Thermal Evaporation Synthesis of Vertically Aligned Zn2SnO4/ZnO Radial Heterostructured Nanowires Array
Abstract
1. Introduction
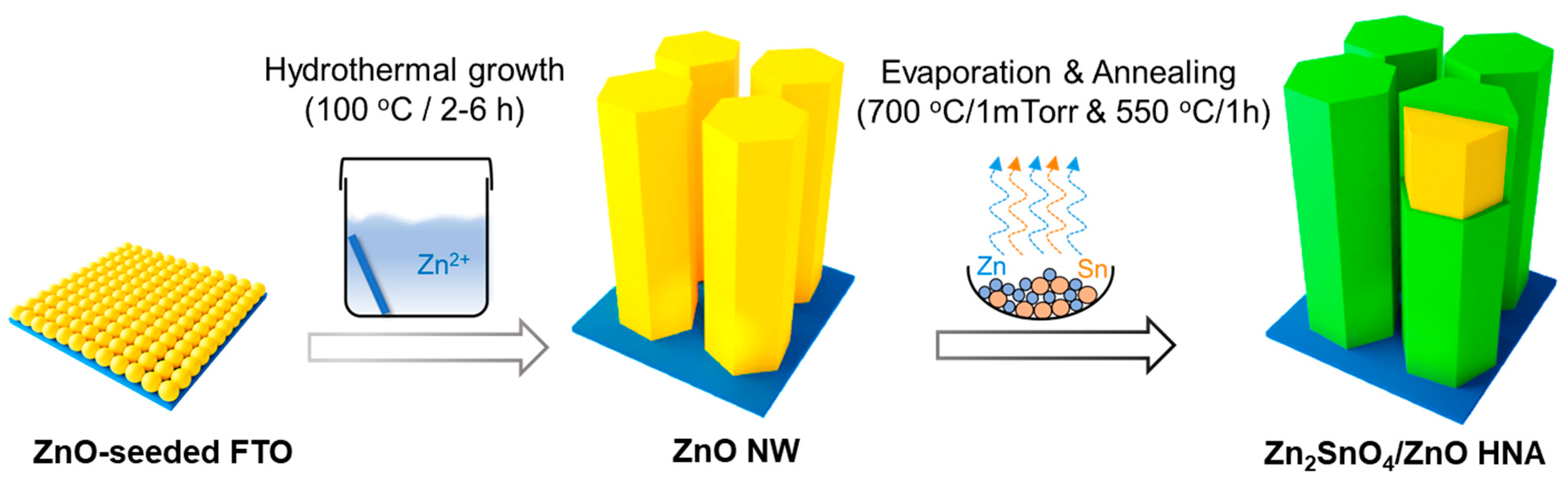
2. Experimental Section
2.1. Deposition of ZnO Seed Layer
2.2. Hydrothermal Growth of ZnO Nanowires Array
2.3. Synthesis of ZSO/ZnO Heterostructure Nanowires Array
2.4. Characterization and Measurement of Materials
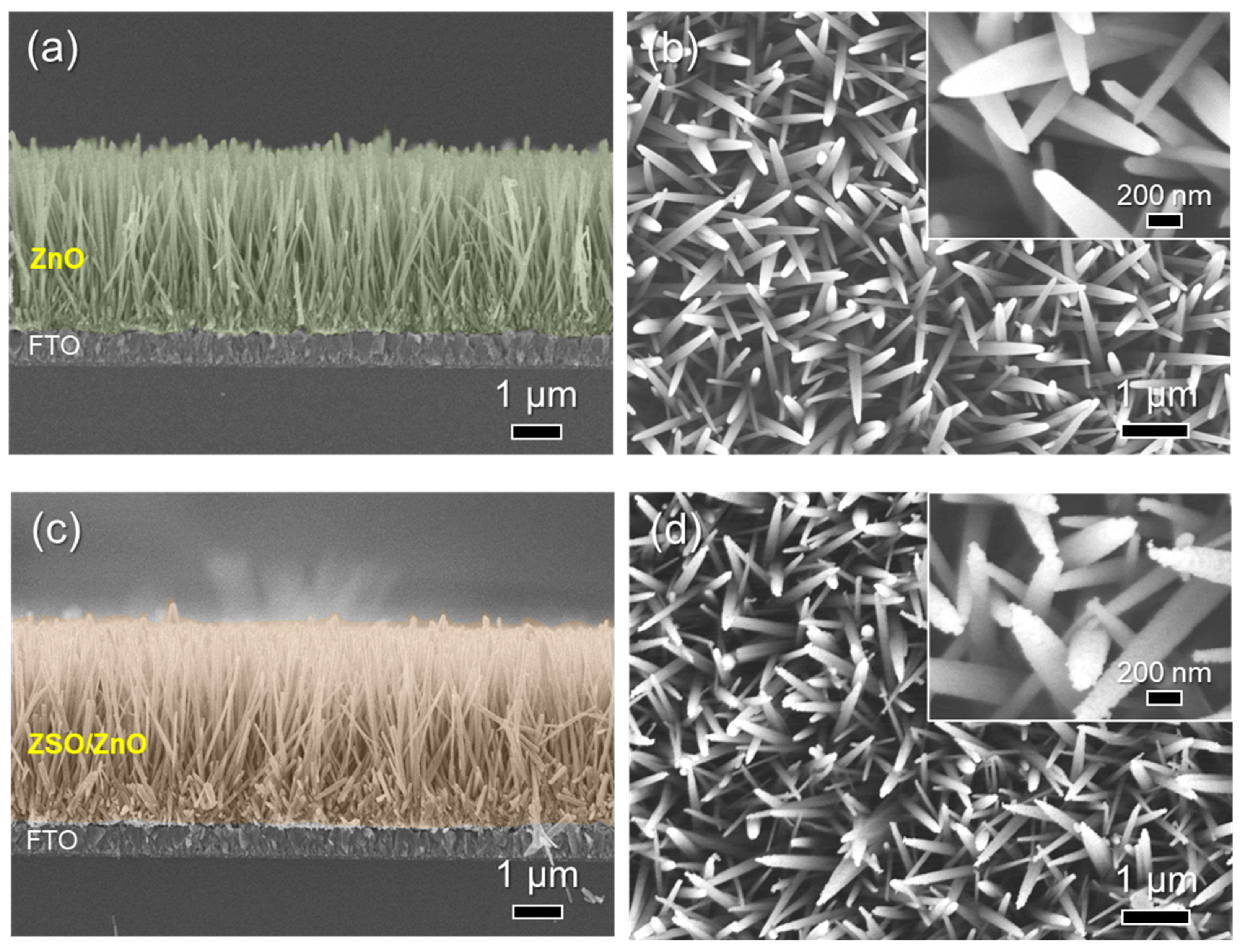
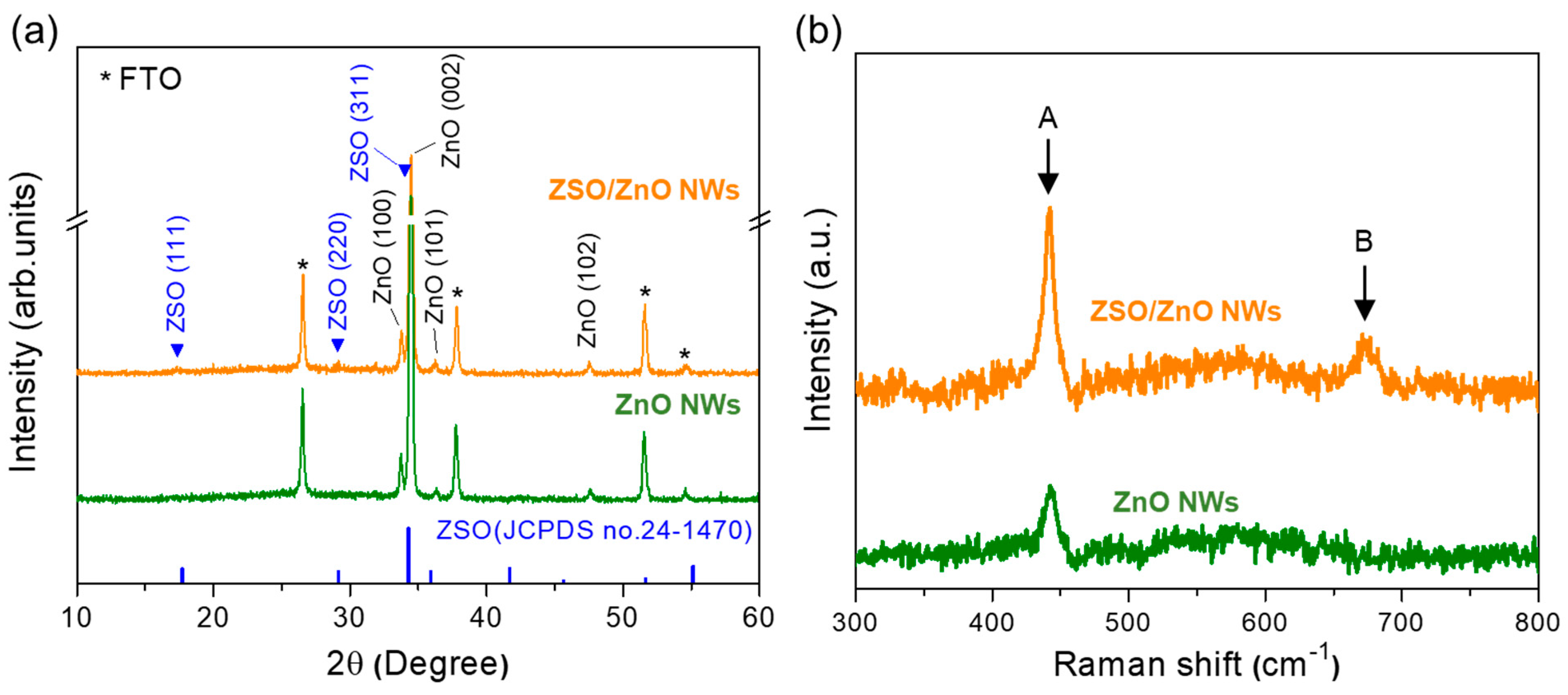
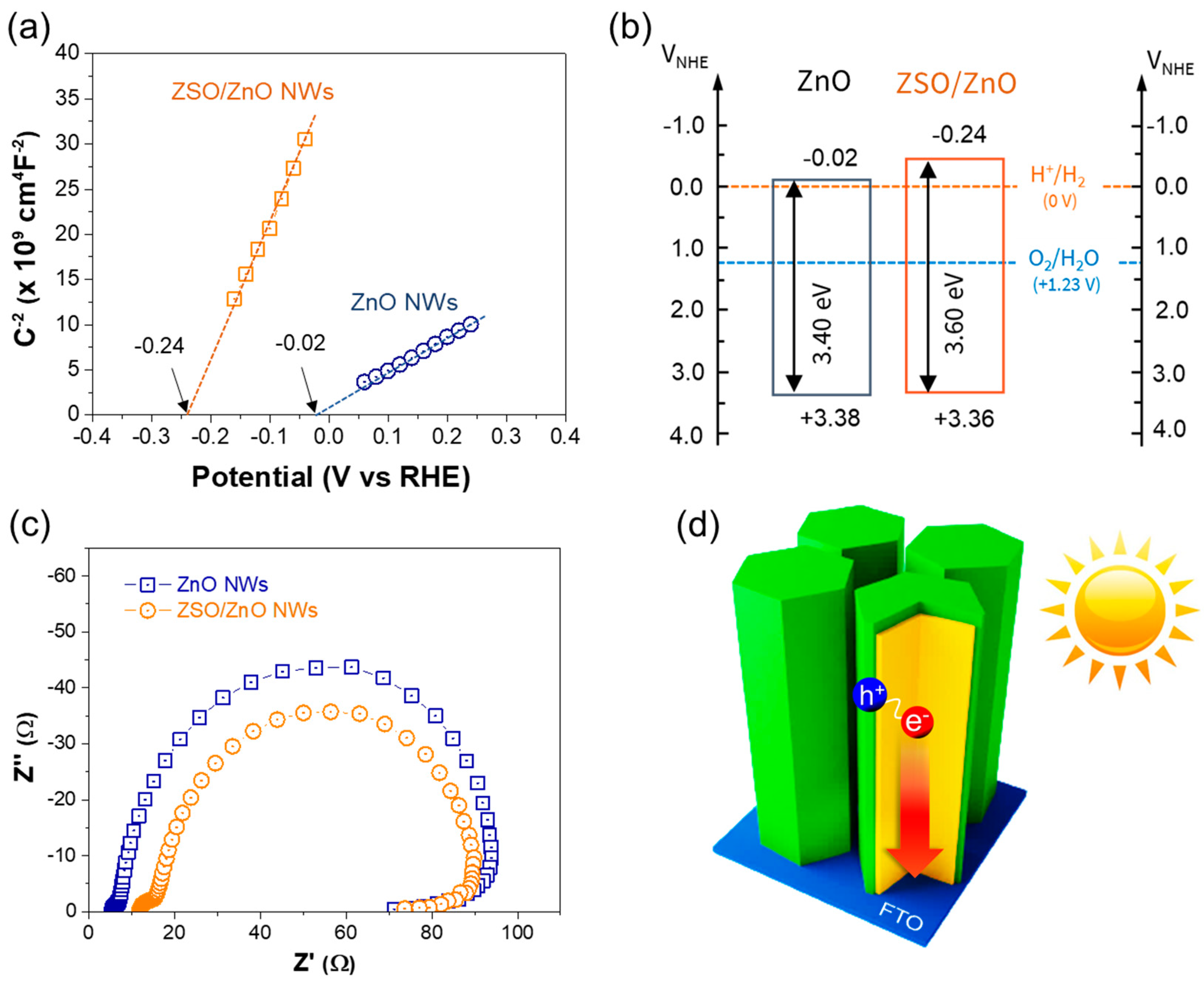
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xia, X.; Tu, J.; Zhang, Y.; Wang, X.; Gu, C.; Zhao, X.-B.; Fan, H.J. High-Quality Metal Oxide Core/Shell Nanowire Arrays on Conductive Substrates for Electrochemical Energy Storage. ACS Nano 2012, 6, 5531–5538. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Chen, P.-C.; Ryu, K.; Zhou, C. Devices and chemical sensing applications of metal oxide nanowires. J. Mater. Chem. 2008, 19, 828–839. [Google Scholar] [CrossRef]
- Yuhas, B.D.; Yang, P. Nanowire-Based All-Oxide Solar Cells. J. Am. Chem. Soc. 2009, 131, 3756–3761. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.; Xu, L.; Han, C.; Xu, X.; Luo, Y.; Zhao, S.; Zhao, Y. Electrospun Ultralong Hierarchical Vanadium Oxide Nanowires with High Performance for Lithium Ion Batteries. Nano Lett. 2010, 10, 4750–4755. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Fang, X.; Liao, M.; Xu, X.; Zeng, H.; Yoshio, B.; Golberg, D. A Comprehensive Review of One-Dimensional Metal-Oxide Nanostructure Photodetectors. Sensors 2009, 9, 6504–6529. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Su, Y.; Liu, D.; Yang, P.; Liu, B.; Liu, C. Nanowire Photoelectrochemistry. Chem. Rev. 2019, 119, 9221–9259. [Google Scholar] [CrossRef]
- Cho, I.S.; Chen, Z.; Forman, A.J.; Kim, D.R.; Rao, P.M.; Jaramillo, T.F.; Zheng, X.L. Branched TiO2Nanorods for Photoelectrochemical Hydrogen Production. Nano Lett. 2011, 11, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wolcott, A.; Wang, G.; Sobo, A.; Fitzmorris, R.C.; Qian, F.; Zhang, J.Z.; Li, Y. Nitrogen-Doped ZnO Nanowire Arrays for Photoelectrochemical Water Splitting. Nano Lett. 2009, 9, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ganesan, R.; Shen, H.; Mathur, S. Plasma-Modified SnO2 Nanowires for Enhanced Gas Sensing. J. Phys. Chem. C 2010, 114, 8245–8250. [Google Scholar] [CrossRef]
- Hyun, J.K.; Zhang, S.; Lauhon, L. Nanowire Heterostructures. Annu. Rev. Mater. Res. 2013, 43, 451–479. [Google Scholar] [CrossRef]
- Lauhon, L.J.; Gudiksen, M.S.; Lieber, C.M. Semiconductor nanowire heterostructures. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.M.; Cai, L.; Liu, C.; Cho, I.S.; Lee, C.H.; Weisse, J.M.; Yang, P.; Zheng, X. Simultaneously Efficient Light Absorption and Charge Separation in WO3/BiVO4Core/Shell Nanowire Photoanode for Photoelectrochemical Water Oxidation. Nano Lett. 2014, 14, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, J.; Zhang, T.; Wang, Y.; Liu, D.; Chen, H.; Jiang, W.; Liu, C.; Ahmad, W.; Chen, Z.D.; et al. Perovskite Solar Cells with ZnO Electron-Transporting Materials. Adv. Mater. 2018, 30, 1703737. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, K. Charge Transport and Recombination in Perovskite (CH3NH3)PbI3 Sensitized TiO2 Solar Cells. J. Phys. Chem. Lett. 2013, 4, 2880–2884. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, X.; You, J. SnO2: A Wonderful Electron Transport Layer for Perovskite Solar Cells. Small 2018, 14, e1801154. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Shen, D.; Li, Y.; Abate, A.; Wei, M. Highly Efficient Perovskite Solar Cells Based on a Zn2SnO4 Compact Layer. ACS Appl. Mater. Interfaces 2019, 11, 36553–36559. [Google Scholar] [CrossRef] [PubMed]
- Oh, L.S.; Kim, D.H.; Lee, J.A.; Shin, S.S.; Park, I.J.; Ko, M.J.; Park, N.-G.; Pyo, S.G.; Hong, K.S.; Kim, J.Y. Zn2SnO4-Based Photoelectrodes for Organolead Halide Perovskite Solar Cells. J. Phys. Chem. C 2014, 118, 22991–22994. [Google Scholar] [CrossRef]
- Shin, S.S.; Yeom, E.J.; Yang, W.S.; Hur, S.; Kim, M.G.; Im, J.; Seo, J.; Noh, J.H.; Seok, S.I. Colloidally prepared La-doped BaSnO3 electrodes for efficient, photostable perovskite solar cells. Science 2017, 356, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, Y.; Starr, M.B.; Yin, X.; Li, Z.; Kvit, A.; Wang, S.; Zhao, P.; Wang, X. Ferroelectric Polarization-Enhanced Photoelectrochemical Water Splitting in TiO2–BaTiO3 Core–Shell Nanowire Photoanodes. Nano Lett. 2015, 15, 7574–7580. [Google Scholar] [CrossRef]
- Alpuche-Aviles, M.A.; Wu, Y. Photoelectrochemical Study of the Band Structure of Zn2SnO4 Prepared by the Hydrothermal Method. J. Am. Chem. Soc. 2009, 131, 3216–3224. [Google Scholar] [CrossRef]
- Tharsika, T.; Haseeb, A.; Akbar, S.; Sabri, M.; Wong, Y.H. Gas sensing properties of zinc stannate (Zn2SnO4) nanowires prepared by carbon assisted thermal evaporation process. J. Alloy. Compd. 2015, 618, 455–462. [Google Scholar] [CrossRef]
- Pang, C.; Yan, B.; Liao, L.; Liu, B.; Zheng, Z.; Wu, T.; Sun, H.; Yu, T. Synthesis, characterization and opto-electrical properties of ternary Zn2SnO4 nanowires. Nanotechnology 2010, 21, 465706. [Google Scholar] [CrossRef]
- He, L.; Luan, C.; Wang, D.; Le, Y.; Feng, X.; Ma, J. Preparation and characterization of heteroepitaxial Zn2SnO4 single crystalline films prepared on MgO (100) substrates. J. Am. Ceram. Soc. 2020, 103, 2555–2561. [Google Scholar] [CrossRef]
- Rong, A.; Gao, X.P.; Li, G.R.; Yan, T.Y.; Zhu, H.Y.; Qu, J.Q.; Song, D.Y. Hydrothermal Synthesis of Zn2SnO4 as Anode Materials for Li-Ion Battery. J. Phys. Chem. B 2006, 110, 14754–14760. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, X.; Long, J.; Ding, Z.; Yan, T.; Zhang, G.; Zhang, Z.; Lin, H.; Fu, X. Hydrothermal synthesis, characterization, and photocatalytic properties of Zn2SnO4. J. Solid State Chem. 2009, 182, 517–524. [Google Scholar] [CrossRef]
- Chen, C.; Li, G.; Li, J.; Liu, Y. One-step synthesis of 3D flower-like Zn2SnO4 hierarchical nanostructures and their gas sensing properties. Ceram. Int. 2015, 41, 1857–1862. [Google Scholar] [CrossRef]
- Danwittayakul, S.; Jaisai, M.; Koottatep, T.; Dutta, J. Enhancement of Photocatalytic Degradation of Methyl Orange by Supported Zinc Oxide Nanorods/Zinc Stannate (ZnO/ZTO) on Porous Substrates. Ind. Eng. Chem. Res. 2013, 52, 13629–13636. [Google Scholar] [CrossRef]
- Bao, S.; Wu, J.; He, X.; Tu, Y.; Wang, S.; Huang, M.; Lan, Z. Mesoporous Zn2SnO4 as effective electron transport materials for high-performance perovskite solar cells. Electrochim. Acta 2017, 251, 307–315. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Liao, X.; Yang, W. A simple method to synthesize single-crystalline Zn2SnO4(ZTO) nanowires and their photoluminescence properties. Nanotechnology 2005, 16, 2928–2931. [Google Scholar] [CrossRef]
- Mali, S.S.; Shim, C.S.; Hong, C.K. Highly porous Zinc Stannate (Zn2SnO4) nanofibers scaffold photoelectrodes for efficient methyl ammonium halide perovskite solar cells. Sci. Rep. 2015, 5, 11424. [Google Scholar] [CrossRef] [PubMed]
- Bora, T.; Kyaw, H.H.; Dutta, J. Zinc oxide–zinc stannate core–shell nanorod arrays for CdS quantum dot sensitized solar cells. Electrochim. Acta 2012, 68, 141–145. [Google Scholar] [CrossRef]
- Siwatch, S.; Kundu, V.S.; Kumar, A.; Kumar, S.; Chauhan, N.; Kumari, M. Effect of novel ZnO/Zn2SnO4 photoanode on the performance of dye sensitized solar cell. Optik 2019, 194, 163117. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, L.; Li, G.; Shen, W. Fabrication and optical properties of large-scale uniform zinc oxide nanowire arrays by one-step electrochemical deposition technique. Chem. Phys. Lett. 2002, 363, 123–128. [Google Scholar] [CrossRef]
- Mereu, R.; Le Donne, A.; Trabattoni, S.; Acciarri, M.; Binetti, S. Comparative study on structural, morphological and optical properties of Zn2SnO4 thin films prepared by r.f. sputtering using Zn and Sn metal targets and ZnO–SnO2 ceramic target. J. Alloy. Compd. 2015, 626, 112–117. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Garcia-Belmonte, G.; Bisquert, J.; Bogdanoff, P.; Zaban, A. Mott-Schottky analysis of nanoporous semiconductor electrodes in dielectric state deposited on SnO2 (F) conducting substrates. J. Electrochem. Soc. 2003, 150, E293. [Google Scholar] [CrossRef]
- Rana, A.U.H.S.; Shaikh, S.F.; Al-Enizi, A.M.; Agyeman, D.A.; Ghani, F.; Nah, I.W.; Shahid, A. Intrinsic Control in Defects Density for Improved ZnO Nanorod-Based UV Sensor Performance. Nanomater 2020, 10, 142. [Google Scholar] [CrossRef]
- Cheng, B.; Ouyang, Z.; Chen, C.; Xiao, Y.; Lei, S. Individual Zn2SnO4-sheathed ZnO heterostructure nanowires for efficient resistive switching memory controlled by interface states. Sci. Rep. 2013, 3, 3249. [Google Scholar] [CrossRef] [PubMed]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2019, 3, 66–98. [Google Scholar] [CrossRef]
- Hwang, S.W.; Kim, J.U.; Baek, J.H.; Kalanur, S.S.; Jung, H.S.; Seo, H.; Cho, I.S. Solution-processed TiO2/BiVO4/SnO2 triple-layer photoanode with enhanced photoelectrochemical performance. J. Alloy. Compd. 2019, 785, 1245–1252. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, G.; Kang, M.; Jeong, Y.; Lee, S.; Cho, I. Thermal Evaporation Synthesis of Vertically Aligned Zn2SnO4/ZnO Radial Heterostructured Nanowires Array. Nanomaterials 2021, 11, 1500. https://doi.org/10.3390/nano11061500
Han G, Kang M, Jeong Y, Lee S, Cho I. Thermal Evaporation Synthesis of Vertically Aligned Zn2SnO4/ZnO Radial Heterostructured Nanowires Array. Nanomaterials. 2021; 11(6):1500. https://doi.org/10.3390/nano11061500
Chicago/Turabian StyleHan, Gillsang, Minje Kang, Yoojae Jeong, Sangwook Lee, and Insun Cho. 2021. "Thermal Evaporation Synthesis of Vertically Aligned Zn2SnO4/ZnO Radial Heterostructured Nanowires Array" Nanomaterials 11, no. 6: 1500. https://doi.org/10.3390/nano11061500
APA StyleHan, G., Kang, M., Jeong, Y., Lee, S., & Cho, I. (2021). Thermal Evaporation Synthesis of Vertically Aligned Zn2SnO4/ZnO Radial Heterostructured Nanowires Array. Nanomaterials, 11(6), 1500. https://doi.org/10.3390/nano11061500







