PVA Films with Mixed Silver Nanoparticles and Gold Nanostars for Intrinsic and Photothermal Antibacterial Action
Abstract
1. Introduction
2. Materials and Methods
2.1. TEM Imaging
2.2. SEM Imaging
2.3. Thermograms
2.4. Ag and Au Release
2.5. Mechanical Properties
2.6. Syntheses
2.6.1. AgNP
2.6.2. GNS
2.6.3. PVA Films
2.7. Microbiological Experiments
2.7.1. Antimicrobial Activity on Planktonic cells
2.7.2. Photothermal Antimicrobial Activity
2.8. Cytotoxicity Tests
3. Results and Discussion
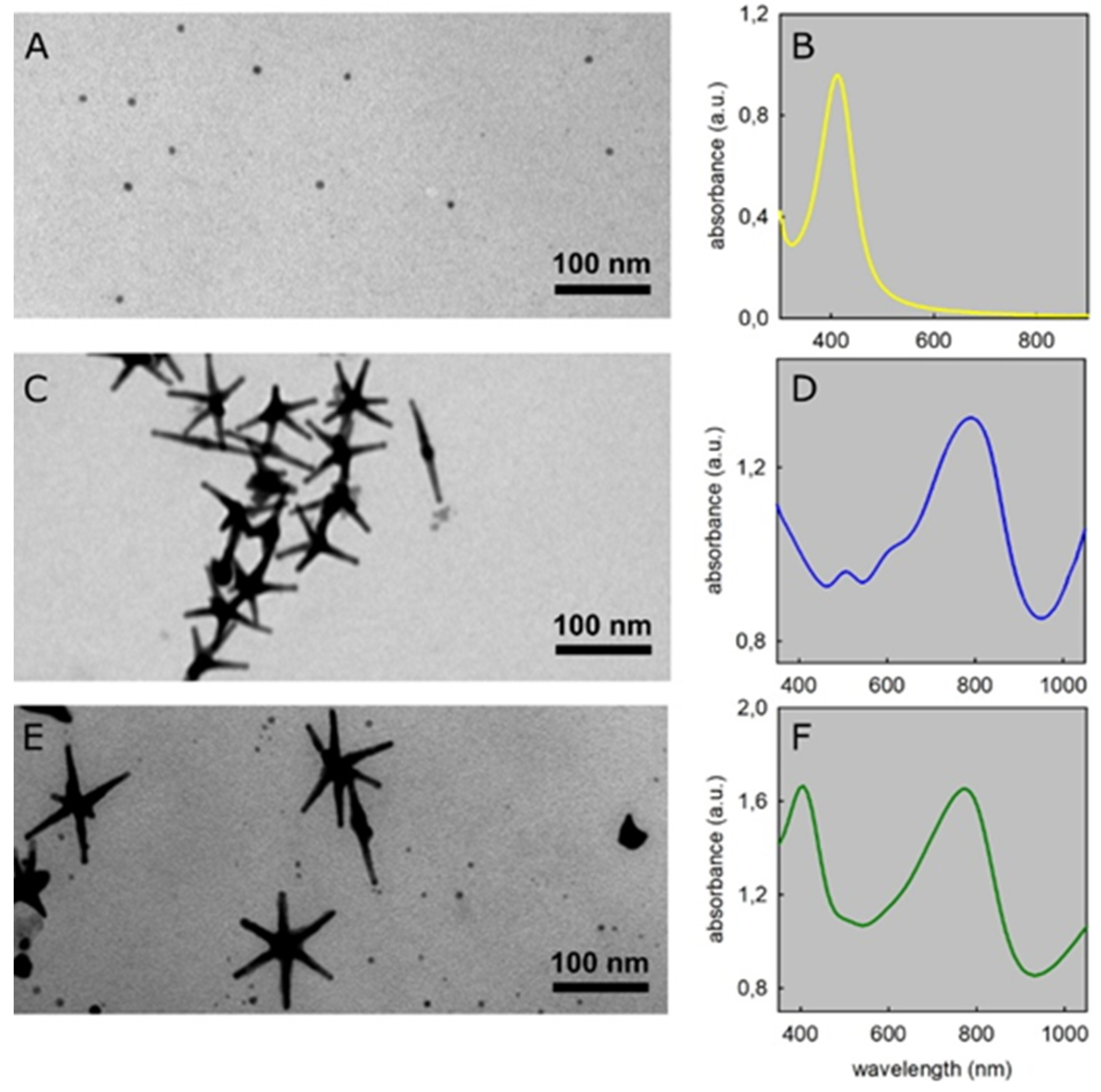
3.1. AgNP and GNS
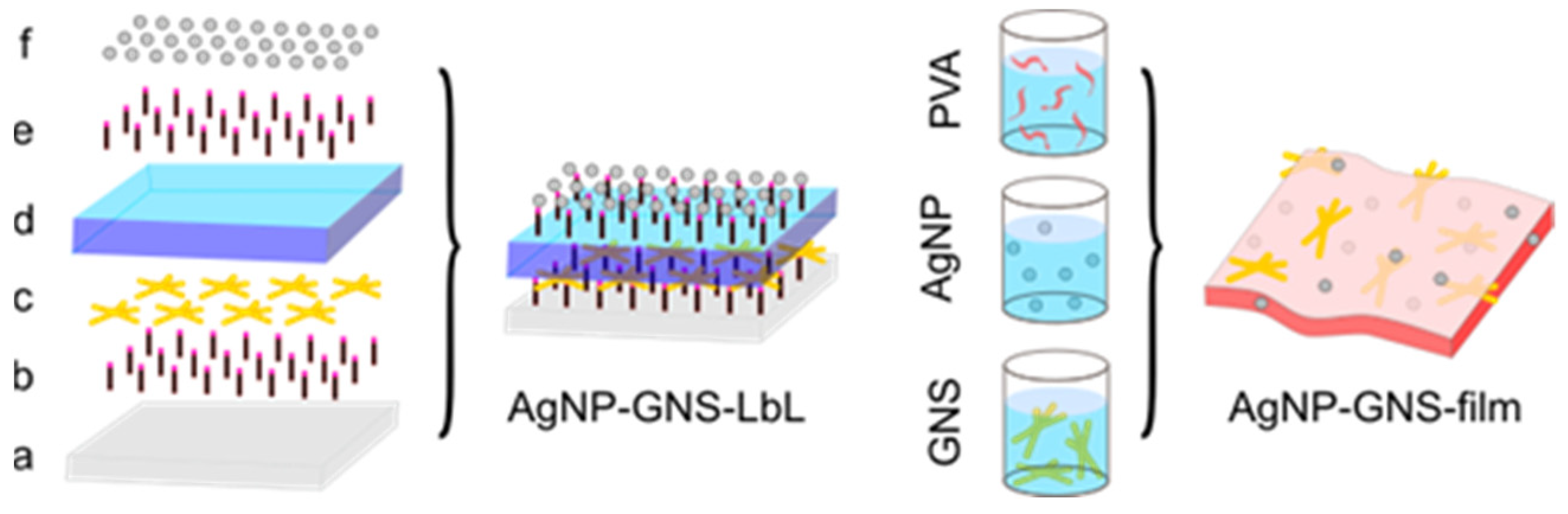
3.2. AgNP + GNS Mixtures and Films Preparation
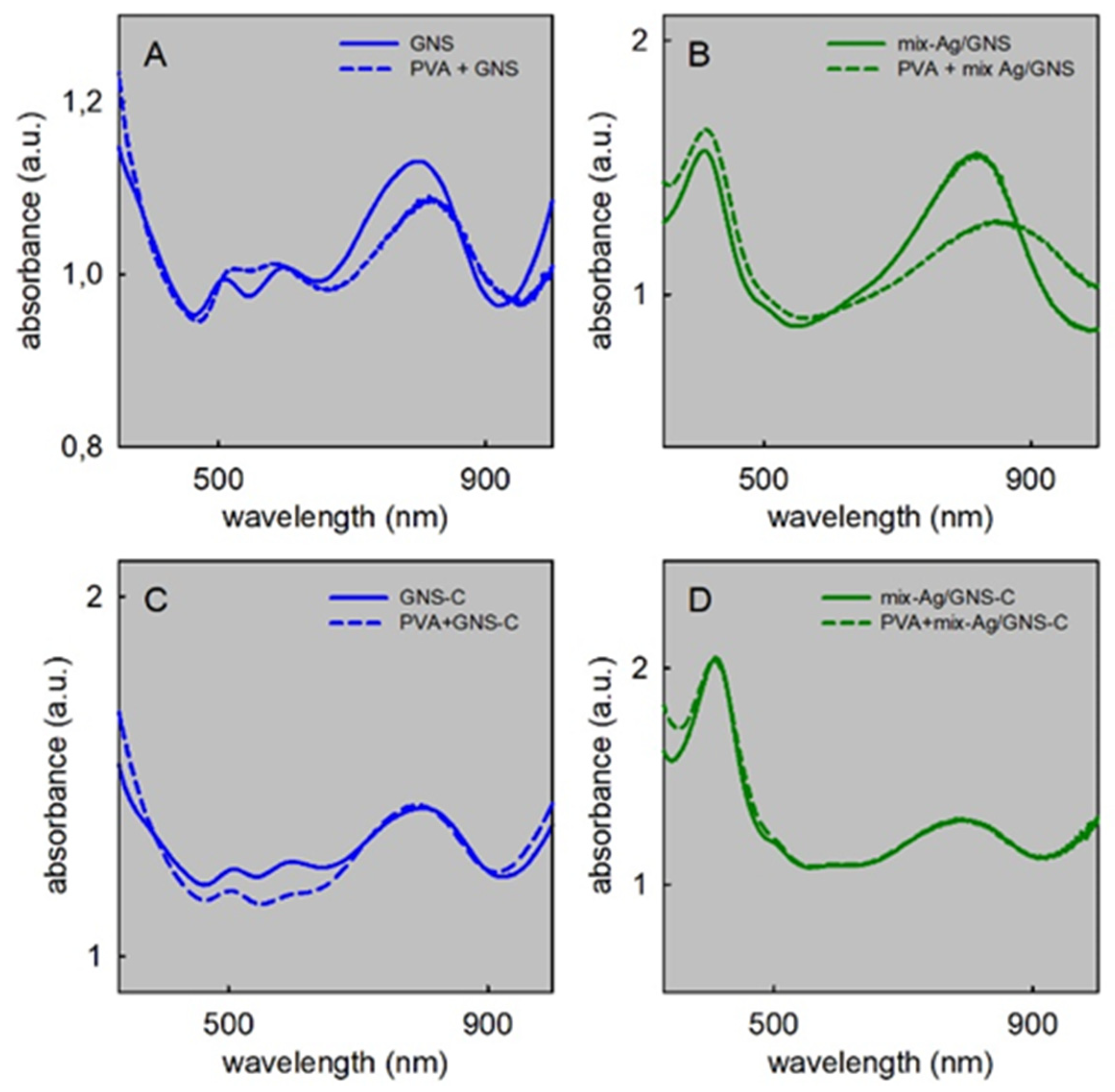
3.3. Effect on AgNP and GNS of PVA Addition and Film Formation
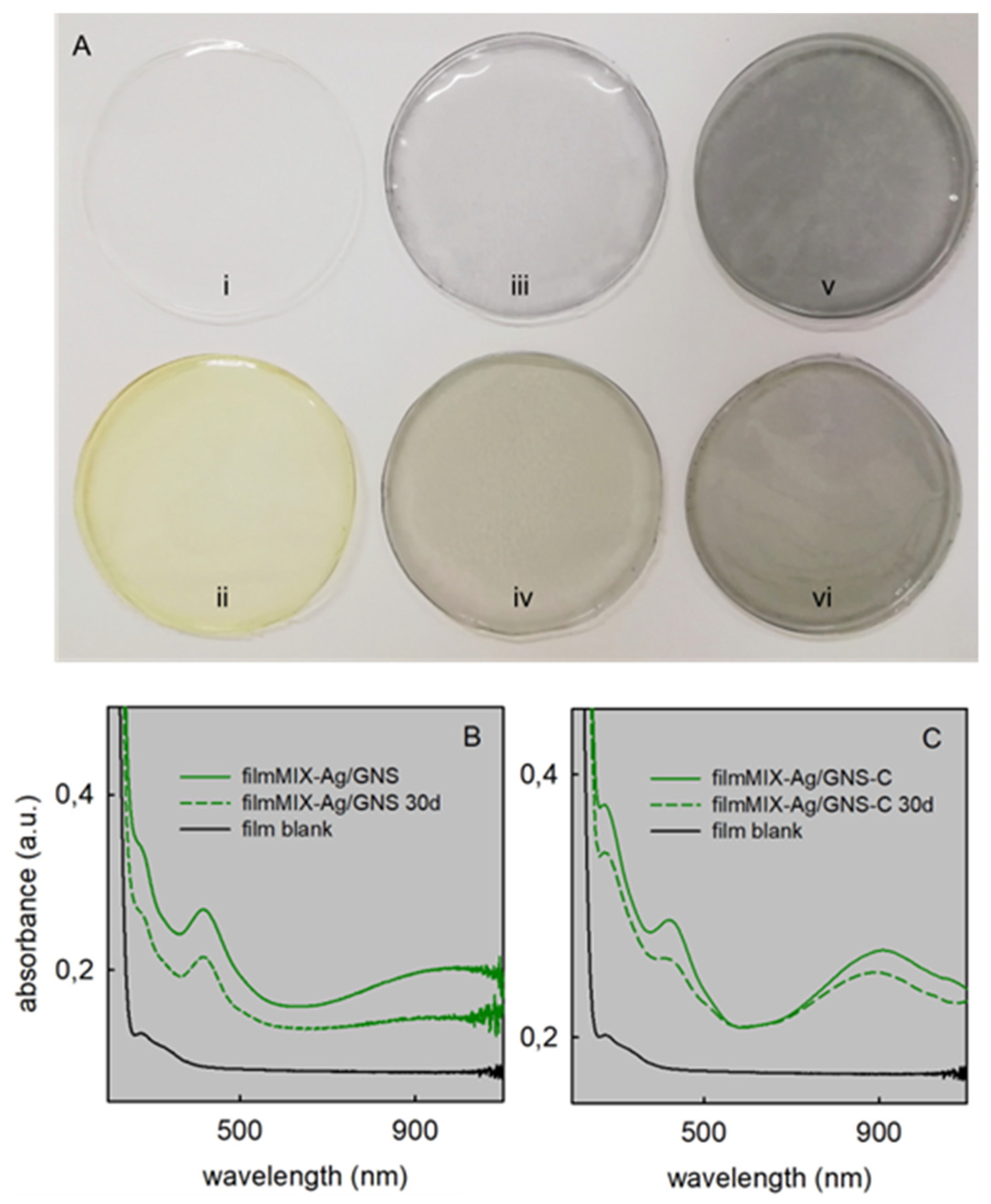
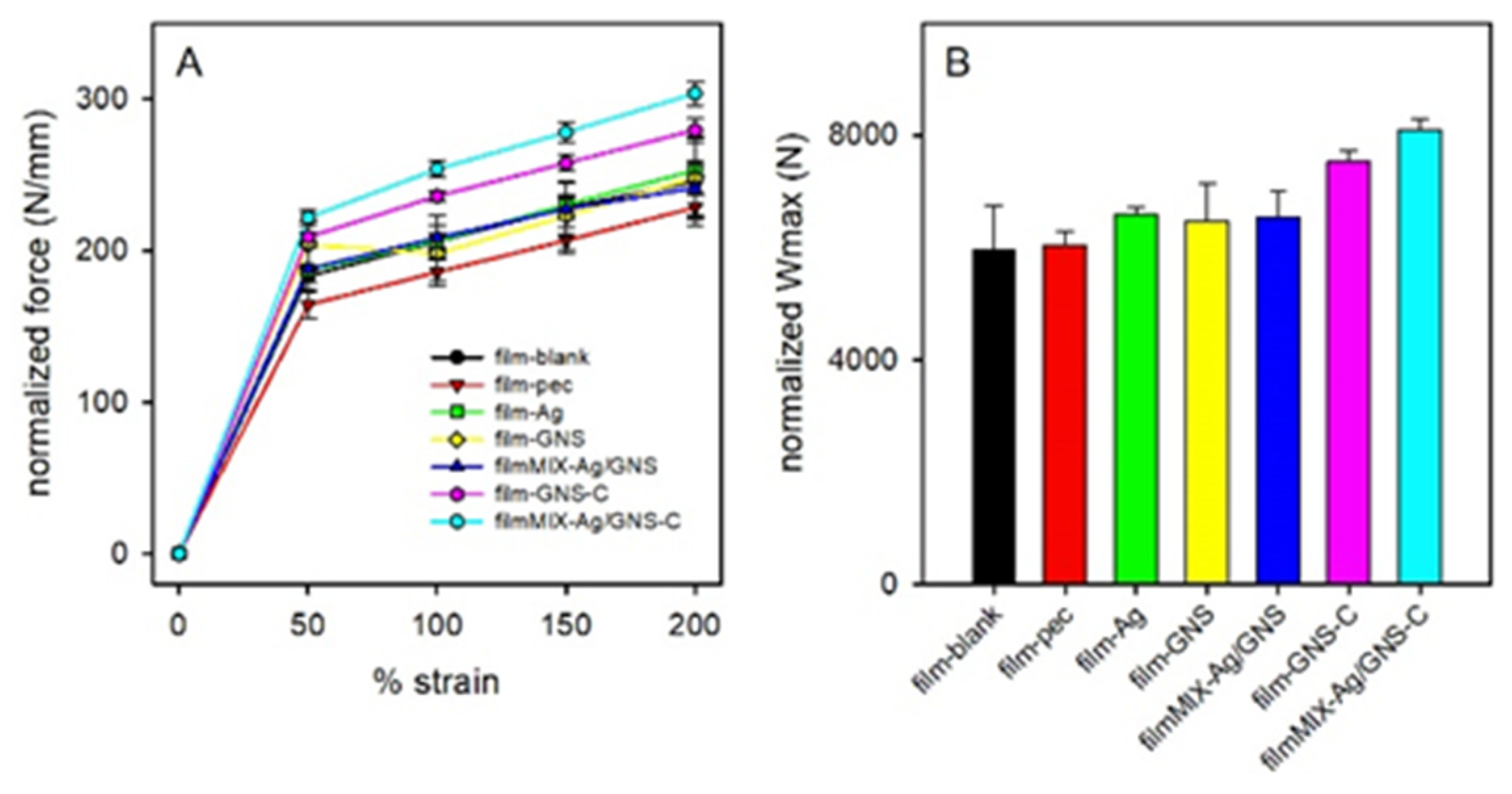
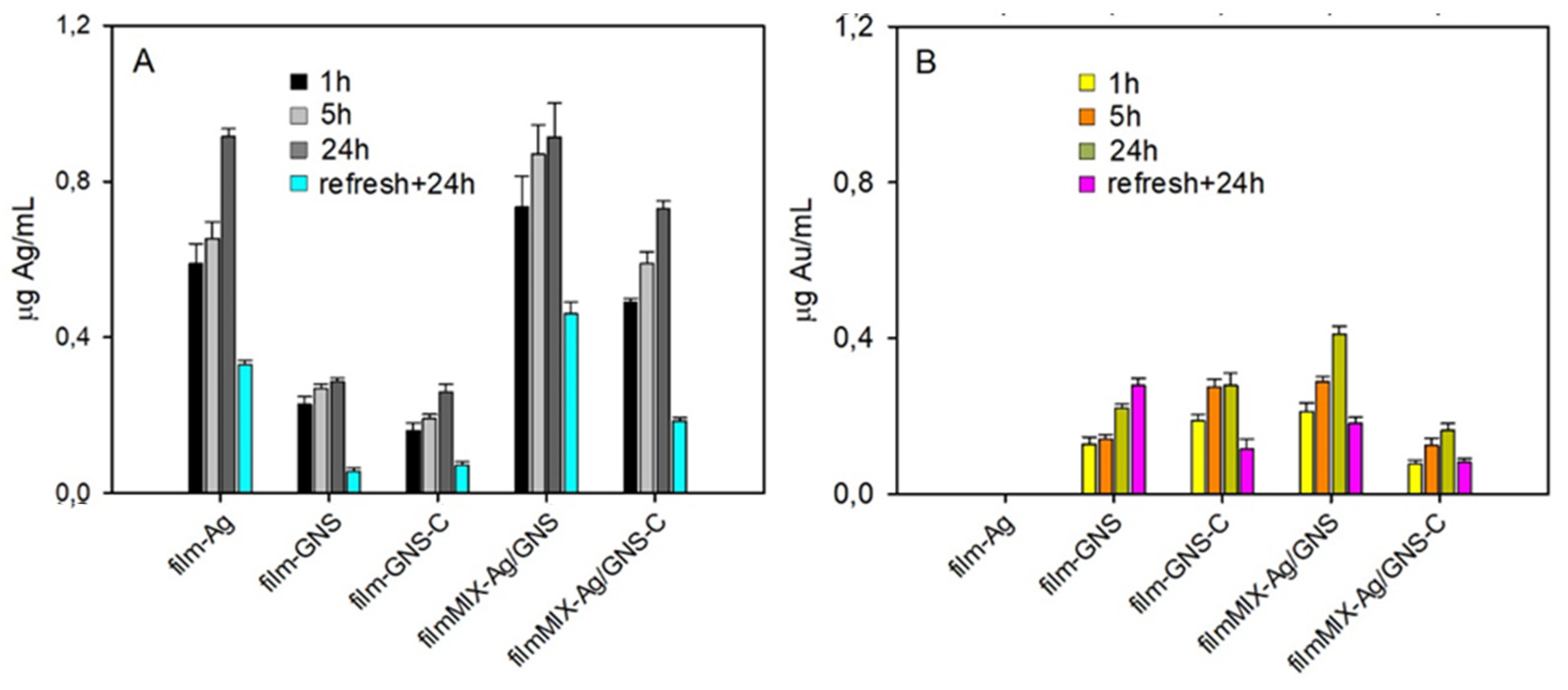
3.4. Films Characterization
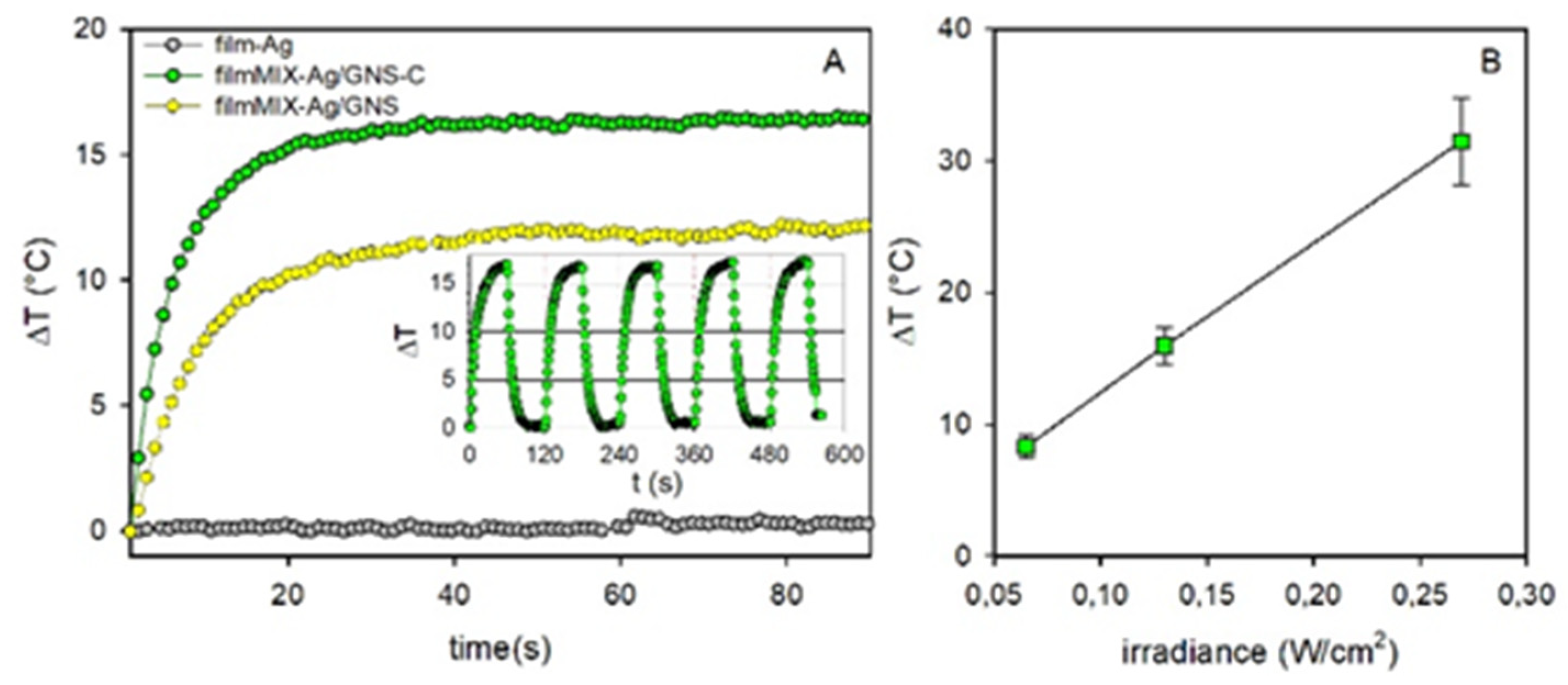
3.5. Photothermal Effect
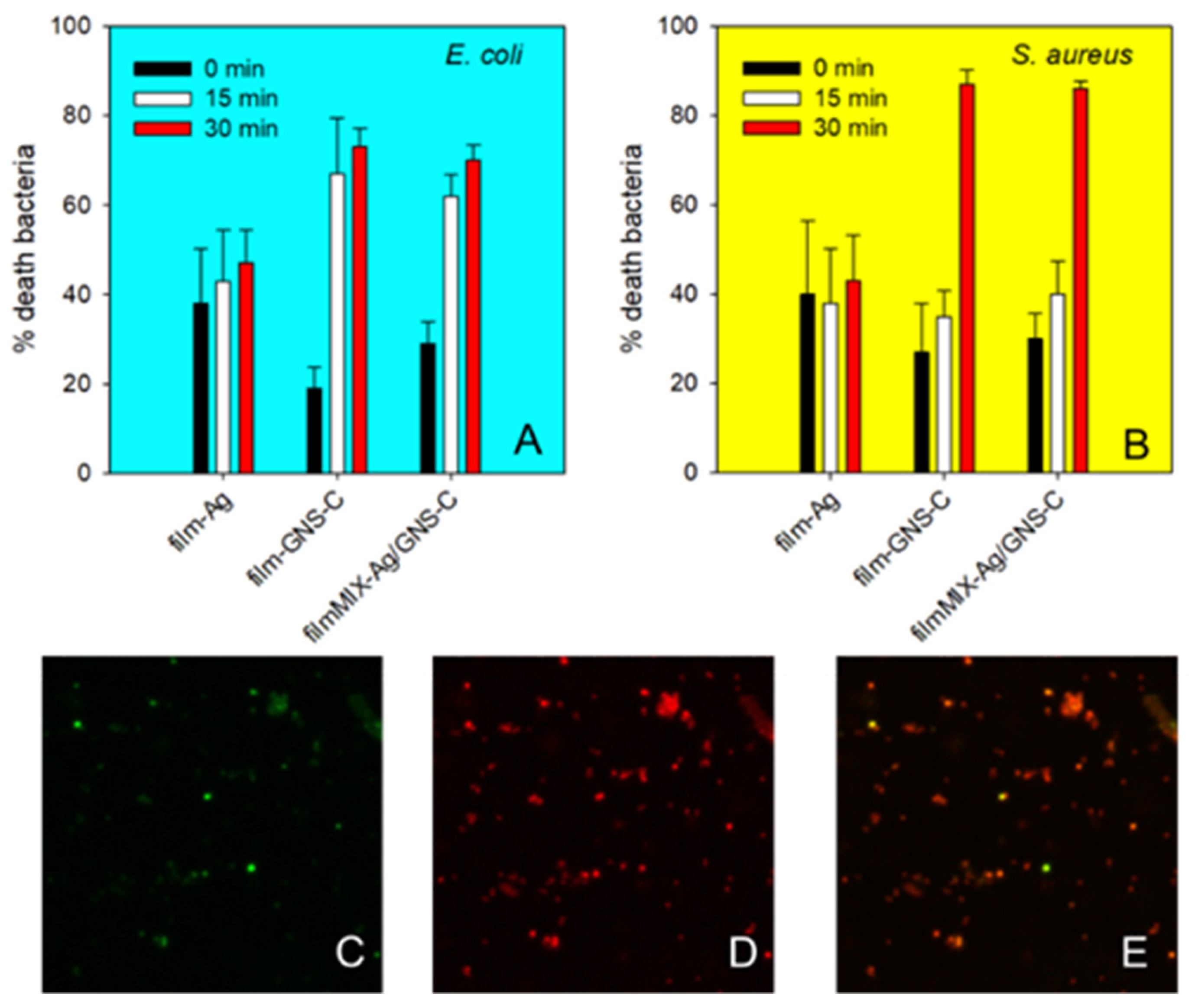
3.6. Antibacterial and Biocompatibility Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- 14969 Results in a Search on Web of Science for “Silver Nanoparticles” and “Antibacterial”. Available online: https://apps.webofknowledge.com/Search.do?product=WOS&SID=C1pxSv3oyYkVWtEzrhJ&search_mode=GeneralSearch&prID=83eb815b-a5a1-4bd9-9903-8212cbd1ba45 (accessed on 22 April 2021).
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Cook, J.; Griesser, H.J. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 2009, 6, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Dacarro, G.; Taglietti, A. Self-Assembled Monolayers of Silver Nanoparticles: From Intrinsic to Switchable Inorganic Antibacterial Surfaces. Eur. J. Inorg. Chem. 2018, 2018, 4846–4855. [Google Scholar] [CrossRef]
- D’Agostino, A.; Taglietti, A.; Desando, R.; Bini, M.; Patrini, M.; Dacarro, G.; Cucca, L.; Pallavicini, P.; Grisoli, P. Bulk surfaces coated with triangular silver nanoplates: Antibacterial action based on silver release and photo-thermal effect. Nanomaterials 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Dacarro, G.; Cucca, L.; Grisoli, P.; Pallavicini, P.; Patrini, M.; Taglietti, A. Monolayers of polyethilenimine on flat glass: A versatile platform for cations coordination and nanoparticles grafting in the preparation of antibacterial surfaces. Dalton Trans. 2012, 41, 2456. [Google Scholar] [CrossRef]
- Salomé Veiga, A.; Schneider, J.P. Antimicrobial hydrogels for the treatment of infection. Biopolymers 2013, 100, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- de Azeredo, H.M.C. Antimicrobial nanostructures in food packaging. Trends Food Sci. Technol. 2013, 30, 56–69. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Lau, W.J.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Paul Chen, J.; Ismail, A.F. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Rajendran, N.K.; Houreld, N.N.; Abrahamse, H. Recent advances on silver nanoparticle and biopolymer-based biomaterials for wound healing applications. Int. J. Biol. Macromol. 2018, 115, 165–175. [Google Scholar] [CrossRef]
- Laser Institute of America. American National Standard for Safe Use of Lasers; Laser Institute of America: Orlando, FL, USA, 2007. [Google Scholar]
- Yuan, H.; Khoury, C.G.; Wilson, C.M.; Grant, G.A.; Bennett, A.J.; Vo-Dinh, T. In vivo particle tracking and photothermal ablation using plasmon-resonant gold nanostars. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1355–1363. [Google Scholar] [CrossRef]
- Cennamo, N.; Donà, A.; Pallavicini, P.; D’Agostino, G.; Dacarro, G.; Zeni, L.; Pesavento, M.; D’Agostino, G.; Dacarro, G.; Zeni, L.; et al. Sensitive detection of 2, 4, 6-trinitrotoluene by tridimensional monitoring of molecularly imprinted polymer with optical fiber and five-branched gold nanostars. Sens. Actuators B Chem. 2015, 208, 291–298. [Google Scholar] [CrossRef]
- Sironi, L.; Freddi, S.; Caccia, M.; Pozzi, P.; Rossetti, L.; Pallavicini, P.; Donà, A.; Cabrini, E.; Gualtieri, M.; Rivolta, I.; et al. Gold Branched Nanoparticles for Cellular Treatments. J. Phys. Chem. C 2012, 116, 18407–18418. [Google Scholar] [CrossRef]
- Montalti, M.; Prodi, L.; Rampazzo, E.; Zaccheroni, N. Dye-doped silica nanoparticles as luminescent organized systems for nanomedicine. Chem. Soc. Rev. 2014, 43, 4243–4268. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Alkilany, A.M.; Khalil, E.A.; Al-Bakri, A.G. Nano-Photothermal ablation effect of Hydrophilic and Hydrophobic Functionalized Gold Nanorods on Staphylococcus aureus and Propionibacterium acnes. Sci. Rep. 2018, 8, 6881. [Google Scholar] [CrossRef]
- Bermúdez-Jiménez, C.; Romney, M.G.; Roa-Flores, S.A.; Martínez-Castañón, G.; Bach, H. Hydrogel-embedded gold nanorods activated by plasmonic photothermy with potent antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2019, 22, 102093. [Google Scholar] [CrossRef]
- Pallavicini, P.; Dona, A.; Taglietti, A.; Minzioni, P.; Patrini, M.; Dacarro, G.; Chirico, G.; Sironi, L.; Bloise, N.; Visaie, L.; et al. Self-assembled monolayers of gold nanostars: A convenient tool for near-IR photothermal biofilm eradication. Chem. Commun. 2014, 50, 1969–1971. [Google Scholar] [CrossRef]
- Borzenkov, M.; Moros, M.; Tortiglione, C.; Bertoldi, S.; Contessi, N.; Faré, S.; Taglietti, A.; D’Agostino, A.; Pallavicini, P.; Collini, M.; et al. Fabrication of photothermally active poly(vinyl alcohol) films with gold nanostars for antibacterial applications. Beilstein J. Nanotechnol. 2018, 9, 2040–2048. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion Release Kinetics and Particle Persistence in Aqueous Nano-Silver Colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Preti, L.; De Vita, L.; Dacarro, G.; Diaz Fernandez, Y.A.; Merli, D.; Rossi, S.; Taglietti, A.; Vigani, B. Fast dissolution of silver nanoparticles at physiological pH. J. Colloid Interface Sci. 2020, 563, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Bright, R.M.; Musick, M.D.; Natan, M.J. Preparation and characterization of Ag colloid monolayers. Langmuir 1998, 14, 5695. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Gentry, S.T.; Kendra, S.F.; Bezpalko, M.W. Ostwald Ripening in Metallic Nanoparticles: Stochastic Kinetics. J. Phys. Chem. C 2011, 115, 12736–12741. [Google Scholar] [CrossRef]
- Zhang, Q.; Jing, H.; Li, G.G.; Lin, Y.; Blom, D.A.; Wang, H. Intertwining Roles of Silver Ions, Surfactants, and Reducing Agents in Gold Nanorod Overgrowth: Pathway Switch between Silver Underpotential Deposition and Gold-Silver Codeposition. Chem. Mater. 2016, 28, 2728–2741. [Google Scholar] [CrossRef]
- Attia, Y.A.; Buceta, D.; Requejo, F.G.; Giovanetti, L.J.; López-Quintela, M.A. Photostability of gold nanoparticles with different shapes: The role of Ag clusters. Nanoscale 2015, 7, 11273–11279. [Google Scholar] [CrossRef]
- Attia, Y.A.; Buceta, D.; Blanco-Varela, C.; Mohamed, M.B.; Barone, G.; López-Quintela, M.A. Structure-Directing and High-Efficiency Photocatalytic Hydrogen Production by Ag Clusters. J. Am. Chem. Soc. 2014, 136, 1182–1185. [Google Scholar] [CrossRef]
- Pallavicini, P.; Bassi, B.; Chirico, G.; Collini, M.; Dacarro, G.; Fratini, E.; Grisoli, P.; Patrini, M.; Sironi, L.; Taglietti, A.; et al. Modular approach for bimodal antibacterial surfaces combining photo-switchable activity and sustained biocidal release. Sci. Rep. 2017, 7, 5259. [Google Scholar] [CrossRef]
- Pallavicini, P.; Arciola, C.R.; Bertoglio, F.; Curtosi, S.; Dacarro, G.; D’Agostino, A.; Ferrari, F.; Merli, D.; Milanese, C.; Rossi, S.; et al. Silver nanoparticles synthesized and coated with pectin: An ideal compromise for anti-bacterial and anti-biofilm action combined with wound-healing properties. J. Colloid Interface Sci. 2017, 498, 271–281. [Google Scholar] [CrossRef]
- Bertoglio, F.; de Vita, L.; D’Agostino, A.; Fernandez, Y.D.; Falqui, A.; Casu, A.; Merli, D.; Milanese, C.; Rossi, S.; Taglietti, A.; et al. Increased antibacterial and antibiofilm properties of silver nanoparticles using silver fluoride as precursor. Molecules 2020, 25, 3494. [Google Scholar] [CrossRef]
- Hajji, S.; Chaker, A.; Jridi, M.; Maalej, H.; Jellouli, K.; Boufi, S.; Nasri, M. Structural analysis, and antioxidant and antibacterial properties of chitosan-poly (vinyl alcohol) biodegradable films. Environ. Sci. Pollut. Res. 2016, 23, 15310–15320. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Preparation and physicochemical evaluation of chitosan/poly(vinyl alcohol)/pectin ternary film for food-packaging applications. Carbohydr. Polym. 2010, 79, 711–716. [Google Scholar] [CrossRef]
- Rossi, S.; Mori, M.; Vigani, B.; Bonferoni, M.C.; Sandri, G.; Riva, F.; Caramella, C.; Ferrari, F. A novel dressing for the combined delivery of platelet lysate and vancomycin hydrochloride to chronic skin ulcers: Hyaluronic acid particles in alginate matrices. Eur. J. Pharm. Sci. 2018, 118, 87–95. [Google Scholar] [CrossRef]
- Pallavicini, P.; Basile, S.; Chirico, G.; Dacarro, G.; D’Alfonso, L.; Donà, A.; Patrini, M.; Falqui, A.; Sironi, L.; Taglietti, A. Monolayers of gold nanostars with two near-IR LSPRs capable of additive photothermal response. Chem. Commun. 2015, 51, 12928–12930. [Google Scholar] [CrossRef]
- Fraise, A.P.; Lambert, P.A.; Maillard, J.-Y. (Eds.) Russell, Hugo and Ayliffe’s Principles and Practice of Disinfection, Preservation & Sterilization; Blackwell: Oxford, UK, 2004; ISBN 1405101997. [Google Scholar]
- Borzenkov, M.; D’Alfonso, L.; Polissi, A.; Sperandeo, P.; Collini, M.; Dacarro, G.; Taglietti, A.; Chirico, G.; Pallavicini, P.; D’Alfonso, L.; et al. Novel photo-thermally active polyvinyl alcohol-Prussian blue nanoparticles hydrogel films capable of eradicating bacteria and mitigating biofilms. Nanotechnology 2019, 30, 295702. [Google Scholar] [CrossRef]
- Evanoff, D.D.; Chumanov, G. Synthesis and optical properties of silver nanoparticles and arrays. ChemPhysChem 2005, 6, 1221–1231. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, J.; Li, J.-J.; Li, X.; Zhao, J.-W. Improve the Plasmonic Spectral Detection of Alpha-Fetoprotein: The Effect of Branch Length on the Coagulation of Gold Nanostars. Plasmonics 2016, 11, 1175–1182. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, M.-J.; Li, J.-J.; Li, X.; Zhao, J.-W. Multi-branched gold nanostars with fractal structure for SERS detection of the pesticide thiram. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 586–593. [Google Scholar] [CrossRef]
- Dacarro, G.; Pallavicini, P.; Bertani, S.M.; Chirico, G.; D’Alfonso, L.; Falqui, A.; Marchesi, N.; Pascale, A.; Sironi, L.; Taglietti, A.; et al. Synthesis of reduced-size gold nanostars and internalization in SH-SY5Y cells. J. Colloid Interface Sci. 2017, 505, 1055–1064. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Musetti, A.; Paderni, K.; Fabbri, P.; Pulvirenti, A.; Al-Moghazy, M.; Fava, P.; Al-Moghazy, M.; Fava, P.; Al-Moghazy, M.; Fava, P. Poly (vinyl alcohol)-based film potentially suitable for antimicrobial packaging applications. J. Food Sci. 2014, 79, E577–E582. [Google Scholar] [CrossRef] [PubMed]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Pallavicini, P.; Bernhard, C.; Chirico, G.; Dacarro, G.; Denat, F.; Donà, A.; Milanese, C.; Taglietti, A. Gold nanostars co-coated with the Cu (II) complex of a tetraazamacrocyclic ligand. Dalton Trans. 2015, 44, 5652–5661. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; De Vita, L.; Merlin, F.; Milanese, C.; Borzenkov, M.; Taglietti, A.; Chirico, G. Suitable polymeric coatings to avoid localized surface plasmon resonance hybridization in printed patterns of photothermally responsive gold nanoinks. Molecules 2020, 25, 2499. [Google Scholar] [CrossRef]
- Schnepf, M.J.; Mayer, M.; Kuttner, C.; Tebbe, M.; Wolf, D.; Dulle, M.; Altantzis, T.; Formanek, P.; Förster, S.; Bals, S.; et al. Nanorattles with tailored electric field enhancement. Nanoscale 2017, 9, 9376–9385. [Google Scholar] [CrossRef]
- Halas, N.J.; Lal, S.; Chang, W.-S.; Link, S.; Nordlander, P. Plasmons in strongly coupled metallic nanostructures. Chem. Rev. 2011, 111, 3913–3961. [Google Scholar] [CrossRef]
- Liu, S.-D.D.; Cheng, M.-T.T. Linear plasmon ruler with tunable measurement range and sensitivity. J. Appl. Phys. 2010, 108, 34313. [Google Scholar] [CrossRef]
- Pallavicini, P.; Taglietti, A.; Dacarro, G.; Diaz-Fernandez, Y.A.; Galli, M.; Grisoli, P.; Patrini, M.; Santucci De Magistris, G.; Zanoni, R. Self-assembled monolayers of silver nanoparticles firmly grafted on glass surfaces: Low Ag+ release for an efficient antibacterial activity. J. Colloid Interface Sci. 2010, 350, 110–116. [Google Scholar] [CrossRef]
- Christian, S.D.; Smith, G.A.; Tucker, E.E.; Scamehorn, J.F. Semiequilibrium dialysis: A new method for measuring the solubilization of organic solutes by aqueous surfactant solutions. Langmuir 1985, 1, 564–567. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Duan, S.-S.; Ouyang, Y.-S.; Chen, Y.-B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. BioMetals 2011, 24, 135–141. [Google Scholar] [CrossRef]

| PECTIN 1 | AgNP 2 | GNS@PEG 3 | GNS@PEG-C 4 | CA 5 | TOTAL Ag 6(MOL; μg) | TOTAL Au 7(MOL; μg) | CALCULATED FILM MASS 8 (mg) | DRY FILM MASS 9 (mg) | |
|---|---|---|---|---|---|---|---|---|---|
| FILM-BLANK | - | - | - | - | ✓ | - | - | 732 | 731(±5) |
| FILM-PEC | 1.0 | - | - | - | ✓ | - | - | 742 | 740(±8) |
| FILM-Ag | - | 1.0 | - | - | ✓ | 8.82 × 10−7 95.1 | - | 742 | 748(±10) |
| FILM-GNS | - | - | 0.5 | - | ✓ | 9.7 × 10-7 104 | 4.34 × 10−6 855 | 733 | 728(±12) |
| FILM-GNS-C | - | - | - | 0.5 | - | 9.3 × 10-7 99 | 4.26 × 10−6 840 | 667 | 660(±11) |
| FILM-MIX-Ag/GNS | - | 1.0 | 0.5 | - | ✓ | 1.85 × 10−6 199 | 4.34 × 10−6 855 | 743 | 746(±9) |
| FILM-MIX-Ag/GNS-C | - | 1.0 | - | 0.5 | - | 1.81 × 10−6 194 | 4.26 × 10−6 840 | 677 | 686(±8) |
| E. coli | ||
| 5 h | 24 h | |
| Film-blank | 1.2(0.2) | 2.3(0.2) |
| Film-pec | 1.7(0.3) | 3.0(0.2) |
| Film-Ag | 5.5(0.3) | > 7 |
| Film-GNS | 2.2(0.2) | 4.9(0.3) |
| Film-GNS-C | 0.8(0.1) | 2.3(0.2) |
| FilmMIX-Ag/GNS | 5.6(0.3) | >7 |
| FilmMIX-Ag/GNS-C | 0.2(0.1) | 4.4(0.3) |
| S. aureus | ||
| 5 h | 24 h | |
| Film-blank | 2.9(0.2) | 3.2(0.2) |
| Film-pec | 3.1(0.2) | 3.7(0.3) |
| Film-Ag | 3.1(0.3) | >7 |
| Film-GNS | 3.7(0.2) | 4.6(0.3) |
| Film-GNS-C | 1.1(0.2) | 2.3(0.2) |
| FilmMIX-Ag/GNS | 3.5(0.3) | >7 |
| FilmMIX-Ag/GNS-C | 1.3(0.2) | 4.7(0.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grisoli, P.; De Vita, L.; Milanese, C.; Taglietti, A.; Diaz Fernandez, Y.; Bouzin, M.; D’Alfonso, L.; Sironi, L.; Rossi, S.; Vigani, B.; et al. PVA Films with Mixed Silver Nanoparticles and Gold Nanostars for Intrinsic and Photothermal Antibacterial Action. Nanomaterials 2021, 11, 1387. https://doi.org/10.3390/nano11061387
Grisoli P, De Vita L, Milanese C, Taglietti A, Diaz Fernandez Y, Bouzin M, D’Alfonso L, Sironi L, Rossi S, Vigani B, et al. PVA Films with Mixed Silver Nanoparticles and Gold Nanostars for Intrinsic and Photothermal Antibacterial Action. Nanomaterials. 2021; 11(6):1387. https://doi.org/10.3390/nano11061387
Chicago/Turabian StyleGrisoli, Pietro, Lorenzo De Vita, Chiara Milanese, Angelo Taglietti, Yuri Diaz Fernandez, Margaux Bouzin, Laura D’Alfonso, Laura Sironi, Silvia Rossi, Barbara Vigani, and et al. 2021. "PVA Films with Mixed Silver Nanoparticles and Gold Nanostars for Intrinsic and Photothermal Antibacterial Action" Nanomaterials 11, no. 6: 1387. https://doi.org/10.3390/nano11061387
APA StyleGrisoli, P., De Vita, L., Milanese, C., Taglietti, A., Diaz Fernandez, Y., Bouzin, M., D’Alfonso, L., Sironi, L., Rossi, S., Vigani, B., Sperandeo, P., Polissi, A., & Pallavicini, P. (2021). PVA Films with Mixed Silver Nanoparticles and Gold Nanostars for Intrinsic and Photothermal Antibacterial Action. Nanomaterials, 11(6), 1387. https://doi.org/10.3390/nano11061387













