-1 to Form Cu2−xS/CNFs Intertwining Network for Efficient Electrocatalytic Hydrogen Evolution. Nanomaterials 2021, 11, 1505. https://doi.org/10.3390/nano11061505
Bai Y, Li Y, Liu G, Hu J. Assembly of Copolymer and Metal−Organic Framework HKUST-1 to Form Cu2−xS/CNFs Intertwining Network for Efficient Electrocatalytic Hydrogen Evolution. Nanomaterials. 2021; 11(6):1505. https://doi.org/10.3390/nano11061505
Chicago/Turabian StyleBai, Yuanjuan, Yanran Li, Gonggang Liu, and Jinbo Hu. 2021. "Assembly of Copolymer and Metal−Organic Framework HKUST-1 to Form Cu2−xS/CNFs Intertwining Network for Efficient Electrocatalytic Hydrogen Evolution" Nanomaterials 11, no. 6: 1505. https://doi.org/10.3390/nano11061505
APA StyleBai, Y., Li, Y., Liu, G., & Hu, J. (2021). Assembly of Copolymer and Metal−Organic Framework HKUST-1 to Form Cu2−xS/CNFs Intertwining Network for Efficient Electrocatalytic Hydrogen Evolution. Nanomaterials, 11(6), 1505. https://doi.org/10.3390/nano11061505






