The Concentration of C(sp3) Atoms and Properties of an Activated Carbon with over 3000 m2/g BET Surface Area
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Equipment Details
3. Results and Discussion
3.1. Specific Surface Area
3.2. Elemental Analysis
3.3. TEM and ED
3.4. XRD
3.5. Raman Spectra
3.6. XPS
3.7. XAES
3.8. Plasmonic REELS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Quinlivan, P.A.; Knappe, D.R.U. Effects of Activated Carbon Surface Chemistry and Pore Structure on the Adsorption of Organic Contaminants from Aqueous Solution. Carbon 2002, 40, 2085–2100. [Google Scholar] [CrossRef]
- Quinlivan, P.A.; Li, L.; Knappe, D.R.U. Effects of Activated Carbon Characteristics on the Simultaneous Adsorption of Aqueous Organic Micropollutants and Natural Organic Matter. Water Res. 2005, 39, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.J.; Yüzak, Y.; Thomas, K.M. Adsorption and Desorption Kinetics for Hydrophilic and Hydrophobic Vapors on Activated Carbon. Carbon 2006, 44, 989–1004. [Google Scholar] [CrossRef]
- Furuya, E.G.; Chang, H.T.; Miura, Y.; Noll, K.E. A Fundamental Analysis of the Isotherm for the Adsorption of Phenolic Compounds on Activated Carbon. Sep. Purif. Technol. 1997, 11, 69–78. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.L.; Simon, P.; Fauvarque, J.F.; Chesneau, M. Studies and Characterisations of Various Activated Carbons Used for Carbon/carbon Supercapacitors. J. Power Sources 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Du Pasquier, A.; Plitz, I.; Menocal, S.; Amatucci, G. A Comparative Study of Li-Ion Battery, Supercapacitor and Nonaqueous Asymmetric Hybrid Devices for Automotive Applications. J. Power Sources 2003, 115, 171–178. [Google Scholar] [CrossRef]
- Chen, J.; Gao, X.; Song, W. Effect of Various Carbon Nanofillers and Different Filler Aspect Ratios on the Thermal Conductivity of Epoxy Matrix Nanocomposites. Results Phys. 2019, 15, 102771. [Google Scholar] [CrossRef]
- Zhang, W.; Dehghani-Sanij, A.A.; Blackburn, R.S. Carbon Based Conductive Polymer Composites. J. Mater. Sci. 2007, 42, 3408–3418. [Google Scholar] [CrossRef]
- Lebedev, S.M.; Gefle, O.S. Electrical and Thermal Properties of Polymer Composites Based on Polyvinylidene Fluoride. Russ. Phys. J. 2017, 60, 115–121. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Matranga, K.R.; Myers, A.L.; Glandt, E.D. Storage of Natural Gas by Adsorption on Activated Carbon. Chem. Eng. Sci. 1992, 47, 1569–1579. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Chai, Z.; Hu, W. Low-Temperature Plasma Synthesis of Carbon Nanotubes and Graphene Based Materials and Their Fuel Cell Applications. Chem. Soc. Rev. 2013, 42, 8821–8834. [Google Scholar] [CrossRef]
- Chae, H.K.; Siberio-Pérez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A Route to High Surface Area, Porosity and Inclusion of Large Molecules in Crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef]
- Kaneko, K.; Ishii, C.; Ruike, M.; Kuwabara, H. Origin of Superhigh Surface Area and Microcrystalline Graphitic Structures of Activated Carbons. Carbon 1992, 30, 1075–1088. [Google Scholar] [CrossRef]
- Cranford, S.W.; Buehler, M.J. Packing Efficiency and Accessible Surface Area of Crumpled Graphene. Phys. Rev. B Condens. Matter 2011, 84, 205451. [Google Scholar] [CrossRef]
- Gauden, P.A.; Terzyk, A.P.; Furmaniak, S.; Harris, P.J.F.; Kowalczyk, P. BET Surface Area of Carbonaceous Adsorbents—Verification Using Geometric Considerations and GCMC Simulations on Virtual Porous Carbon Models. Appl. Surf. Sci. 2010, 256, 5204–5209. [Google Scholar] [CrossRef]
- De Lange, M.F.; Lin, L.-C.; Gascon, J.; Vlugt, T.J.H.; Kapteijn, F. Assessing the Surface Area of Porous Solids: Limitations, Probe Molecules, and Methods. Langmuir 2016, 32, 12664–12675. [Google Scholar] [CrossRef]
- Murali, S.; Potts, J.R.; Stoller, S.; Park, J.; Stoller, M.D.; Zhang, L.L.; Zhu, Y.; Ruoff, R.S. Preparation of Activated Graphene and Effect of Activation Parameters on Electrochemical Capacitance. Carbon 2012, 50, 3482–3485. [Google Scholar] [CrossRef]
- Kierzek, K.; Frackowiak, E.; Lota, G.; Gryglewicz, G.; Machnikowski, J. Electrochemical Capacitors Based on Highly Porous Carbons Prepared by KOH Activation. Electrochim. Acta 2004, 49, 515–523. [Google Scholar] [CrossRef]
- Alonso, A.; Ruiz, V.; Blanco, C.; Santamaría, R.; Granda, M.; Menéndez, R.; de Jager, S.G.E. Activated Carbon Produced from Sasol-Lurgi Gasifier Pitch and Its Application as Electrodes in Supercapacitors. Carbon 2006, 44, 441–446. [Google Scholar] [CrossRef]
- Du, S.-H.; Wang, L.-Q.; Fu, X.-T.; Chen, M.-M.; Wang, C.-Y. Hierarchical Porous Carbon Microspheres Derived from Porous Starch for Use in High-Rate Electrochemical Double-Layer Capacitors. Bioresour. Technol. 2013, 139, 406–409. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Marco-Lozar, J.P.; Cazorla-Amorós, D.; Linares-Solano, A. Activated Carbons Prepared by Pyrolysis of Mixtures of Carbon Precursor/alkaline Hydroxide. J. Anal. Appl. Pyrolysis 2007, 80, 166–174. [Google Scholar] [CrossRef]
- Xu, B.; Wu, F.; Chen, R.; Cao, G.; Chen, S.; Yang, Y. Mesoporous Activated Carbon Fiber as Electrode Material for High-Performance Electrochemical Double Layer Capacitors with Ionic Liquid Electrolyte. J. Power Sources 2010, 195, 2118–2124. [Google Scholar] [CrossRef]
- Yang, C.-S.; Jang, Y.S.; Jeong, H.K. Bamboo-Based Activated Carbon for Supercapacitor Applications. Curr. Appl. Phys. 2014, 14, 1616–1620. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, W.; Yue, Q.; Gao, B.; Sun, Y.; Kong, J.; Zhao, P. Simple Synthesis of Hierarchical Porous Carbon from Enteromorpha Prolifera by a Self-Template Method for Supercapacitor Electrodes. J. Power Sources 2014, 270, 403–410. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Geng, Z.; Cai, M. High-Pressure Hydrogen Storage and Optimizing Fabrication of Corncob-Derived Activated Carbon. Microporous Mesoporous Mater. 2014, 194, 60–65. [Google Scholar] [CrossRef]
- Wang, D.; Geng, Z.; Li, B.; Zhang, C. High Performance Electrode Materials for Electric Double-Layer Capacitors Based on Biomass-Derived Activated Carbons. Electrochim. Acta 2015, 173, 377–384. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, W.; Lin, H.; Li, Y.; Lu, H.; Wang, Y. A Green Technology for the Preparation of High Capacitance Rice Husk-Based Activated Carbon. J. Clean. Prod. 2016, 112, 1190–1198. [Google Scholar] [CrossRef]
- Yu, Y.; Qiao, N.; Wang, D.; Zhu, Q.; Fu, F.; Cao, R.; Wang, R.; Liu, W.; Xu, B. Fluffy Honeycomb-like Activated Carbon from Popcorn with High Surface Area and Well-Developed Porosity for Ultra-High Efficiency Adsorption of Organic Dyes. Bioresour. Technol. 2019, 285, 121340. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Yang, X.; Long, G.; Wu, Y.; Zhang, T.; Leng, K.; Huang, Y.; Ma, Y.; Yu, A.; et al. Porous 3D Graphene-Based Bulk Materials with Exceptional High Surface Area and Excellent Conductivity for Supercapacitors. Sci. Rep. 2013, 3, 1408. [Google Scholar] [CrossRef]
- Fung, A.W.P.; Rao, A.M.; Kuriyama, K.; Dresselhaus, M.S.; Dresselhaus, G.; Endo, M.; Shindo, N. Raman Scattering and Electrical Conductivity in Highly Disordered Activated Carbon Fibers. J. Mater. Res. 1993, 8, 489–500. [Google Scholar] [CrossRef]
- Koh, K.; Wong-Foy, A.G.; Matzger, A.J. A Porous Coordination Copolymer with over 5000 m2/g BET Surface Area. J. Am. Chem. Soc. 2009, 131, 4184–4185. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.H.; Lin, J.; Young, C.; Matsagar, B.M.; Wu, K.C.W.; Dhepe, P.L.; Islam, M.T.; Rahman, M.M.; Shrestha, L.K.; Alshehri, S.M.; et al. High Surface Area Nanoporous Carbon Derived from High Quality Jute from Bangladesh. Mater. Chem. Phys. 2018, 216, 491–495. [Google Scholar] [CrossRef]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous Activated Coconut Shell-Derived Hydrochar Prepared via Hydrothermal Carbonization-NaOH Activation for Methylene Blue Adsorption. J. Environ. Manag. 2017, 203, 237–244. [Google Scholar] [CrossRef]
- Nowicki, P.; Kazmierczak-Razna, J.; Pietrzak, R. Physicochemical and Adsorption Properties of Carbonaceous Sorbents Prepared by Activation of Tropical Fruit Skins with Potassium Carbonate. Mater. Des. 2016, 90, 579–585. [Google Scholar] [CrossRef]
- Reffas, A.; Bernardet, V.; David, B.; Reinert, L.; Lehocine, M.B.; Dubois, M.; Batisse, N.; Duclaux, L. Carbons Prepared from Coffee Grounds by H3PO4 Activation: Characterization and Adsorption of Methylene Blue and Nylosan Red N-2RBL. J. Hazard. Mater. 2010, 175, 779–788. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Kumar, K.V.; Preuss, K.; Titirici, M.-M.; Rodríguez-Reinoso, F. Nanoporous Materials for the Onboard Storage of Natural Gas. Chem. Rev. 2017, 117, 1796–1825. [Google Scholar] [CrossRef]
- Düren, T.; Sarkisov, L.; Yaghi, O.M.; Snurr, R.Q. Design of New Materials for Methane Storage. Langmuir 2004, 20, 2683–2689. [Google Scholar] [CrossRef]
- Herdes, C.; Sarkisov, L. Computer Simulation of Volatile Organic Compound Adsorption in Atomistic Models of Molecularly Imprinted Polymers. Langmuir 2009, 25, 5352–5359. [Google Scholar] [CrossRef] [PubMed]
- Düren, T.; Millange, F.; Férey, G.; Walton, K.S.; Snurr, R.Q. Calculating Geometric Surface Areas as a Characterization Tool for Metal- Organic Frameworks. J. Phys. Chem. C 2007, 111, 15350–15356. [Google Scholar] [CrossRef]
- Walton, K.S.; Snurr, R.Q. Applicability of the BET Method for Determining Surface Areas of Microporous Metal- Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-S.; Yazaydin, A.O.; Snurr, R.Q. Evaluation of the BET Method for Determining Surface Areas of MOFs and Zeolites That Contain Ultra-Micropores. Langmuir 2010, 26, 5475–5483. [Google Scholar] [CrossRef] [PubMed]
- Sarkisov, L. Accessible Surface Area of Porous Materials: Understanding Theoretical Limits. Adv. Mater. 2012, 24, 3130–3133. [Google Scholar] [CrossRef]
- Titantah, J.T.; Lamoen, D. sp3/sp2 Characterization of Carbon Materials from First-Principles Calculations: X-Ray Photoelectron versus High Energy Electron Energy-Loss Spectroscopy Techniques. Carbon 2005, 43, 1311–1316. [Google Scholar] [CrossRef]
- Sun, J.; Klechikov, A.; Moise, C.; Prodana, M.; Enachescu, M.; Talyzin, A.V. Molecular Pillar Approach to Grow Vertical Covalent Organic Framework Nanosheets on Graphene: New Hybrid Materials for Energy Storage. Angew. Chem. Int. Ed. 2018, 57, 1034–1038. [Google Scholar] [CrossRef]
- Bellucci, L.; Tozzini, V. Engineering 3D Graphene-Based Materials: State of the Art and Perspectives. Molecules 2020, 25, 339. [Google Scholar] [CrossRef]
- William, S.; Hummers, J.R.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar]
- Shulga, Y.M.; Baskakov, S.A.; Smirnov, V.A.; Shulga, N.Y.; Belay, K.G.; Gutsev, G.L. Graphene Oxide Films as Separators of Polyaniline-Based Supercapacitors. J. Power Sources 2014, 245, 33–36. [Google Scholar] [CrossRef]
- Baskakov, S.A.; Baskakova, Y.V.; Lyskov, N.V.; Dremova, N.N.; Irzhak, A.V.; Kumar, Y.; Michtchenok, A.; Shulga, Y.M. Fabrication of Current Collector Using a Composite of Polylactic Acid and Carbon Nano-Material for Metal-Free Supercapacitors with Graphene Oxide Separators and Microwave Exfoliated Graphite Oxide Electrodes. Electrochim. Acta 2018, 260, 557–563. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Végh, J. The Shirley Background Revised. J. Electron Spectrosc. Relat. Phenom. 2006, 151, 159–164. [Google Scholar] [CrossRef]
- Shulga, Y.M.; Kabachkov, E.N.; Baskakov, S.A.; Baskakova, Y.V. Doping Graphene Oxide Aerogel with Nitrogen during Reduction with Hydrazine and Low Temperature Annealing in Air. Russ. J. Phys. Chem. A 2019, 93, 296–300. [Google Scholar] [CrossRef]
- Jurkiewicz, K.; Pawlyta, M.; Zygadło, D.; Chrobak, D.; Duber, S.; Wrzalik, R.; Ratuszna, A.; Burian, A. Evolution of Glassy Carbon under Heat Treatment: Correlation Structure–Mechanical Properties. J. Mater. Sci. 2018, 53, 3509–3523. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman Spectroscopy of Amorphous, Nanostructured, Diamond-like Carbon, and Nanodiamond. Philos. Trans. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Kūrti, J.; Magyar, C.; Balázs, A.; Rajczy, P. Vibrational Analysis for Short Carbon Chains with Alternating and Cumulenic Structure. Synth. Met. 1995, 71, 1865–1866. [Google Scholar] [CrossRef]
- Kavan, L.; Hlavatý, J.; Kastner, J.; Kuzmany, H. Electrochemical Carbyne from Perfluorinated Hydrocarbons: Synthesis and Stability Studied by Raman Scattering. Carbon 1995, 33, 1321–1329. [Google Scholar] [CrossRef]
- Lucotti, A.; Tommasini, M.; Zoppo, M.D.; Castiglioni, C.; Zerbi, G.; Cataldo, F.; Casari, C.S.; Bassi, A.L.; Russo, V.; Bogana, M.; et al. Raman and SERS Investigation of Isolated sp Carbon Chains. Chem. Phys. Lett. 2006, 417, 78–82. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B Condens. Matter 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Kavan, L.; Kastner, J. Carbyne Forms of Carbon: Continuation of the Story. Carbon N. Y. 1994, 32, 1533–1536. [Google Scholar] [CrossRef]
- Zhao, X.; Ando, Y.; Liu, Y.; Jinno, M.; Suzuki, T. Carbon Nanowire Made of a Long Linear Carbon Chain Inserted inside a Multiwalled Carbon Nanotube. Phys. Rev. Lett. 2003, 90, 187401. [Google Scholar] [CrossRef] [PubMed]
- Ravagnan, L.; Piseri, P.; Bruzzi, M.; Miglio, S.; Bongiorno, G.; Baserga, A.; Casari, C.S.; Li Bassi, A.; Lenardi, C.; Yamaguchi, Y.; et al. Influence of Cumulenic Chains on the Vibrational and Electronic Properties of S P-S p2 Amorphous Carbon. Phys. Rev. Lett. 2007, 98, 216103. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Speranza, G. Tuning the Oxygen Content of Reduced Graphene Oxide and Effects on Its Properties. ACS Omega 2021, 6, 6195–6205. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying Disorder in Graphite-Based Systems by Raman Spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Barclay, M.; Hill, S.B.; Fairbrother, D.H. Use of X-Ray Photoelectron Spectroscopy and Spectroscopic Ellipsometry to Characterize Carbonaceous Films Modified by Electrons and Hydrogen Atoms. Appl. Surf. Sci. 2019, 479, 557–568. [Google Scholar] [CrossRef]
- Lei, Y.; Jiang, J.; Wang, Y.; Bi, T.; Zhang, L. Structure Evolution and Stress Transition in Diamond-like Carbon Films by Glancing Angle Deposition. Appl. Surf. Sci. 2019, 479, 12–19. [Google Scholar] [CrossRef]
- Lesiak, B.; Kövér, L.; Tóth, J.; Zemek, J.; Jiricek, P.; Kromka, A.; Rangam, N. C sp2/sp3 Hybridisations in Carbon Nanomaterials--XPS and(X) AES Study. Appl. Surf. Sci. 2018, 452, 223–231. [Google Scholar] [CrossRef]
- Lascovich, J.C.; Scaglione, S. Comparison among XAES, PELS and XPS Techniques for Evaluation of Sp2 Percentage in a-C:H. Appl. Surf. Sci. 1994, 78, 17–23. [Google Scholar] [CrossRef]
- Bhaumik, A.; Sachan, R.; Narayan, J. Tunable Charge States of Nitrogen-Vacancy Centers in Diamond for Ultrafast Quantum Devices. Carbon 2019, 142, 662–672. [Google Scholar] [CrossRef]
- Haque, A.; Sachan, R.; Narayan, J. Synthesis of Diamond Nanostructures from Carbon Nanotube and Formation of Diamond-CNT Hybrid Structures. Carbon N. Y. 2019, 150, 388–395. [Google Scholar] [CrossRef]
- Zhang, X.; Schneider, R.; Müller, E.; Gerthsen, D. Practical Aspects of the Quantification of sp2-Hybridized Carbon Atoms in Diamond-like Carbon by Electron Energy Loss Spectroscopy. Carbon 2016, 102, 198–207. [Google Scholar] [CrossRef]
- Bruley, J.; Williams, D.B.; Cuomo, J.J.; Pappas, D.P. Quantitative near-Edge Structure Analysis of Diamond-like Carbon in the Electron Microscope Using a Two-Window Method. J. Microsc. 1995, 180, 22–32. [Google Scholar] [CrossRef]
- Titantah, J.T.; Lamoen, D. Technique for the p2p3 Characterization of Carbon Materials: Ab Initio Calculation of near-Edge Structure in Electron-Energy-Loss Spectra. Phys. Rev. B Condens. Matter 2004, 70, 075115. [Google Scholar] [CrossRef]
- Bernier, N.; Bocquet, F.; Allouche, A.; Saikaly, W.; Brosset, C.; Thibault, J.; Charai, A. A methodology to optimize the quantification of sp2 carbon fraction from K edge EELS spectra. J. Electron Spectrosc. Relat. Phenom. 2008, 164, 34–43. [Google Scholar] [CrossRef]
- Lajaunie, L.; Pardanaud, C.; Martin, C.; Puech, P.; Hu, C.; Biggs, M.J.; Arenal, R. Advanced spectroscopic analyses on a:C-H materials: Revisiting the EELS characterization and its coupling with multi-wavelength Raman spectroscopy. Carbon 2017, 112, 149–161. [Google Scholar] [CrossRef]
- Pelaez-Fernandez, M.; Bermejo, A.; Benito, A.M.; Maser, W.K.; Arenal, R. Detailed thermal reduction analyses of Graphene Oxide via in-situ TEM/EELS studies. Carbon 2021, 178, 477–487. [Google Scholar] [CrossRef]
- Taft, E.A.; Philipp, H.R. Optical Properties of Graphite. Phys. Rev. 1965, 138, A197–A202. [Google Scholar] [CrossRef]
- Liang, W.Y.; Cundy, S.L. Electron Energy Loss Studies of the Transition Metal Dichalcogenides. Philos. Mag. 1969, 19, 1031–1043. [Google Scholar] [CrossRef]
- Caputi, L.S.; Papagno, L. Plasmon Excitation in Graphite by Electron Energy Loss. Phys. Lett. A 1983, 93, 417–418. [Google Scholar] [CrossRef]
- Pflüger, J.; Fink, J.; Weber, W.; Bohnen, K.P.; Crecelius, G. Dielectric Properties of TiC X, TiN X, VC X, and VN X from 1.5 to 40 eV Determined by Electron-Energy-Loss Spectroscopy. Phys. Rev. B Condens. Matter Mater. Phys. 1984, 30, 1155. [Google Scholar] [CrossRef]
- Martin, P.J.; Filipczuk, S.W.; Netterfield, R.P.; Field, J.S.; Whitnall, D.F.; McKenzie, D.R. Structure and Hardness of Diamond-like Carbon Films Prepared by Arc Evaporation. J. Mater. Sci. Lett. 1988, 7, 410–412. [Google Scholar] [CrossRef]
- Berger, S.D.; McKenzie, D.R.; Martin, P.J. EELS Analysis of Vacuum Arc-Deposited Diamond-like Films. Philos. Mag. Lett. 1988, 57, 285–290. [Google Scholar] [CrossRef]
- Saito, Y.; Shinohara, H.; Ohshita, A. Bulk Plasmons in Solid C60. Jpn. J. Appl. Phys. 1991, 30, L1068. [Google Scholar] [CrossRef]
- Jost, M.B.; Troullier, N.; Poirier, D.M.; Martins, J.L.; Weaver, J.H.; Chibante, L.P.F.; Smalley, R.E. Band Dispersion and Empty Electronic States in Solid C60: Inverse Photoemission and Theory. Phys. Rev. B Condens. Matter 1991, 44, 1966–1969. [Google Scholar] [CrossRef]
- Sohmen, E.; Fink, J.; Krätschmer, W. Electron Energy-Loss Spectroscopy Studies on C 60 and C 70 Fullerite. Z. Phys. B Condens. Matter 1992, 86, 87–92. [Google Scholar] [CrossRef]
- Gass, M.H.; Bangert, U.; Bleloch, A.L.; Wang, P.; Nair, R.R.; Geim, A.K. Free-Standing Graphene at Atomic Resolution. Nat. Nanotechnol. 2008, 3, 676–681. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 7th ed.; Wiley: New York, NY, USA, 1996; p. 673. ISBN 978-0-471-11181-8. [Google Scholar]
- Shulga, Y.M.; Rubtsov, V.I.; Lobach, A.S. Reflection Electron Energy-Loss Spectra of the Fullerenes C 60 and C 70. Z. Phys. B Condens. Matter 1994, 93, 327–331. [Google Scholar] [CrossRef]
- Doniach, S.; Sunjic, M. Many-Electron Singularity in X-Ray Photoemission and X-Ray Line Spectra from Metals. J. Phys. C Solid State Phys. 1970, 3, 285. [Google Scholar] [CrossRef]
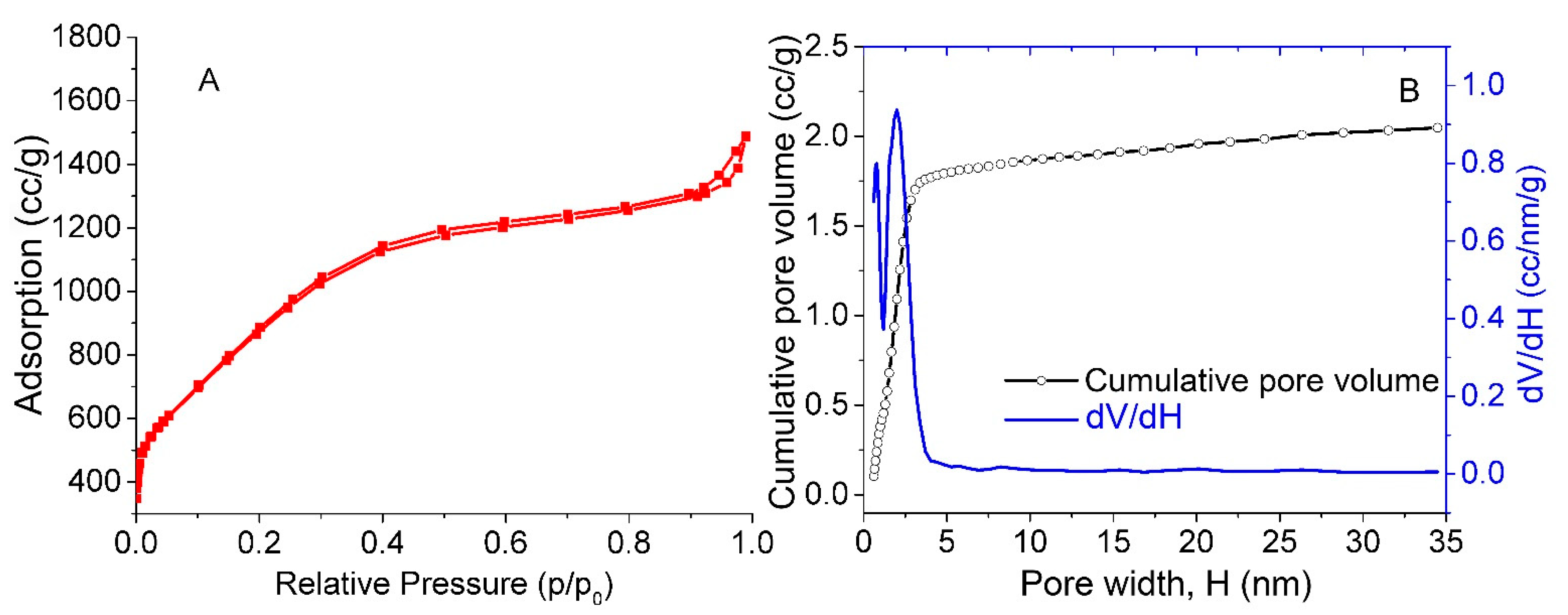
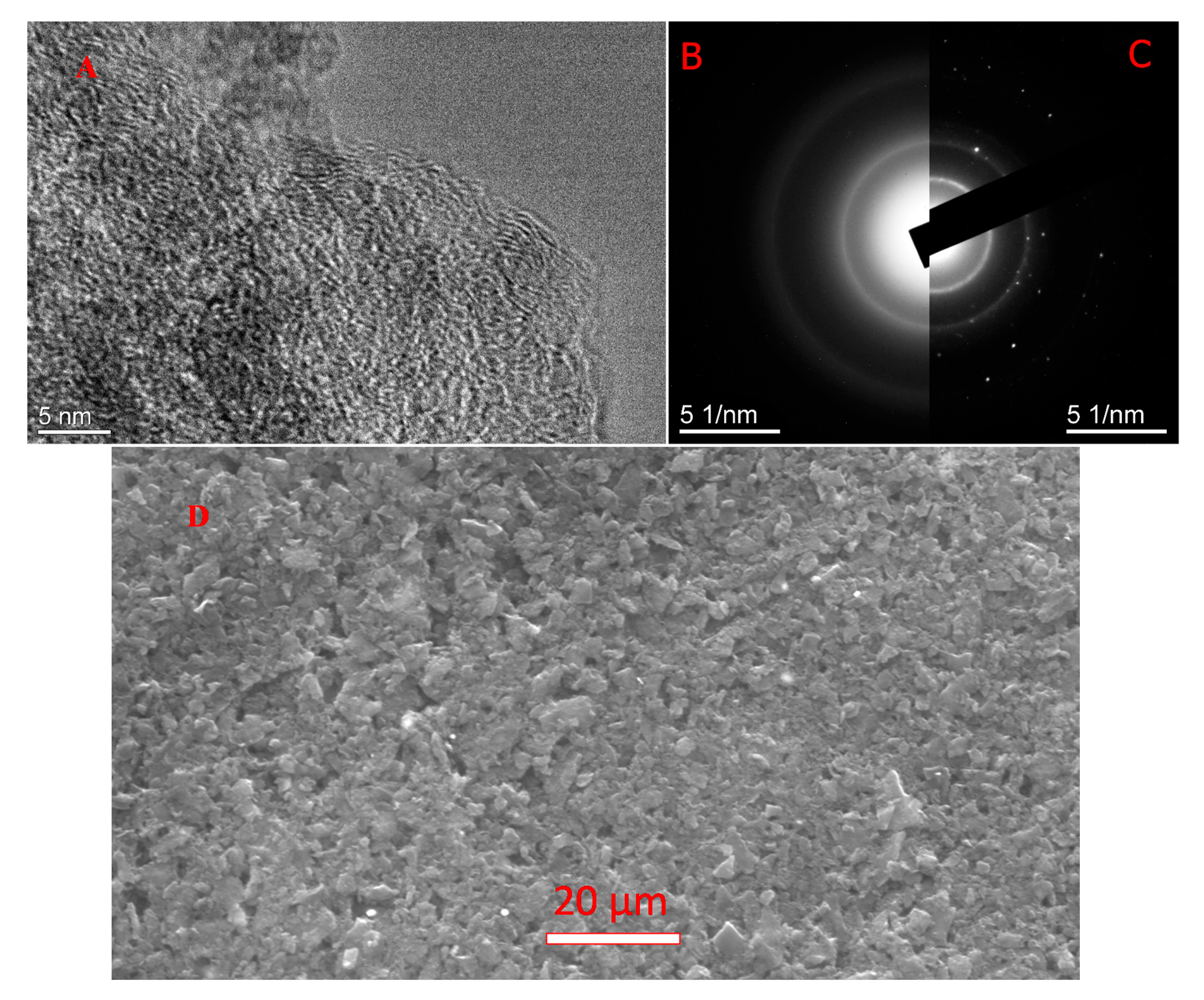
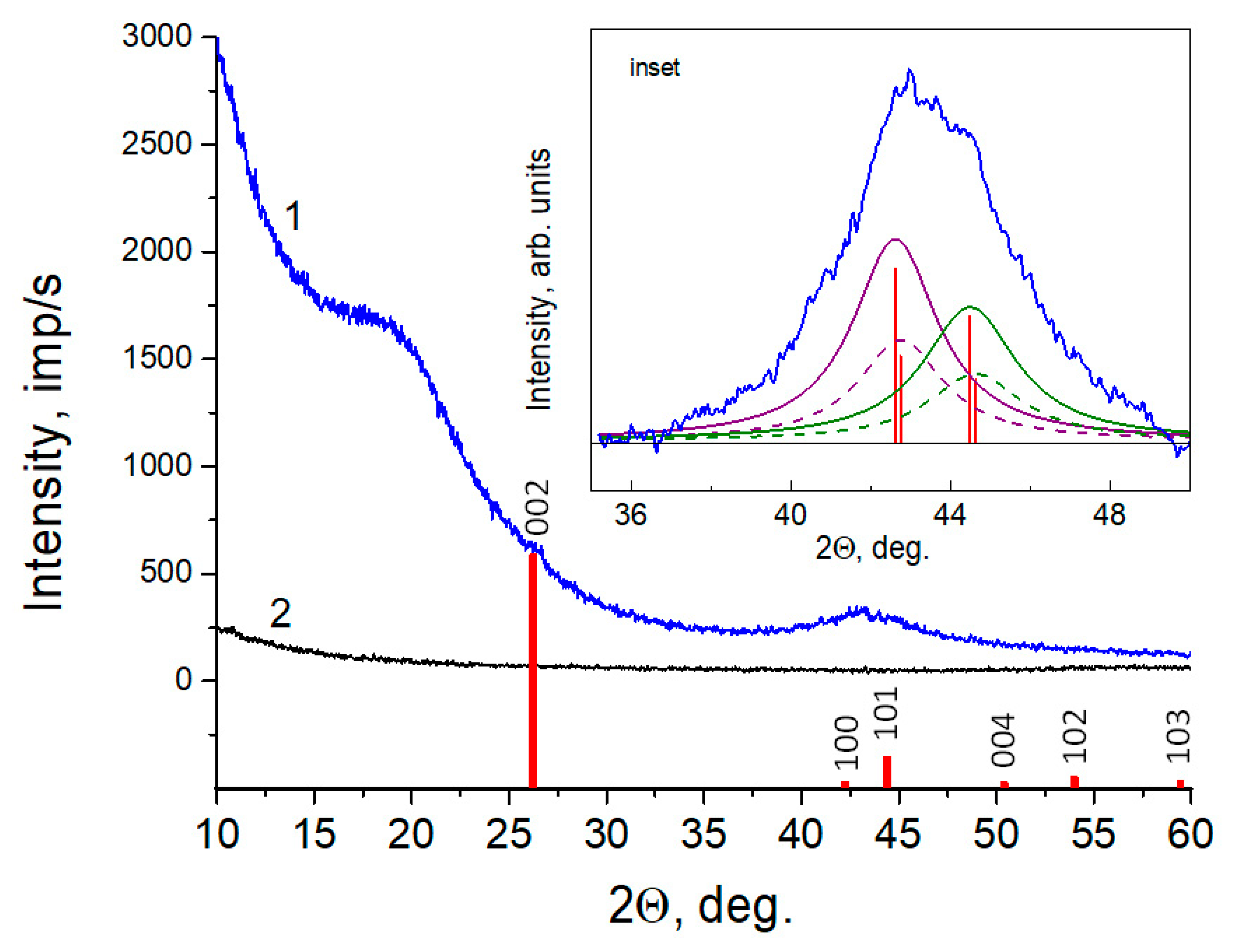
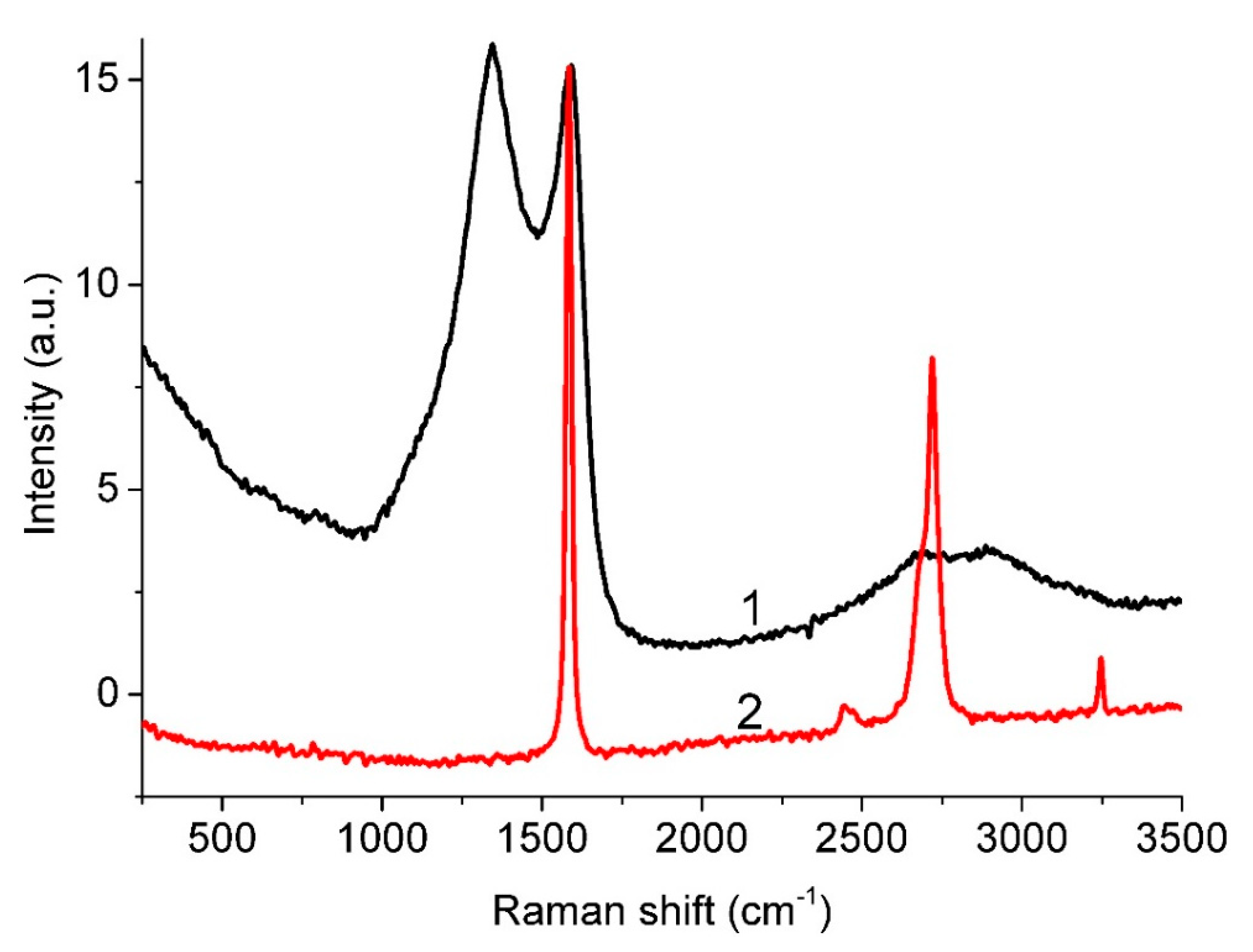


| Material and Fabrication Method | Specific Surface Area, m2/g | References |
|---|---|---|
| alkaline activation of MEGO | 3100 | [11] |
| graphene (calculated data) | 2620–2675 | [12,13,14,15,16,19] |
| alkali-activated carbon | From 3026 to 3708 | [20,21,22,23,24,25,26,27,28,29,30,31] |
| oxidative activation of pitch-derived carbon fiber | From 1000 to 3000 | [32] |
| polymer derived from zinc-mediated coordination copolymerization of a dicarboxylic and tricarboxylic acid | >5000 | [33] |
| alkaline activation of a mixture graphene oxide and dextrin (AC-TR) carbonized at 750 °C | 3167 | This work |
| AC used in supercapacitors | 1000–2000 | [34,35,36,37] |
| Content | C | H | N | S | Cl | O | K |
|---|---|---|---|---|---|---|---|
| wt % a | 92.97 ± 0.03 | 0.451 ± 0.069 | 0.65 ± 0.07 | 0.164 ± 0.026 | |||
| at % b | 94.6 5 | 0.15 | >0.1 | 0.31 | 4.69 | >0.2 |
| Peak | Pos, cm−1 | FWHM, cm−1 | Int, % |
|---|---|---|---|
| D * | 1125.2 | 160.0 | 4.9 |
| D | 1346.0 | 220.0 | 63.1 |
| D” | 1537.5 | 161.0 | 20.3 |
| G | 1592.0 | 81.8 | 10.3 |
| D’ | 1608.0 | 48.0 | 1.4 |
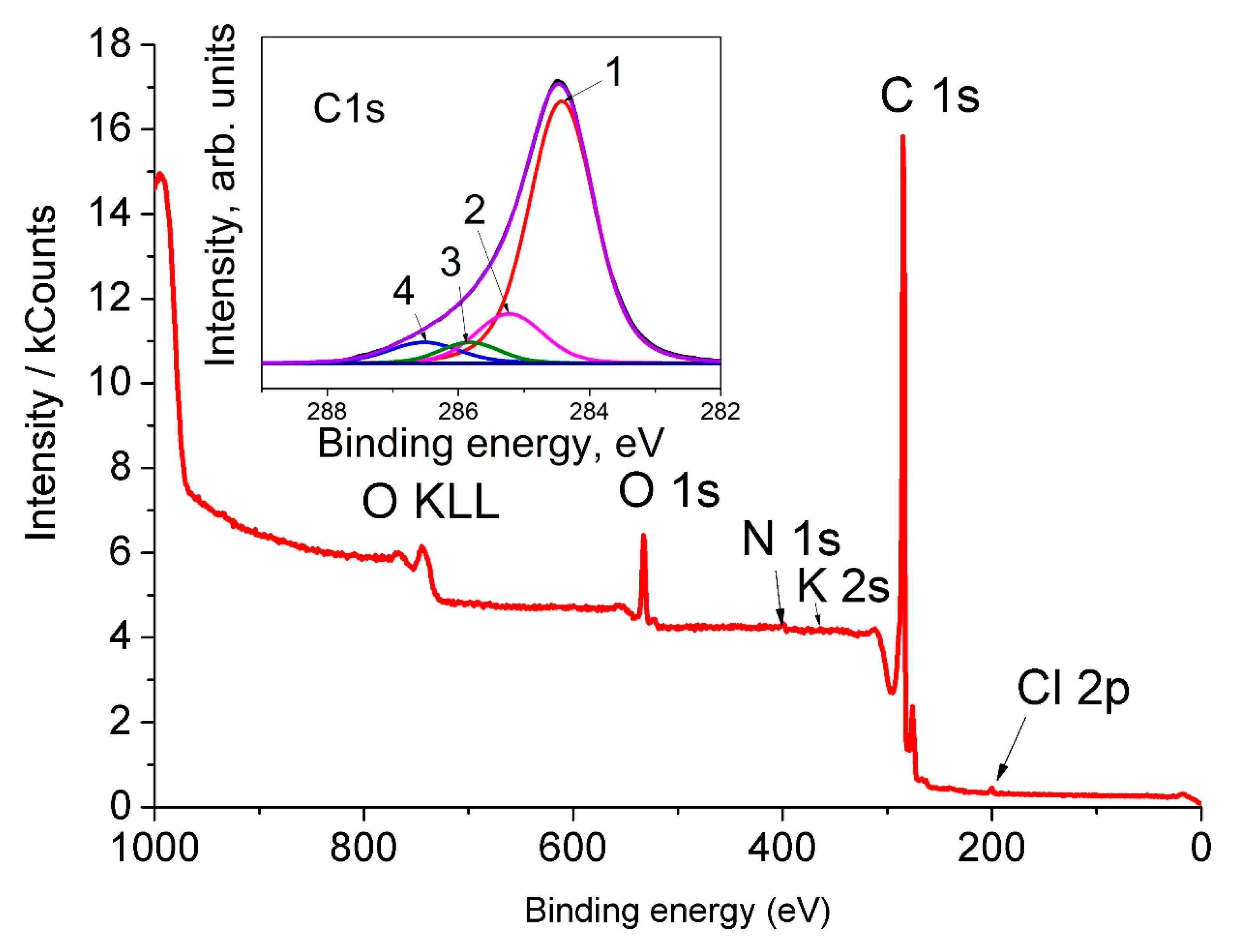
| Peak | Eb, eV | I, % | Assignment |
|---|---|---|---|
| 1 | 284.4 | 75.87 | C (sp2) |
| 2 | 285.1 | 15.23 | C (sp3) |
| 3 | 285.9 | 4.38 | C (sp3)–OH |
| 4 | 286.5 | 4.51 | C (sp2) = O |
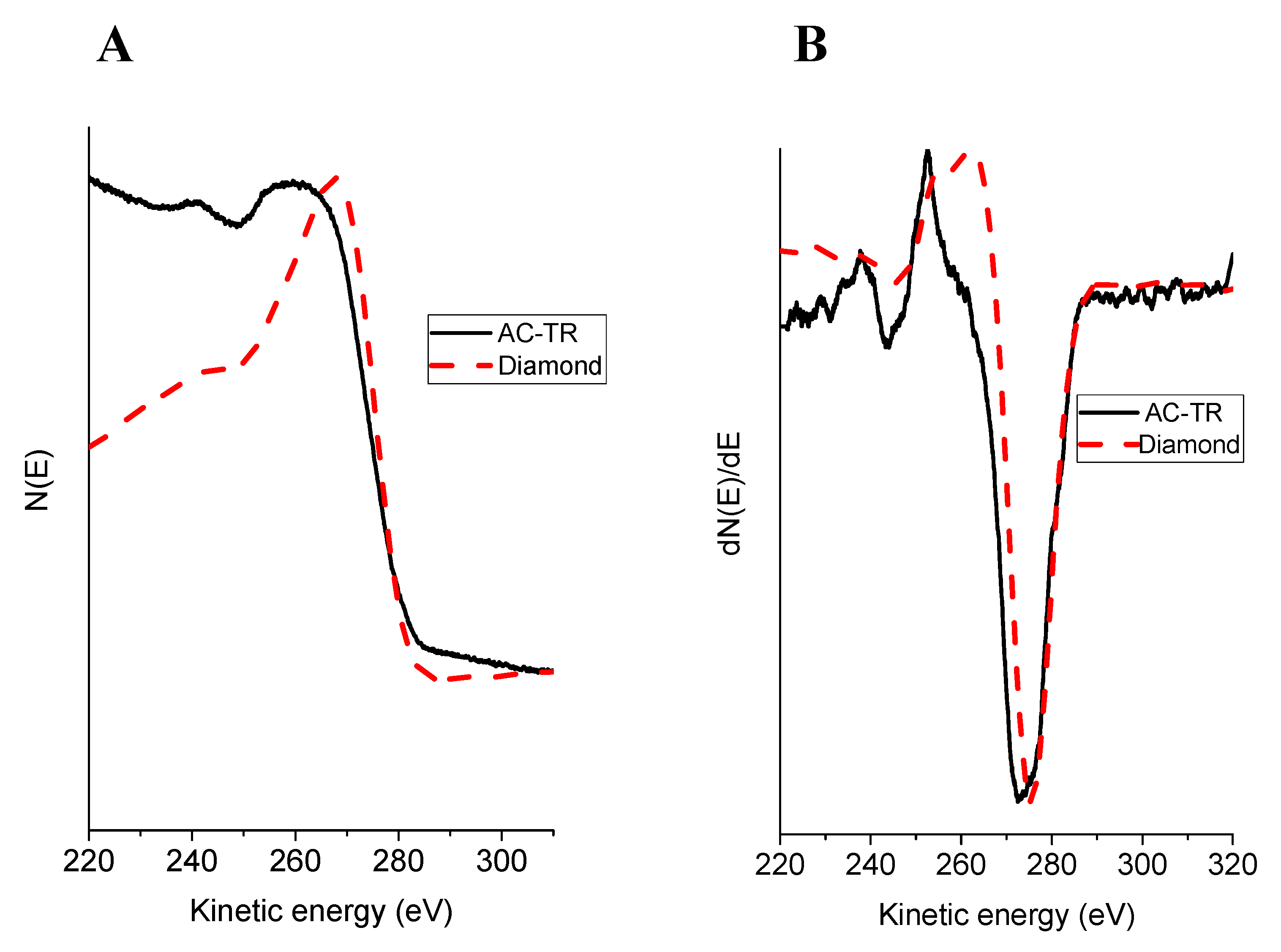
| Sample | π, eV | (σ + π), eV | Iπ/Iσ + π |
|---|---|---|---|
| AC-TR | 5.2 | 25.0 | 0.134 |
| graphite | 6.5 | 26.5 | 0.145 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shulga, Y.M.; Kabachkov, E.N.; Korepanov, V.I.; Khodos, I.I.; Kovalev, D.Y.; Melezhik, A.V.; Tkachev, A.G.; Gutsev, G.L. The Concentration of C(sp3) Atoms and Properties of an Activated Carbon with over 3000 m2/g BET Surface Area. Nanomaterials 2021, 11, 1324. https://doi.org/10.3390/nano11051324
Shulga YM, Kabachkov EN, Korepanov VI, Khodos II, Kovalev DY, Melezhik AV, Tkachev AG, Gutsev GL. The Concentration of C(sp3) Atoms and Properties of an Activated Carbon with over 3000 m2/g BET Surface Area. Nanomaterials. 2021; 11(5):1324. https://doi.org/10.3390/nano11051324
Chicago/Turabian StyleShulga, Yury M., Eugene N. Kabachkov, Vitaly I. Korepanov, Igor I. Khodos, Dmitry Y. Kovalev, Alexandr V. Melezhik, Aleksei G. Tkachev, and Gennady L. Gutsev. 2021. "The Concentration of C(sp3) Atoms and Properties of an Activated Carbon with over 3000 m2/g BET Surface Area" Nanomaterials 11, no. 5: 1324. https://doi.org/10.3390/nano11051324
APA StyleShulga, Y. M., Kabachkov, E. N., Korepanov, V. I., Khodos, I. I., Kovalev, D. Y., Melezhik, A. V., Tkachev, A. G., & Gutsev, G. L. (2021). The Concentration of C(sp3) Atoms and Properties of an Activated Carbon with over 3000 m2/g BET Surface Area. Nanomaterials, 11(5), 1324. https://doi.org/10.3390/nano11051324









