Abstract
Tantalum (Ta)-doped titanium oxide (TiO2) thin films are grown by plasma enhanced atomic layer deposition (PEALD), and used as both an electron transport layer and hole blocking compact layer of perovskite solar cells. The metal precursors of tantalum ethoxide and titanium isopropoxide are simultaneously injected into the deposition chamber. The Ta content is controlled by the temperature of the metal precursors. The experimental results show that the Ta incorporation introduces oxygen vacancies defects, accompanied by the reduced crystallinity and optical band gap. The PEALD Ta-doped films show a resistivity three orders of magnitude lower than undoped TiO2, even at a low Ta content (0.8–0.95 at.%). The ultraviolet photoelectron spectroscopy spectra reveal that Ta incorporation leads to a down shift of valance band and conduction positions, and this is helpful for the applications involving band alignment engineering. Finally, the perovskite solar cell with Ta-doped TiO2 electron transport layer demonstrates significantly improved fill factor and conversion efficiency as compared to that with the undoped TiO2 layer.
1. Introduction
Titanium dioxide (TiO2) thin films are widely studied owing to their optoelectrical properties that fulfill the requirements of numerous applications such as dye-sensitized photovoltaic devices [1], photocatalysis [2], and perovskite solar cells (PSCs) [3]. In order to enhance properties, the TiO2 films are commonly doped with transition metal, rare metal, or noble metal ions [4,5,6,7,8,9]. Some studies indicate that the electron-hole recombination could be reduced due to the generation of the charge trapping centers by foreign ions [10]. A moderate amount of metal dopants promotes the excitons separation and thus improves the mobility of photogenerated carriers [11,12,13]. Due to the wide forbidden energy gap and the absorption only at UV-light region, the TiO2 films are intentionally doped in order to reduce the energy gap and extend the light absorption to visible light in some applications [14]. The reduced band gap results from the generation of oxygen vacancies produced simultaneously through doping, introducing shallow energy levels below the conduction band edge [15,16]. Zhao et al. reported that doped TiO2 with suitable metal ions can effectively improve the electron transport of TiO2 [17]. Among various dopants, Ta5+ has an ionic radius (0.64 Å) very close to Ti4+ (0.61 Å), and thus it is able to be incorporated into the TiO2 lattice without severe strain or secondary phase production [18]. Doping with Ta is also more effective than with niobium, and has lower formation energy consumption and superior thermodynamic stability compared to doping with nitrogen [13]. Ta-doped TiO2 thin films can be prepared by various methods such as sputtering [19], sol-gel spin-coating [13], and chemical vapor deposition [20]. Recently, due to the demands of slim and thin electronics or the requirements of depositing films on high aspect ratio substrates, atomic layer deposition (ALD) technique receives great attention because of its pinhole-free deposition process, accurate thickness control at sub-nanometer level, and high conformality [21]. The Ta incorporation into ALD TiO2 films is mostly carried out by using the Ta2O5/TiO2 multilayer structure, and controlled by the ALD cycle ratio of the two metal oxides [18,22].
As for planar perovskite solar cells, the electron transport layer (ETL) plays a crucial role in electron transport and hole blocking. Due to the fact that ETLs are mostly prepared using the sol-gel process, the layer may contain pinholes or have low density, rendering holes possible to pass the ETL to increase the recombination rate. A compact layer is usually inserted at the sol-gel ETL/transparent electrode interface to improve the hole blocking ability. Thanks to the pinhole-free structure and highly conformal coverage, a thin ALD layer is able to be used as both ETL and compact layer for the perovskite solar cells [23]. However, the conduction band edge of TiO2 (about −3.9 eV) is shallower than that of cesium formamidinium-based CsxFA1–xPb(I1–yBry)3 (about −4.2 eV), which is one of the most promising classes of perovskite light absorber for solar cells due to its wide band gap and higher durability compared to MAPbI3 [24]. The conduction band mismatch at the TiO2 ETL/perovskite interface causes a barrier that is theoretically unfavorable for electron transport. Development of doped TiO2 ETLs is one possible way to have a better band alignment and an improved PSC performance.
In the present study, Ta-doped TiO2 films are grown by using plasma enhanced ALD (PEALD), and used as an ETL for PSCs. The metal precursors tantalum ethoxide (Ta(OEt)5) as Ta dopant source and titanium isopropoxide (TTIP) as Ti source are simultaneously fed into the deposition chamber. The precursor output flows are controlled by the bubbler temperature, which is varied from 70–90 °C to obtain different Ta doping ratio. The effects of the bubbler temperature on the structural, electrical, and optical properties of the Ta-doped TiO2 films are investigated. The adjustments of the band gap, conduction band, and valence band positions are presented. Finally, the Ta-doped TiO2 ETL is applied to PSC fabrication, and the improved conversion efficiency and hysteresis is demonstrated and discussed.
2. Materials and Methods
2.1. Thin Film Preparation
Glass substrates with a size of 20 mm × 20 mm and a thickness of 1 mm were cleaned in an ultrasonic bath with deionized water, acetone, and ethanol for 15 min, and then dried in an oven at 70 °C for 30 min. The substrates were placed on the substrate holder in the deposition chamber of PEALD (R200 advance, Picosun Oy, Espoo, Finland). The used metal precursors were Ta(OEt)5 and TTIP (Aimou Yuan Scientific, Nanjing, China) as the Ta and Ti sources, respectively. Nitrogen with a purity of 99.999% was introduced to the precursor bubblers to transport the precursor vapors to the deposition chamber. The oxidant was provided by inductively coupled oxygen-argon mixed plasma generated by an RF power of 1500 W. The substrate temperature was 250 °C. The temperatures of the Ta(OEt)5 and TTIP bubblers were controlled from 70 to 90 °C by means of a heating jacket. The thickness of the Ta-doped TiO2 films was 60 nm. The undoped films were prepared under identical conditions, except for the use of the TTIP metal precursor alone. The detailed deposition parameters for the PEALD Ta-doped TiO2 films are summarized in Table 1.

Table 1.
Deposition parameters of the PEALD Ta-doped TiO2 films.
2.2. Perovskite Solar Cell Fabrication
The 15 nm-thick ALD TiO2 films were deposited on the cleaned fluorine-doped tin oxide (FTO) substrates. Lead iodide, lead bromide, methylammonium bromide, and formamidinium iodide were dissolved with a molar ratio of 1:1.15:0.2:0.2 in N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) with 4:1 volume ratio. The CsI dissolved in DMSO as 1.5 mol stock solution was further poured to give Cs0.1(FA0.83MA0.17)0.9Pb(I0.83Br0.17)3. The perovskite precursor solution was then spin-coated on ALD doped or undoped TiO2 ETL in two steps, the first spreading step with 1000 rpm for 10 s and the second step with 6000 rpm for 25 s. Chlorobenzene of 110 μL was sprayed on the spinning substrate at five seconds before the end of the second-step spin-coating process. Afterwards, an annealing process at 100 °C was performed for 60 min. Spiro-OMeTAD (Lumtec, New Taipei, Taiwan) of 50 uL was spin-coated on the perovskite with 4000 rpm for 30 s following cool down of the substrate back to room temperature. Finally, the cell fabrication was finished by evaporating gold films on the Spiro-OMeTAD. The devices had an active area of 0.1 cm2.
2.3. Characterization
The thickness of the films was determined using an ellipsometer (M-2000, J. A. Woollan Co., Lincoln, NE, USA). The ultraviolet-visible spectrometer (MFS630, Hong-Ming, New Taipei City, Taiwan) was employed to obtain the transmittance spectra. The X-ray diffraction apparatus (XRD, Rigaku TTRAXIII, Ibaraki, Japan) was used for characterizing the crystalline structure of the films. The X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Fisher, Waltham, MA, USA) was used in order to investigate the chemical states and elemental composition of the films. The Fermi-level and valance band position of the Ta-doped TiO2 films were obtained using UV photoelectron spectroscopy (UPS, ESCALAB Xi+, Thermo Fisher Scientific, Gloucester, UK) with He I source (photon energy of 21.2 eV). The resistivity of the films was examined by using a four-point probe (T2001A3, Ossila, Sheffield, UK). The current density–voltage (J–V) curves and solar cell external parameters such as open-circuit voltage (Voc), short-circuit current density (Jsc), fill factor (FF), and conversion efficiency (η) were measured at AM1.5G (100 mW/cm2) using a solar simulator (Newport Oriel, Irvine, CA, USA).
3. Results and Discussion
In this work, Ta(OEt)5 precursor is used as the Ta dopant source, and co-injected with TTIP precursor into the deposition zone. The amount of the output precursor molecules is determined by the bubbler temperature controlling the vapor pressure of the metal precursors. Figure 1a shows the temperature-dependent vapor pressures of TTIP and Ta(OEt)5 as given by:
where P is the vapor pressure (unit in mmHg), and T is the bubbler temperature. Both of the metal vapor pressures increase with the bubbler temperature. It is believed that the film growth is related to the output vapors of metal precursors, which can be controlled by either varying the carrier gas flows or the bubbler temperature. The latter is adopted in this study. Figure 1b shows the deposition rates of the PEALD Ta-doped TiO2 films prepared at different bubbler temperatures. The value of the undoped film is also indicated for comparison. It is seen that the TiO2 has a deposition rate of 0.252 Å/cycle, which is similar to that reported by other groups [25,26]. The deposition rate is not much affected at the bubbler temperature of 70 °C, and then increases from 0.252 to 0.284 Å/cycle when the bubbler temperature increases from 70 to 90 °C. It is reported that the deposition rate of the ALD tantalum oxide is 0.5–1 Å/cycle [27,28], which is nearly two to four times higher than that of TiO2. This may be one explanation for the increased deposition rate for the Ta-doped TiO2. Another reason is that Ta incorporation leads to a less dense film structure due to the donor doping induced oxygen vacancies defects to maintain charge neutral as commonly seen in doped metal oxides [16]. The relatively loose film structure of the Ta-doped TiO2 contributes to a higher film thickness.
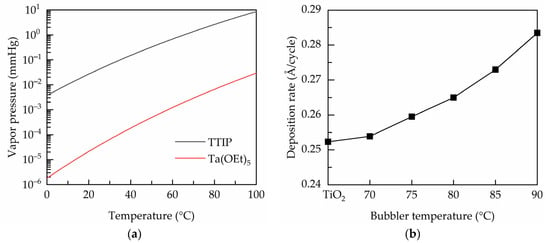
Figure 1.
(a) Temperature-dependent vapor pressures of the TTIP and Ta(OEt)5 metal precursors. (b) Deposition rate of the PEALD films prepared at various bubbler temperatures.
Figure 2a shows the transmittance over a wavelength range from 300 to 800 nm for the PEALD films prepared at various bubbler temperatures. The lower transmittance at the short wavelength region is related to the band-to-band absorption of the TiO2 films. The transmittance decreases with increasing the bubbler temperature, indicating that the increase of Ta concentration enhances the absorption. The reduced transmittance could also be a consequence of the light scattering caused by the oxygen vacancies [29]. The inset shows the transmittance in the short-wavelength region (300–400 nm) to evaluate the absorption edge. It can be seen that the edge shifts towards longer wavelength direction when the bubbler temperature increases, inferring the reduced band gap. Figure 2b shows the band gap deduced from Tauc’s equation [30]:
where α is the absorption coefficient, hv is the photon energy, A is the material-dependent constant, and Eg is the band gap. The n value is taken as 1/2 for indirect band gap anatase TiO2 [31]. The band gap values of the PEALD films are evaluated by plotting (αhv)1/2 versus hv and extrapolating the linear region of the resultant curves to obtain an interception with the hv-axis. The band gap of the TiO2 is 3.17 eV, and it decreases from 3.09 to 3 eV by increasing the bubbler temperature from 70 to 90 °C. This is possibly related to the band gap narrowing effect due to the formation of the oxygen vacancies defects that introduce shallow donor levels below the conduction band. Similar effect can also be observed elsewhere [32].
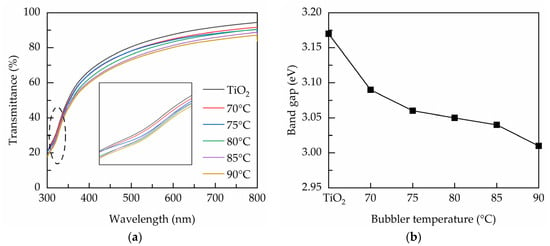
Figure 2.
(a) Transmittance spectra and (b) band gap of the PEALD films prepared with various bubbler temperatures.
Figure 3a shows the XRD patterns of the PEALD undoped and Ta-doped TiO2 films. The peaks labeled are well matched to anatase TiO2 (JCPSD#83-2243). With Ta doping, the (103), (004), and (112) peaks vanish, but no additional peak such as Ta or Ta2O5 is found, indicating that Ta atoms are well-incorporated into the TiO2 crystalline structure by replacing Ti4+ at random lattice sites. The full width at half maximum (FWHM) of the most intense (101) orientation is extracted to calculate crystallite size according to the Scherrer equation:
with L the crystallite size, K the shape factor taken as 0.9, λ the X-ray wavelength (0.154 nm), β is the width of the observed diffraction line at its half intensity maximum, and θ is the Bragg angle. Figure 3b illustrates the FWHM and crystallite size as function of bubbler temperature. It is seen that the FWHM increases when Ta is substituted into TiO2, suggesting that the crystallinity is diminished. The crystallite size reduces from 28 nm for undoped TiO2 to 23 nm for the film deposited at the bubbler temperature of 70 °C. Further increasing the bubbler temperature up to 90 °C results in a slight reduction in crystallite size. Although Ta5+ has a similar ionic radius to Ti4+, the electron density around Ta and the doping induced oxygen vacancies cause the rearrangement of the nearby atoms [16,33], which limits crystal growth.
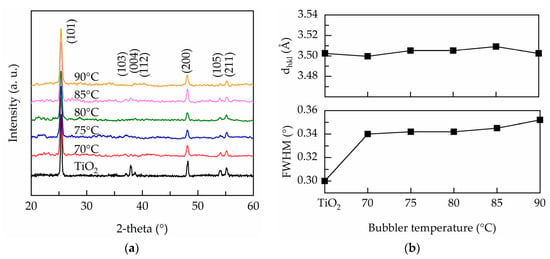
Figure 3.
(a) XRD pattern, (b) interplanar distance and FWHM of the PEALD Ta-doped TiO2 films prepared at various bubbler temperatures.
Figure 4 shows the composition and chemical states measured by XPS for the PEALD Ta-doped TiO2 films prepared at different bubbler temperatures. Figure 4a shows the high-resolution Ta 4f for the PEALD films, evidencing the Ta incorporation into the TiO2 films. The Ta peaks can be deconvoluted into two Gaussian components at 21.7 and 23.7 eV, corresponding to TaOx (x < 2.5) [34].The atomic ratios of the Ti, O, and Ta elements are depicted in Figure 4b. The TiO2 film has an oxygen-deficient structure. The Ta content is varied from 0.82 to 0.95 at.% with increase in bubbler temperature from 70 to 90 °C. The XPS spectra over the biding energy of 450 to 470 eV corresponding to Ti 2p are shown in Figure 4c. The binding energies of around 453–460 eV and 460–466 eV belong to Ti 2p3/2 and Ti 2p1/2, respectively [35]. Each spin state can further be deconvoluted into Ti4+, Ti3+ and Ti2+ peaks [36]. The Ti 2p3/2 peaks are analyzed, and the peak ratios of Ti2+, Ti3+ and Ti4+ to total are shown in Figure 4d. The TiO2 has the highest Ti4+ peak ratio of about 60%. At the bubbler temperature of 70 °C, the Ti4+ ratio decreases and Ti3+ ratio increases, indicating that Ta dopants create oxygen vacancies and reduce Ti4+ to Ti3+. At the bubbler temperature of 90 °C, the Ti4+ ratio further reduces and the Ti2+ ratio increases. This result suggests that the higher Ta incorporation causes Ti4+ to reduce to not only Ti3+ but also Ti2+. Figure 4e shows the high-resolution O 1s spectra for the PEALD films. The peaks are further split into two peaks, one at the lower binding energies (530–531 eV) assigned to lattice oxygen and the other at the higher binding energies of 531–532 eV ascribed to oxygen ions in oxygen-deficient regions in the lattice [37]. The latter is usually deemed to reflect the presence of oxygen vacancies. The ratio of each component to total is calculated as shown in Figure 4f. The lattice oxygen shows an opposite trend to defective oxygen. The increased defective oxygen ratio confirms that the Ta doping leads to the generation of oxygen vacancies defects.
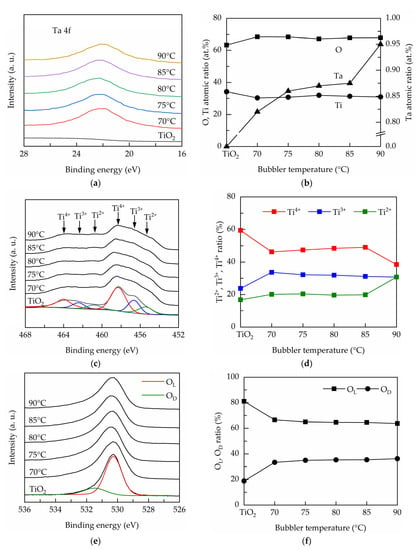
Figure 4.
(a) Ta 4f spectra, (b) O, Ti and Ta atomic ratios, (c) O 1s spectra, (d) OL and OD peak area ratios, (e) Ti 2p3/2 spectra, and (f) Ti2+, Ti3+ and Ti4+ peak area ratios for the PEALD Ta-doped TiO2 films.
Figure 5 shows the UPS spectra for the PEALD Ta-doped TiO2 films prepared at different bubbler temperatures. As shown in Figure 5a, the extrapolation of the linear region of the curves to the binding energy axis gives the maximum valance band energy (EVBM) with respective to the Fermi energy. The curves in the high binding energy region are shown in Figure 5b, and similarly, the extrapolation of the linear region of the curves to the x-axis corresponds to the cut-off energy (Ecutoff). Accordingly, the positions of Fermi level (EF) and valance band (Ev) can be calculated as given by [38]:
where the value of 21.2 eV corresponds to the energy of the used UV light source. The position of the conduction band (Ec) is further obtained using: Ec = Ev + Eg with the band gap values shown previously. The calculated Ec and Ev are summarized in Table 2. It is found the difference between the doped films is relatively small; however, the Ta-doped TiO2 films have both deeper conduction band and valence band edges, as compared to the undoped TiO2 film. The deep valance band positions favor the applications requiring hole blocking ability such as the electron transport layer in solar cells [39,40]. The lowered conduction band edges of the Ta-doped films indicate that Ta incorporation can adjust the conduction band position, which is benefit for interfacial band alignment engineering and a very important feature for being used as a selective charge transport layer of optoelectronics.
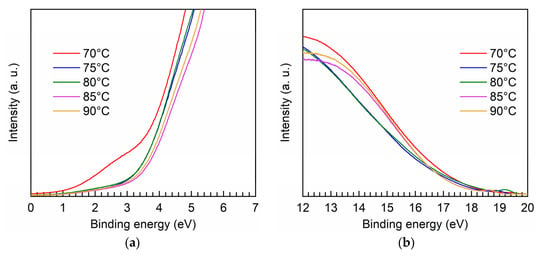
Figure 5.
UPS spectra in the (a) low binding energy region and (b) high binding energy region for the PEALD Ta-doped TiO2 films prepared at different bubbler temperatures.

Table 2.
Band gap, valance band position, and conduction band position for the PEALD Ta-doped TiO2 films with different bubbler temperatures.
Figure 6 shows the resistivity measured by a four-point probe for the PEALD Ta-doped TiO2 films prepared at different bubbler temperatures. The TiO2 exhibits a considerably high resistivity of 3.4 × 102 Ω·cm. The Ta incorporation leads to significant reduction in resistivity. All the Ta-doped TiO2 films have resistivities at the order of 10−1 Ω·cm. The minimum resistivity is 3 × 10−1 Ω·cm at the bubbler temperature of 90 °C. The decrease in resistivity is due to the Ta substitution for Ti atoms in the lattice sites, contributing free electrons. In addition, the Ta incorporation also results in creation of oxygen vacancies (as suggested by XPS), which are widely known as one of the sources of free electrons. Similar resistivity reduction due to the Ta doping is reported by other studies. For example, the resistivity of the Ta-doped TiO2 films prepared using metal organic chemical vapor deposition decreases four to six orders for at a high Ta doping level of 4–5% [17,41]. In the present work, the Ta content for the bubbler temperatures of 70–90 °C is between 0.82 and 0.95 at.%, leading to resistivities three orders lower than that of the undoped TiO2 film.
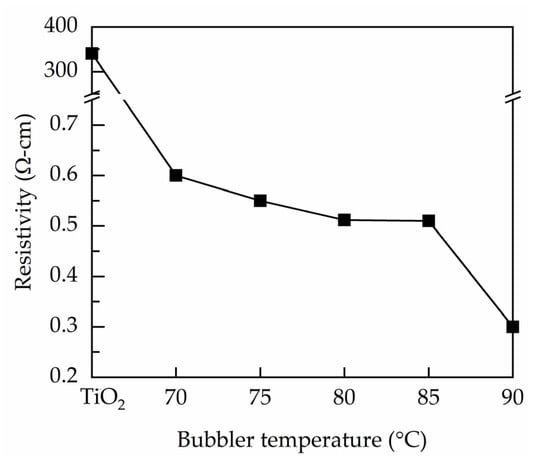
Figure 6.
Four-point probe resistivity of the PEALD Ta-doped TiO2 films prepared at different bubbler temperature.
Figure 7 depicts the isolated energy band diagram of conventional TiO2 ETL-based PSCs. The Ec and Ev values of each layer are experimentally determined. Similar values can be found in other studies [42,43,44,45]. The photogenerated electrons and holes move towards different directions. A downward shift of CBM is generally favorable in the viewpoint of electron transport. However, as shown in the inset of Figure 7, the undoped TiO2 layer has a shallower CBM than the perovskite absorber, which causes the band mismatch and obstacles for electron transport. The charge accumulation at the perovskite/TiO2 interface could resist electrons to flow into FTO. In the case of the adoption of Ta-doped TiO2, with the experimental Ec values around −(4.1–4.23) eV shown in the UPS result, the band mismatch is supposed to be mitigated or absent due to the deeper conduction band edge. Considering the conduction band of the perovskite layer (−4.21 eV), the Ta-doped TiO2 prepared at the bubbler temperature of 85 °C is used for device fabrication due to the least conduction band offset.
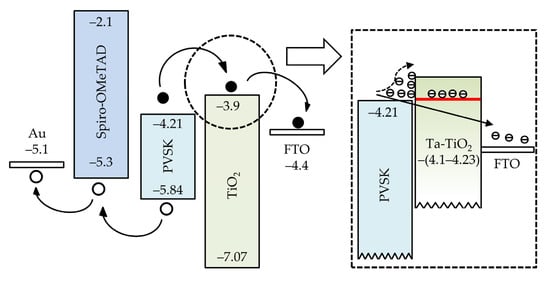
Figure 7.
Isolated energy band diagram of each material involved in a TiO2 ETL-based PSC. The inset indicates the interfaces of perovskite/TiO2 and perovskite/Ta-doped TiO2 ETL.
Figure 8a shows the band-bending of energy levels in the PSCs with undoped TiO2 and Ta-doped TiO2 ETL deposited at the bubbler temperature of 85 °C. The major difference is at the ETL/perovskite interface. A conduction band barrier is clearly presented at the undoped TiO2/perovskite absorber interface; however, the barrier disappears in the case of using Ta-doped TiO2 ETL due to the very little conduction band mismatch. This is expected to be reflected on the improvement in FF of the devices. In addition, as shown in the inset, the slope of the conduction band of the Ta-doped TiO2 device is steeper than that of undoped TiO2 device, and this implies a greater built-in electric field, which could enhance the separation of photo-generated electron-hole pairs. The J–V characteristics of the fabricated PSCs in the forward and reverse scans are shown in Figure 8b, and the corresponded external photovoltaic parameters are listed in Table 3. Apart from the slightly improved Voc and Jsc, the cell with the Ta-doped TiO2 ETL has a considerably enhanced FF. The SEM images of the PEALD TiO2 and Ta-doped TiO2 films are shown in Figure S1. The two samples are compact without observable pinholes, and do not show much difference in surface morphology likely due to the low-Ta doping level. It is believed that the FF improvement of the perovskite solar cells with the Ta-doped TiO2 ETL is due to the reduction of the conduction band mismatch, thereby decreasing the transport barrier of electrons. This demonstrates the significance of the conduction band alignment of the ETL. In addition to the absence of the transport barrier at perovskite/ETL interface, the improved FF of the device with the Ta-doped TiO2 ETL is partially owing to the smaller series resistance as assessed from the inverse slope of the J–V curves at the Voc-point. The hysteresis index of the PSCs is investigated, which is given as [46]:
where the subscript R represents reverse scan, F denotes the forward scan, and H is the unit step function. The result reveals the hysteresis of 1.06% for the device with undoped TiO2 ETL and 0.21% for the cell with Ta-doped TiO2 ETL. This is in agreement with the findings reported by Kim et al. [47], where PSCs with doped metal oxide ETLs exhibit less hysteresis compared to undoped metal oxides. It is worth mentioning that the Ta-doped TiO2 film prepared at the bubbler temperature of 90 °C has been also applied to PSC fabrication as it seems to have the lowest resistivity as well as an appropriate conduction band position. However, the resultant device conversion efficiency is lower than in the case of 85 °C-bubbler temperature. The J–V curve and the corresponded photovoltaic performance of the device at the bubbler temperature of 90 °C are shown in Figure S2. The device has conversion efficiency of 17.3% (Voc of 1.1 V, Jsc of 22.5 mA/cm2, FF of 0.7), lower than that (18.09%) of the device with the Ta-doped TiO2 ETL deposited at 85°C. It is speculated that the performance reduction might be related to the increased defect formation when the impurity doping level increases. This compromises the cell performance, and thus the optimal Ta bubbler temperature occurs at 85 °C.
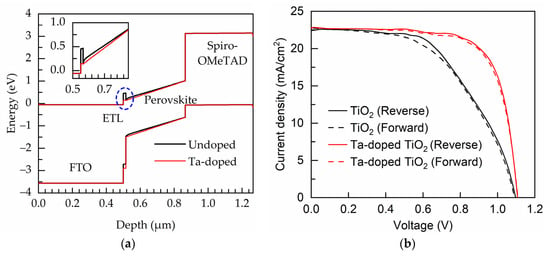
Figure 8.
(a) Band bending of energy levels in PSCs. (b) J–V curves of the PSCs measured in reverse and forward directions. The inset shows the enlarged view at the ETL/perovskite interface.

Table 3.
Performance of PSCs measured in the reverse and forward directions.
The performance of 12 devices under reverse scan is shown in Figure 9 for TiO2 and Ta-doped TiO2 perovskite solar cells. The results demonstrate small standard deviations of the device photovoltaic parameters. For instance, the standard deviation of the device efficiency is less than 0.4% for TiO2 devices and 0.46% for Ta-doped TiO2 devices, indicating high reproducibility. Moreover, the average conversion efficiency has the same trend shown in Figure 9, inferring that the comparison of efficiency among different groups is reliable.
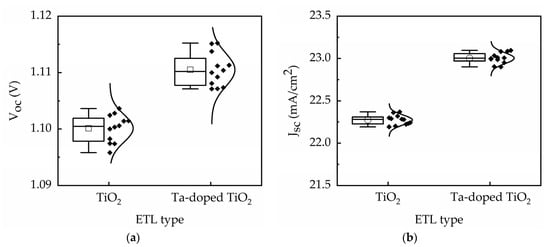
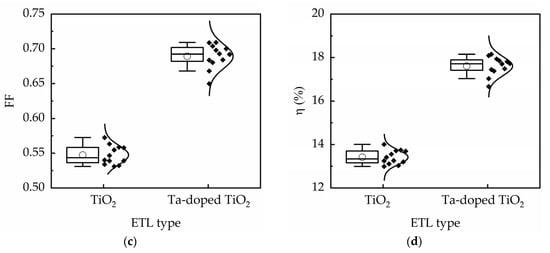
Figure 9.
Performance of 12 devices for TiO2 and Ta-doped TiO2 perovskite solar cells: (a) Voc, (b) Jsc, (c) FF, and (d) η.
4. Conclusions
The Ta-doped TiO2 thin films are prepared using PEALD with co-injected Ta(OEt)5 and TTIP metal precursors as Ta and Ti sources, respectively. The bubbler temperature varies to obtain different Ta content in the films. The XRD result shows that the Ta incorporation does not lead to additional crystalline phases but reduces crystallinity. As confirmed by XPS, the Ta dopants cause Ti4+ ions reducing to Ti3+ and Ti2+, due to the creation of the oxygen vacancies defects. The resistivity significantly reduces by three orders at the Ta content of 0.82–0.95 at.%, as compared to that of the undoped TiO2. The band gap, conduction band, and valence band position can be tailored by the Ta incorporation, and this is favorable for the applications involving interfacial band structure engineering. Finally, the PSC with Ta-doped TiO2 ETL demonstrates a significantly improved FF from 0.54 to 0.71 and conversion efficiency from 13.42% to 18.09% as compared to the PSC with conventionally undoped TiO2 ETL.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano11061504/s1, Figure S1: SEM images of PEALD (a) TiO2 and (b) Ta-doped TiO2 deposited at the bubbler temperature of 85 °C, Figure S2: J–V curve of the perovskite solar cell with the Ta-doped TiO2 ETL deposited at the bubbler temperature of 90 °C.
Author Contributions
Conceptualization, C.-H.H. and S.-Y.L.; methodology, K.-T.C.; formal analysis, C.-H.H., K.-T.C., L.-Y.L., W.-Y.W., L.-S.L., P.G., Y.Q. and W.-Z.Z.; investigation, K.-T.C., X.-Y.Z. and P.-H.H.; writing—original draft preparation, C.-H.H.; writing—review and editing, C.-H.H. and S.-Y.L.; supervision, S.-Y.L.; funding acquisition, C.-H.H., S.-Y.L. and P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by a scientific and technological project in Xiamen (no. 3502ZCQ20191002), scientific research projects of Xiamen University of Technology (no. 405011904, 40199029, YKJ19001R, and XPDKQ19006), the Natural Science Foundation of Fujian Province (no. 2020H0025, 2019J01877, 2020J01930). P.G. knowledge ‘the National Natural Science Foundation of China (Grant No. 21975260), and the Recruitment Program of Global Experts (1000 Talents Plan) of China’. This study is also sponsored by the Fujian Association for Science and Technology.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Q.; Wang, J. Dye-sensitized solar cells based on surficial TiO2 modification. Sol. Energy 2019, 184, 454–465. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, B.; Zhu, Y.; Ma, Z.; Liu, B.; Miao, X.; Ma, R.; Wang, C. Enhanced performance of TiO2-based perovskite solar cells with ru-doped TiO2 electron transport layer. Sol. Energy 2018, 169, 335–342. [Google Scholar] [CrossRef]
- Wu, M.-C.; Chan, S.-H.; Lee, K.-M.; Chen, S.-H.; Jao, M.-H.; Chen, Y.-F.; Su, W.-F. Enhancing the efficiency of perovskite solar cells using mesoscopic zinc-doped TiO2 as the electron extraction layer through band alignment. J. Mater. Chem. A 2018, 6, 16920–16931. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The effect of doping elements on photocatalytic activity. Mater. Adv. 2020, 1, 1193–1201. [Google Scholar] [CrossRef]
- El Ruby Mohamed, A.; Rohani, S. Modified TiO2 nanotube arrays (TNTAs): Progressive Strategies towards visible light responsive photoanode, a review. Energy Environ. Sci. 2011, 4, 1065. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, P.; Bahadur, L. Neetu Hydrothermal synthesized Nd-doped TiO2 with anatase and brookite phases as highly improved photoanode for dye-sensitized solar cell. Sol. Energy 2020, 208, 173–181. [Google Scholar] [CrossRef]
- Sun, J.; Yu, S.; Zheng, Q.; Cheng, S.; Wang, X.; Zhou, H.; Lai, Y.; Yu, J. Improved performance of inverted organic solar cells by using la-doped TiO2 film as electron transport layer. J. Mater Sci. Mater Electron. 2017, 28, 2272–2278. [Google Scholar] [CrossRef]
- Qu, X.; Hou, Y.; Liu, M.; Shi, L.; Zhang, M.; Song, H.; Du, F. Yttrium doped TiO2 porous film photoanode for dye-sensitized solar cells with enhanced photovoltaic performance. Results Phys. 2016, 6, 1051–1058. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. The advancements in sol–gel method of doped-TiO2 photocatalysts. Appl. Catal. A Gen. 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Cui, Q.; Zhao, X.; Lin, H.; Yang, L.; Chen, H.; Zhang, Y.; Li, X. Improved efficient perovskite solar cells based on ta-doped TiO2 nanorod arrays. Nanoscale 2017, 9, 18897–18907. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.J.; McKenna, K.P. Screening doping strategies to mitigate electron trapping at anatase TiO2 surfaces. J. Phys. Chem. C 2019, 123, 22358–22367. [Google Scholar] [CrossRef]
- Ranjan, R.; Prakash, A.; Singh, A.; Singh, A.; Garg, A.; Gupta, R.K. Effect of tantalum doping in a TiO2 compact layer on the performance of planar Spiro-OMeTAD free perovskite solar cells. J. Mater. Chem. A 2018, 6, 1037–1047. [Google Scholar] [CrossRef]
- Xu, C.; Lin, D.; Niu, J.-N.; Qiang, Y.-H.; Li, D.-W.; Tao, C.-X. Preparation of Ta-Doped TiO2 Using Ta2O5 as the doping source. Chin. Phys. Lett. 2015, 32, 088102. [Google Scholar] [CrossRef]
- Alim, M.A. Photocatalytic Properties of Ta-Doped TiO2. Ionics 2017, 23, 1–15. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601. [Google Scholar] [CrossRef]
- Zhao, W.; He, L.; Feng, X.; Luan, C.; Ma, J. Structural, electrical and optical properties of epitaxial ta-doped titania films by MOCVD. Cryst. Eng. Comm. 2018, 20, 5395–5401. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kwon, S.-H.; Jeong, Y.-K.; Kim, I.; Kim, K.-H. Atomic layer deposition of Ta-doped TiO2 electrodes for dye-sensitized solar cells. J. Electrochem. Soc. 2011, 158, B749. [Google Scholar] [CrossRef]
- Hitosugi, T.; Furubayashi, Y.; Ueda, A.; Itabashi, K.; Inaba, K.; Hirose, Y.; Kinoda, G.; Yamamoto, Y.; Shimada, T.; Hasegawa, T. Ta-doped anatase TiO2 epitaxial film as transparent conducting oxide. Jpn. J. Appl. Phys. 2005, 44, L1063–L1065. [Google Scholar] [CrossRef]
- Bawaked, S.M.; Sathasivam, S.; Bhachu, D.S.; Chadwick, N.; Obaid, A.Y.; Al-Thabaiti, S.; Basahel, S.N.; Carmalt, C.J.; Parkin, I.P. Aerosol assisted chemical vapor deposition of conductive and photocatalytically active tantalum doped titanium dioxide films. J. Mater. Chem. A 2014, 2, 12849. [Google Scholar] [CrossRef]
- Dasgupta, N.P.; Lee, H.-B.-R.; Bent, S.F.; Weiss, P.S. Recent advances in atomic layer deposition. Chem. Mater. 2016, 28, 1943–1947. [Google Scholar] [CrossRef]
- Hajibabaei, H.; Zandi, O.; Hamann, T.W. Tantalum nitride films integrated with transparent conductive oxide substrates via atomic layer deposition for photoelectrochemical water splitting. Chem. Sci. 2016, 7, 6760–6767. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Seo, S.; Park, H.; Shin, H. Atomic layer deposition of a SnO2 electron-transporting layer for planar perovskite solar cells with a power conversion efficiency of 18.3%. Chem. Commun. 2019, 55, 2433–2436. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Li, W.; Li, J.; Liang, X.; Wang, L. Enhancement of thermal stability for perovskite solar cells through cesium doping. RSC Adv. 2017, 7, 17473–17479. [Google Scholar] [CrossRef]
- Pfeiffer, K.; Schulz, U.; Tünnermann, A.; Szeghalmi, A. Antireflection coatings for strongly curved glass lenses by atomic layer deposition. Coatings 2017, 7, 118. [Google Scholar] [CrossRef]
- Zhao, C.; Hedhili, M.N.; Li, J.; Wang, Q.; Yang, Y.; Chen, L.; Li, L. Growth and characterization of titanium oxide by plasma enhanced atomic layer deposition. Thin Solid Films 2013, 542, 38–44. [Google Scholar] [CrossRef]
- Heil, S.B.S.; Roozeboom, F.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-assisted atomic layer deposition of Ta2O5 from alkylamide precursor and remote O2 plasma. J. Vac. Sci. Technol. A Vac. Surf. Film. 2008, 26, 472–480. [Google Scholar] [CrossRef][Green Version]
- Maeng, W.J.; Park, S.-J.; Kim, H. Atomic layer deposition of Ta-Based thin films: Reactions of alkylamide precursor with various reactants. J. Vac. Sci. Technol. B 2006, 24, 2276. [Google Scholar] [CrossRef]
- Chen, H.; Gu, H.; Xing, J.; Wang, Z.; Zhou, G.; Wang, S. Correlated evolution of dual-phase microstructures, mutual solubilities and oxygen vacancies in transparent La2-Lu Zr2O7 ceramics. J. Mater. 2021, 7, 185–194. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Deák, P.; Aradi, B.; Frauenheim, T. Band lineup and charge carrier separation in mixed rutile-anatase systems. J. Phys. Chem. C 2011, 115, 3443–3446. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, W.; Wu, P. Electronic Structure and optical properties of Ta-doped and (Ta, N)-codoped SrTiO3 from hybrid functional calculations. J. Appl. Phys. 2017, 121, 075102. [Google Scholar] [CrossRef]
- Rahaman, S.; Maikap, S.; Tien, T.-C.; Lee, H.-Y.; Chen, W.-S.; Chen, F.T.; Kao, M.-J.; Tsai, M.-J. Excellent resistive memory characteristics and switching mechanism using a Ti nanolayer at the Cu/TaOx interface. Nanoscale Res. Lett. 2012, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Park, H.J.; Kim, S.-I.; Thaiyan, M. Magnetic, structural and optical behavior of cupric oxide layers for solar cells. J. Alloy. Compd. 2016, 686, 616–627. [Google Scholar] [CrossRef]
- Kruse, N.; Chenakin, S. XPS Characterization of Au/TiO2 catalysts: Binding energy assessment and irradiation effects. Appl. Catal. A Gen. 2011, 391, 367–376. [Google Scholar] [CrossRef]
- Hannula, M.; Ali-Löytty, H.; Lahtonen, K.; Sarlin, E.; Saari, J.; Valden, M. Improved stability of atomic layer deposited amorphous TiO2 photoelectrode coatings by thermally induced oxygen defects. Chem. Mater. 2018, 30, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, M.; Tao, H.; Ke, W.; Chen, Z.; Wan, J.; Qin, P.; Xiong, L.; Lei, H.; Yu, H.; et al. Performance enhancement of perovskite solar cells with Mg-doped TiO2 compact film as the hole-blocking layer. Appl. Phys. Lett. 2015, 106, 121104. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Lv, L.; Wang, Y.; Hu, Y.; Deng, Z.; Lou, Z.; Hou, Y.; Teng, F. Ligand-free rutile and anatase TiO2 nanocrystals as electron extraction layers for high performance inverted polymer solar cells. RSC Adv. 2017, 7, 20084–20092. [Google Scholar] [CrossRef]
- Man, G.; Schwartz, J.; Sturm, J.C.; Kahn, A. Electronically passivated hole-blocking titanium dioxide/silicon heterojunction for hybrid silicon photovoltaics. Adv. Mater. Interfaces 2016, 3, 1600026. [Google Scholar] [CrossRef]
- Mazzolini, P.; Gondoni, P.; Russo, V.; Chrastina, D.; Casari, C.S.; Li Bassi, A. Tuning of electrical and optical properties of highly conducting and transparent Ta-doped TiO2 polycrystalline films. J. Phys. Chem. C 2015, 119, 6988–6997. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, L.; Li, P.; Li, X.; Qiu, Y. Numerical modeling of inverted perovskite solar cell based on CZTSSe hole transport layer for efficiency improvement. J. Photon. Energy 2019, 9, 1. [Google Scholar] [CrossRef]
- Anwar, F.; Mahbub, R.; Satter, S.S.; Ullah, S.M. Effect of different HTM layers and electrical parameters on ZnO nanorod-based lead-free perovskite solar cell for high-efficiency performance. Int. J. Photoenergy 2017, 2017, 9846310. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, L.; Li, P.; Xiong, H.; Kang, Z.; Fan, B.; Qiu, Y. Simulated development and optimized performance of CsPbI3 based all-inorganic perovskite solar cells. Sol. Energy 2020, 198, 454–460. [Google Scholar] [CrossRef]
- Liang, L.; Luo, H.; Hu, J.; Li, H.; Gao, P. Efficient perovskite solar cells by reducing interface-mediated recombination: A bulky amine approach. Adv. Energy Mater. 2020, 10, 2000197. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, S.-G.; Bae, S.-H.; Lee, D.-K.; Lin, O.; Yang, Y.; Park, N.-G. The interplay between trap density and hysteresis in planar heterojunction perovskite solar cells. Nano Lett. 2017, 17, 4270–4276. [Google Scholar] [CrossRef]
- Kim, J.K.; Chai, S.U.; Ji, Y.; Levy-Wendt, B.; Kim, S.H.; Yi, Y.; Heinz, T.F.; Nørskov, J.K.; Park, J.H.; Zheng, X. Resolving hysteresis in perovskite solar cells with rapid flame-processed cobalt-doped TiO2. Adv. Energy Mater. 2018, 8, 1801717. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).