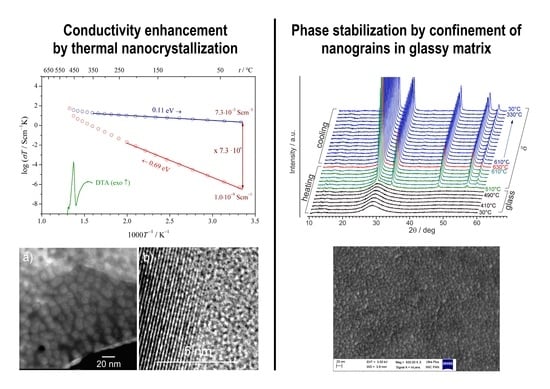
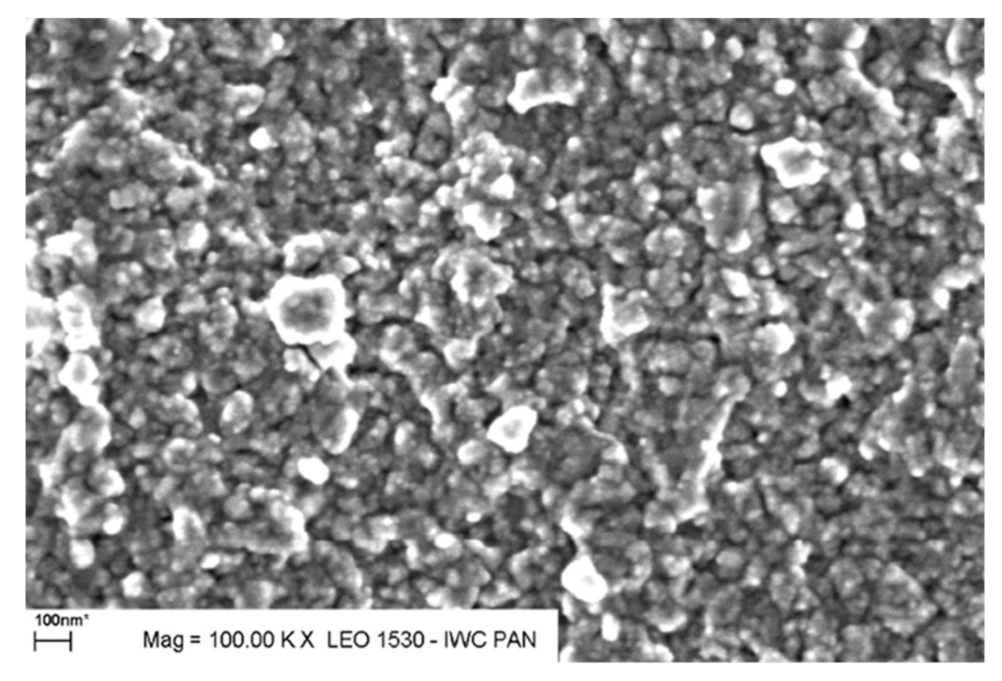
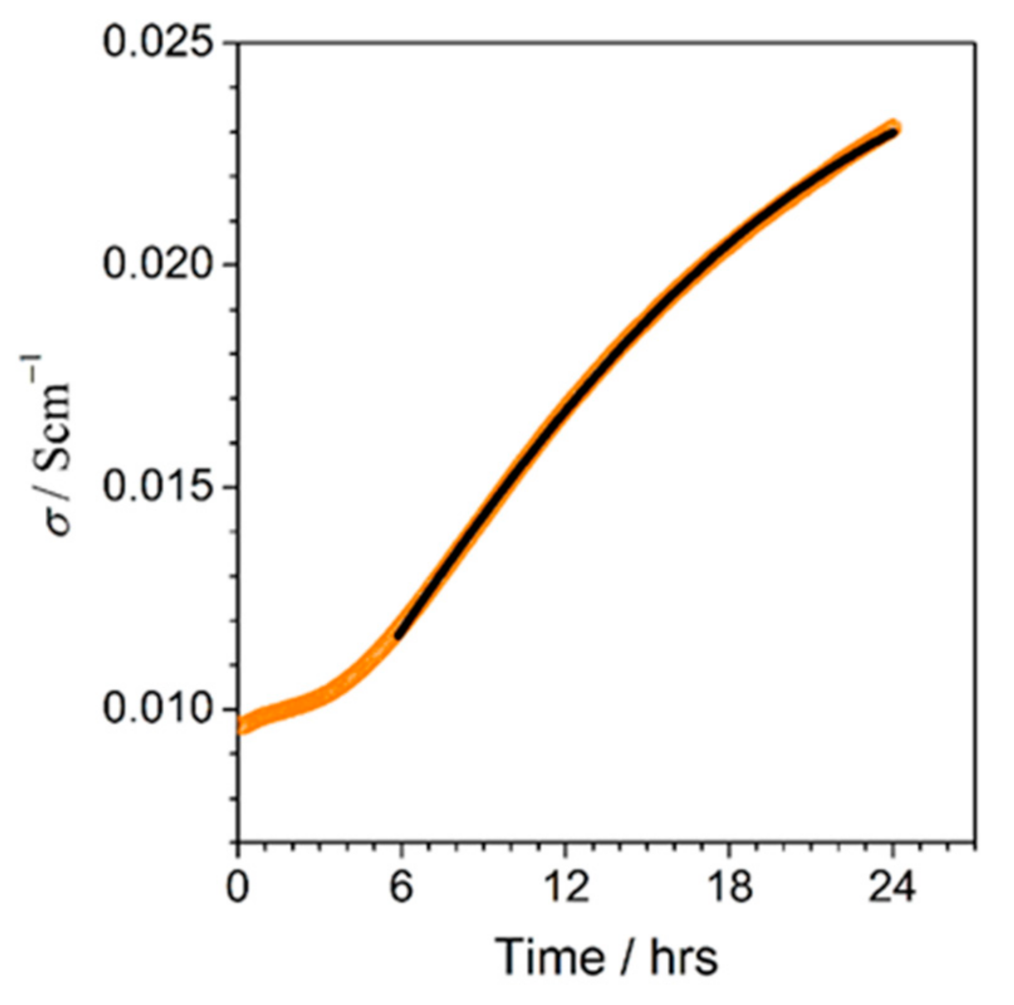
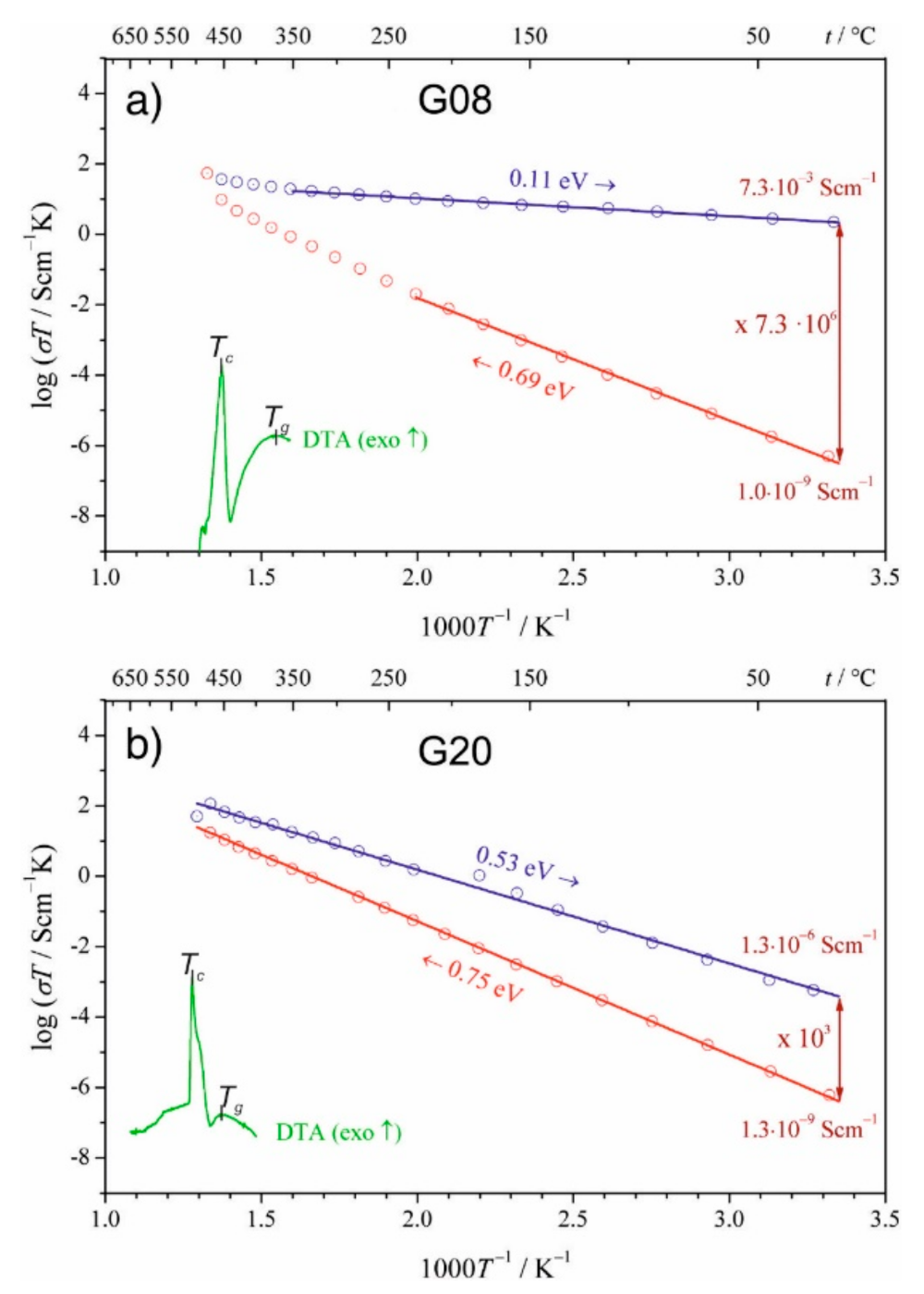
Towards Higher Electric Conductivity and Wider Phase Stability Range via Nanostructured Glass-Ceramics Processing
Abstract
1. Introduction
2. Enhancement of Electrical Conductivity of Glasses by their Nanocrystallization
2.1. Enhancement of Ionic Conductivity
2.2. Enhancement of the Electronic Conductivity
2.2.1. Electronic Conductivity Enhancement in V2O5–P2O5 System
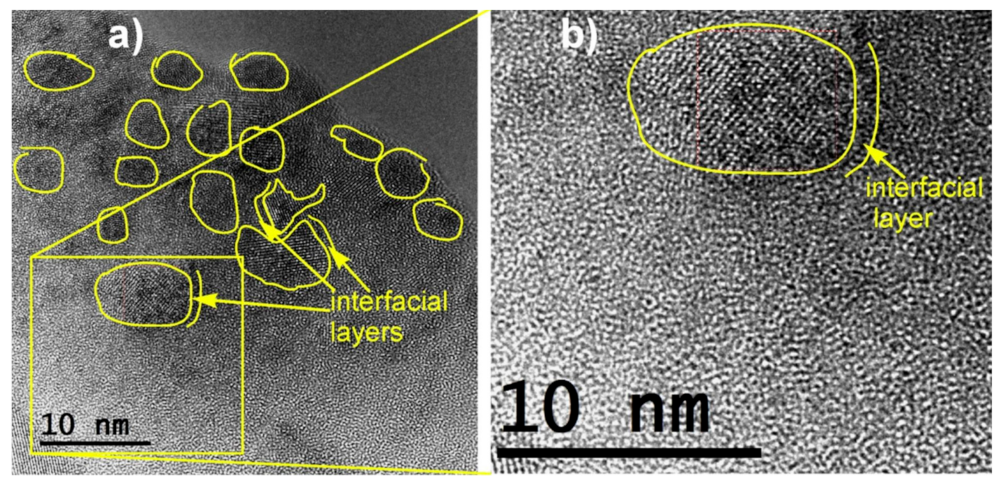
2.2.2. Electronic Conductivity Enhancement in Li–V–Fe–P–O and Other Systems
3. Extension of Thermal Stability Range of High-Temperature Phases by Controlled Nanocrystallization of Glasses
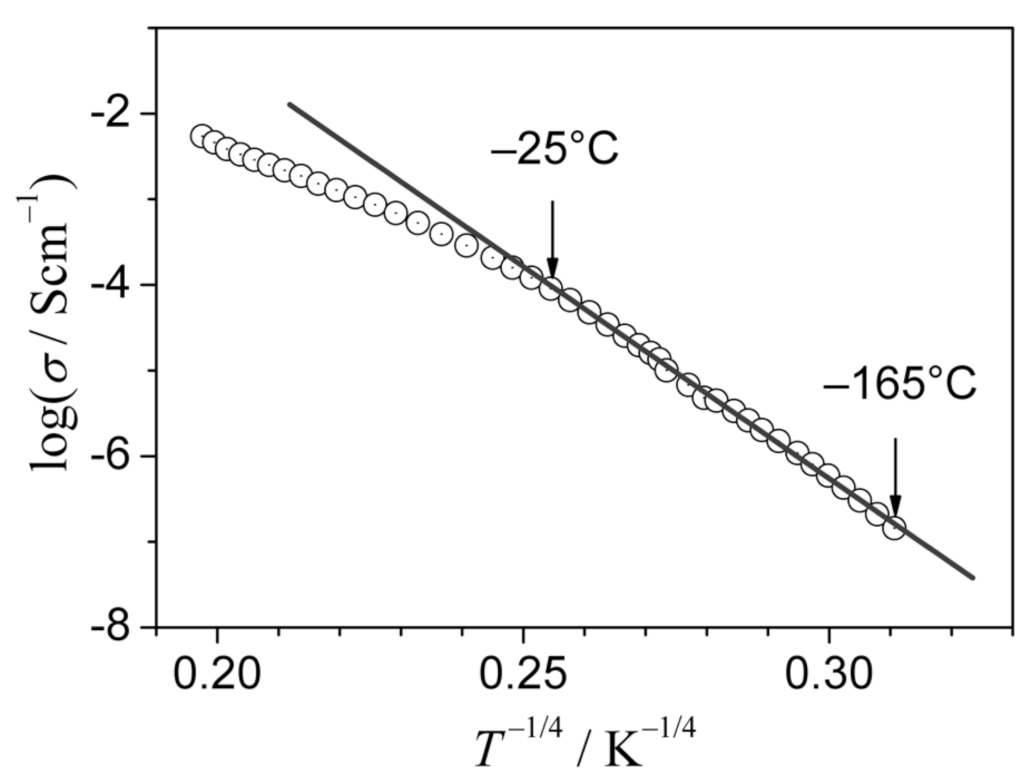
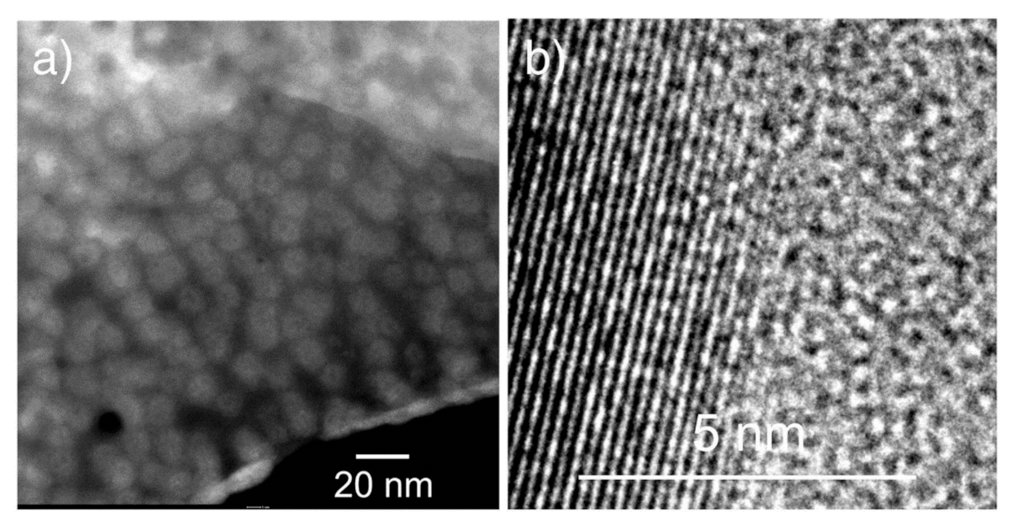
3.1. Stabilization of α-AgI at Room Temperature
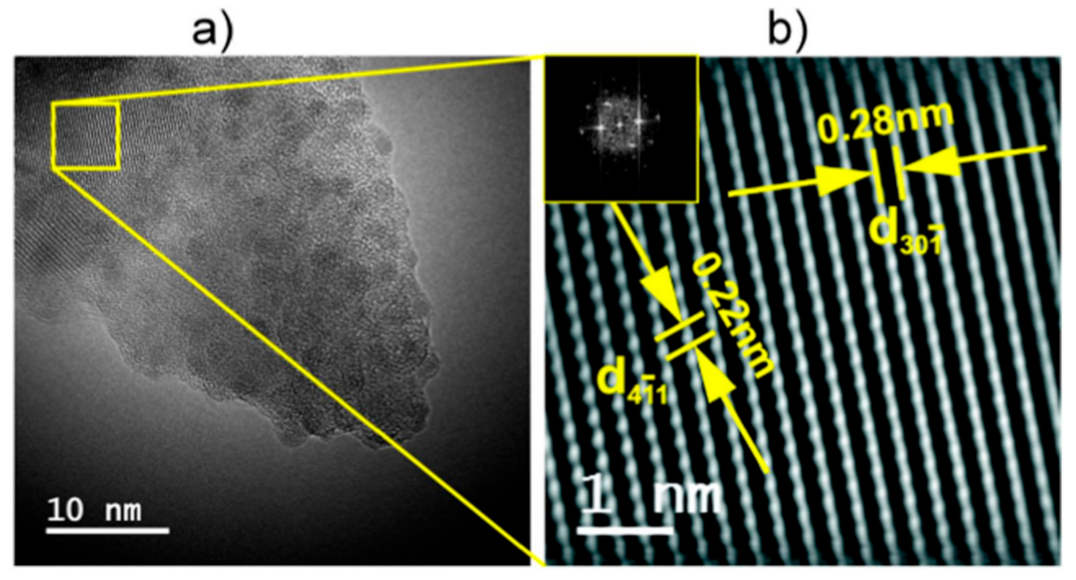
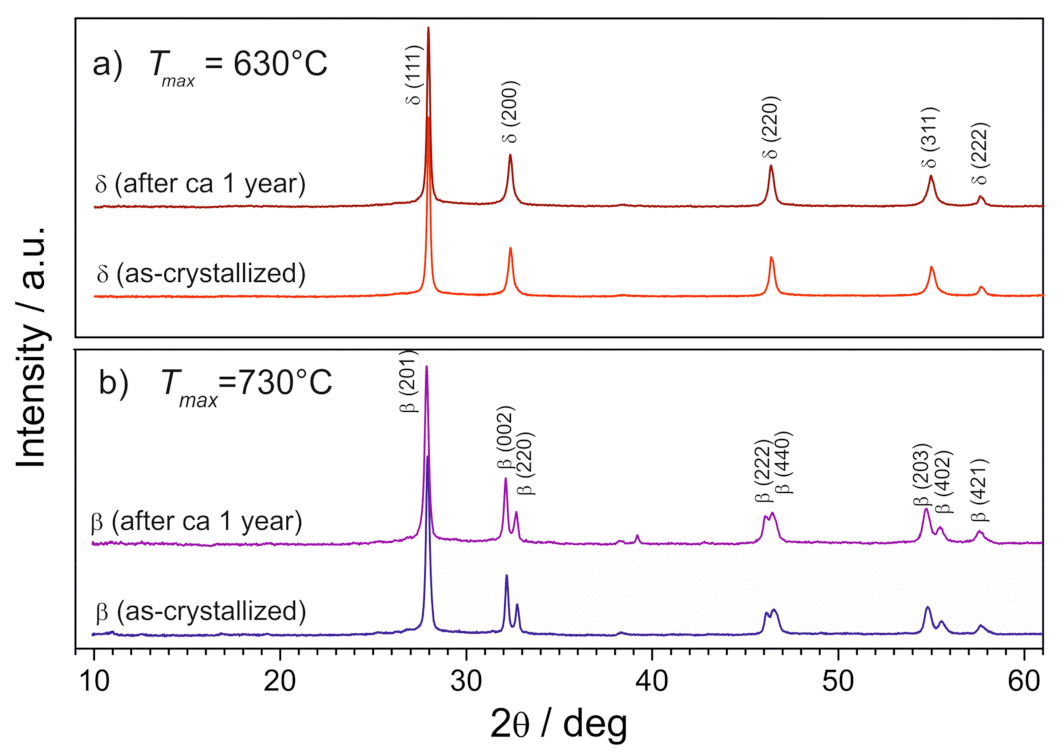
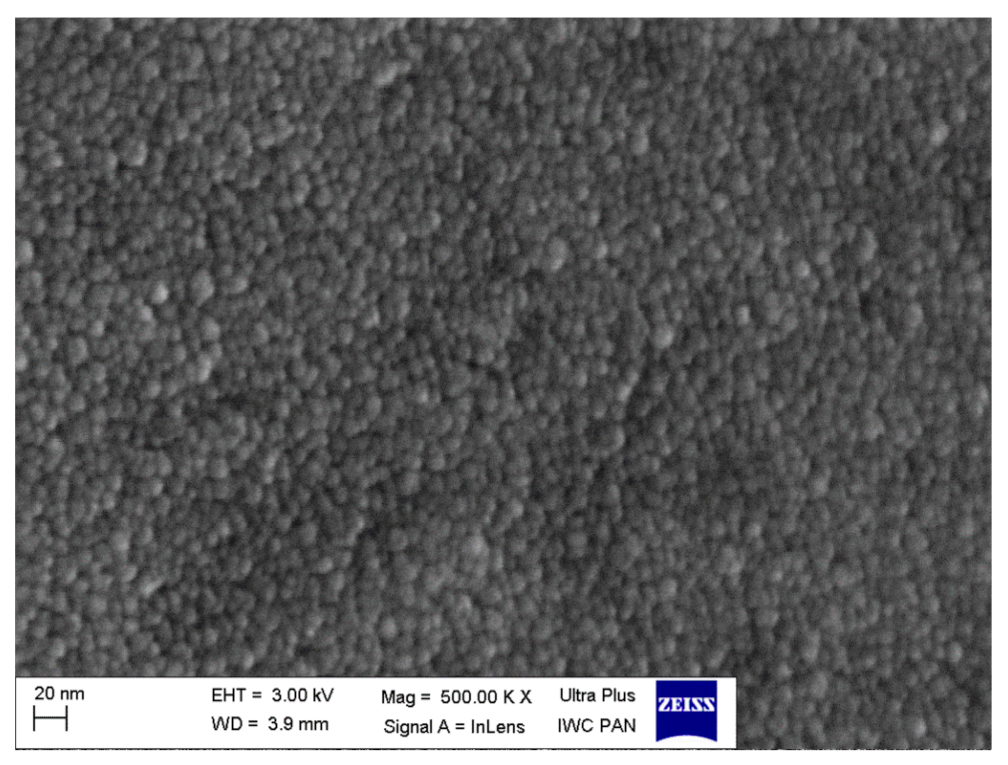
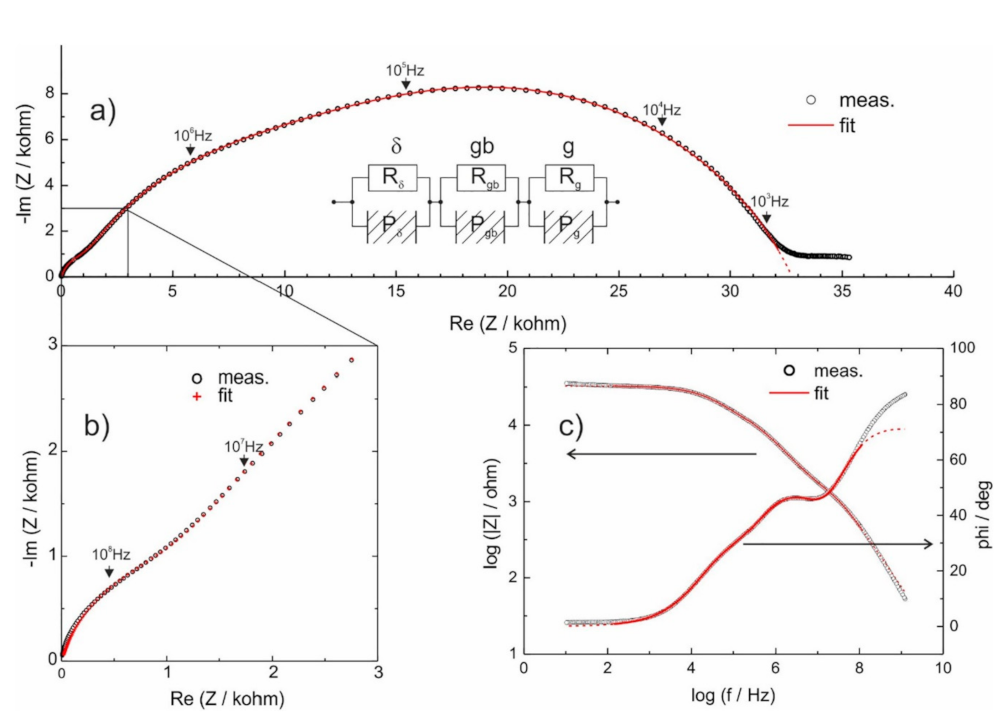
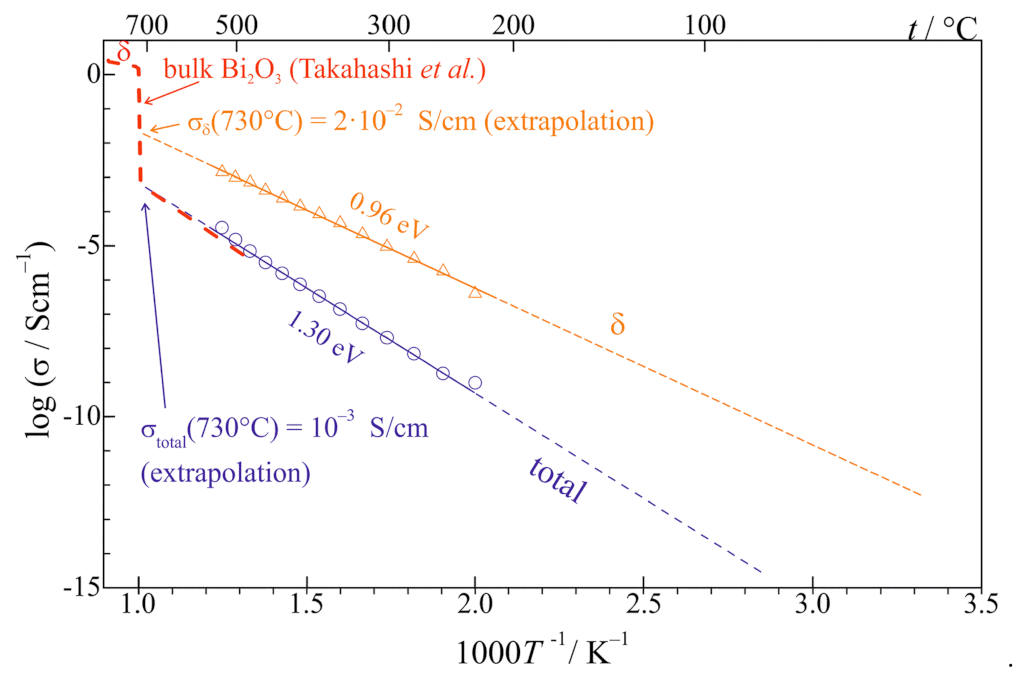
3.2. Stabilization of δ-Bi2O3 at Room Temperature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Cao, G.; Wang, G.; Liu, H.; Liu, J.; Qiao, S.; Lu, G.M.; Munroe, P.; Ahn, H.; Kundu, D.; et al. Mesoporous LiFePO4/C Nanocomposite Cathode Materials for High Power Lithium Ion Batteries with Superior Performance. Adv. Mater. 2009, 21, 9994–10024. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostructured Materials: Basic Concepts and Microstructure. Adv. Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Deubener, J.; Allix, M.; Davis, M.J.; Duran, A.; Höche, T.; Honma, T.; Komatsu, T.; Krüger, S.; Mitra, I.; Müller, R.; et al. Updated definition of glass-ceramics. J. Non. Cryst. Solids 2018, 501, 3–10. [Google Scholar] [CrossRef]
- Sakamoto, A.; Yamamoto, S. Glass-Ceramics: Engineering Principles and Applications. Int. J. Appl. Glas. Sci. 2010, 1, 237–247. [Google Scholar] [CrossRef]
- Comte, M. Commercial applications of glass-ceramics. In From Glass to Crystal: Nucleation, Growth and Phase Separation: From Research to Applications; Neuville, D.R., Cornier, L., Caurant, D., Montagne, L., Eds.; EDP Sciences: Les Ulis, France, 2017; ISBN 9780323393966. [Google Scholar]
- Kwatek, K.; Ślubowska, W.; Trébosc, J.; Lafon, O.; Nowiński, J.L. Structural and electrical properties of ceramic Li-ion conductors based on Li1.3Al0.3Ti1.7(PO4)3-LiF. J. Eur. Ceram. Soc. 2020, 40, 85–93. [Google Scholar] [CrossRef]
- Liang, C.C. Conduction Characteristics of the Lithium Iodide-Aluminum Oxide Solid Electrolytes. J. Electrochem. Soc. 1973, 120, 1289–1292. [Google Scholar] [CrossRef]
- Maier, J. Ionic Conduction In Space Charge Regions. Prog. Solid State Chem. 1995, 23, 171–263. [Google Scholar] [CrossRef]
- Islam, M.M.; Bredow, T.; Indris, S.; Heitjans, P. Enhanced conductivity at the interface of Li2O:B2O3 nanocomposites: Atomistic models. Phys. Rev. Lett. 2007, 99, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Schirmeisen, A.; Taskiran, A.; Fuchs, H.; Bracht, H.; Murugavel, S.; Roling, B. Fast interfacial ionic conduction in nanostructured glass ceramics. Phys. Rev. Lett. 2007, 98, 1–4. [Google Scholar] [CrossRef]
- Knauth, P. Ionic and electronic conduction in nanostructured solids: Concepts and concerns, consensus and controversies. Solid State Ionics 2006, 177, 2495–2502. [Google Scholar] [CrossRef]
- Ulrich, M.; Bunde, A.; Indris, S.; Heitjans, P. Li ion transport and interface percolation in nano- and microcrystalline composites. Phys. Chem. Chem. Phys. 2004, 006, 3680–3683. [Google Scholar] [CrossRef]
- Adams, S.; Hariharan, K.; Maier, J. Crystallization in Fast Ionic Glassy Silver Oxysalt Systems. Solid State Phenom. 1994, 39/40, 285–288. [Google Scholar] [CrossRef]
- Adams, S.; Hariharan, K.; Maier, J. Interface effect on the silver ion conductivity during the crystallization of AgI-Ag2O-V2O5 glasses. Solid State Ionics 1995, 75, 193–201. [Google Scholar] [CrossRef]
- Takahashi, H.; Karasawa, T.; Sakuma, T.; Garbarczyk, J.E. Electrical conduction in the vitreous and crystallized Li2O-V2O5-P2O5 system. Solid State Ionics 2010, 181, 27–32. [Google Scholar] [CrossRef]
- Józwiak, P.; Garbarczyk, J.E.; Wasiucionek, M.; Gorzkowska, I.; Gendron, F.; Mauger, A.; Julien, C. The thermal stability, local structure and electrical properties of lithium-iron phosphate glasses. Mater. Sci. Pol. 2009, 27, 307–318. [Google Scholar]
- Garbarczyk, J.E.; Jozwiak, P.; Wasiucionek, M.; Nowinski, J.L. Enhancement of electrical conductivity in lithium vanadate glasses by nanocrystallization. Solid State Ionics 2004, 175, 691–694. [Google Scholar] [CrossRef]
- Foltyn, M.; Wasiucionek, M.; Garbarczyk, J.; Nowiński, J.L. Effect of nanocrystallization on electrical conductivity of glasses and composites of the AgI-Ag2O-B2O3 system. Solid State Ionics 2005, 176, 2137–2140. [Google Scholar] [CrossRef]
- Nowiński, J.L.; Mroczkowska, M.; Dygas, J.R.; Garbarczyk, J.E.; Wasiucionek, M. Electrical properties and crystallization processes in AgI-Ag2O-P2O5, [Ag2O]/[P2O5] = 3, glasses. Solid State Ionics 2005, 176, 1775–1779. [Google Scholar] [CrossRef]
- Minami, T.; Hayashi, A.; Tatsumisago, M. Recent progress of glass and glass-ceramics as solid electrolytes for lithium secondary batteries. Solid State Ionics 2006, 177, 2715–2720. [Google Scholar] [CrossRef]
- Hayashi, A.; Noi, K.; Sakuda, A.; Tatsumisago, M. Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat. Commun. 2012, 3, 855–856. [Google Scholar] [CrossRef]
- Bradtmüller, H.; Nieto-Muñoz, A.M.; Ortiz-Mosquera, J.F.; Rodrigues, A.C.M.; Eckert, H. Glass-to-crystal transition in the NASICON glass-ceramic system Na1+xAlxM2−x(PO4)3 (M.=Ge, Ti). J. Non. Cryst. Solids 2018, 489, 91–101. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structure. Mater. Res. Bull. 1976, 11, 203. [Google Scholar] [CrossRef]
- Anantharamulu, N.; Koteswara Rao, K.; Rambabu, G.; Vijaya Kumar, B.; Radha, V.; Vithal, M. A wide-ranging review on Nasicon type materials. J. Mater. Sci. 2011, 46, 2821–2837. [Google Scholar] [CrossRef]
- Honma, T.; Okamoto, M.; Togashi, T.; Ito, N.; Shinozaki, K.; Komatsu, T. Electrical conductivity of Na2O–Nb2O5–P2O5 glass and fabrication of glass–ceramic composites with NASICON type Na3Zr2Si2PO12. Solid State Ionics 2015, 269, 19–23. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Shinkuma, Y.; Minami, T. Stabilization of superionic α-Agl at room temperature in a glass matrix. Nature 1991, 354, 217–218. [Google Scholar] [CrossRef]
- Eckert, H.; Martins Rodrigues, A.C. Ion-conducting glass-ceramics for energy-storage applications. MRS Bull. 2017, 42, 206–212. [Google Scholar] [CrossRef]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.-H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef]
- Livage, J. Vanadium Pentoxide Gels. Chem. Mater. 1991, 3, 578–593. [Google Scholar] [CrossRef]
- Livage, J.; Sanchez, C. Vers une nanochimie douce et biomimétique. Actual. Chim. 2005, octobre-no, 72–76. [Google Scholar]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef]
- Tan, H.T.; Rui, X.; Sun, W.; Yan, Q.; Lim, T.M. Vanadium-based nanostructure materials for secondary lithium battery applications. Nanoscale 2015, 7, 14595–14607. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, T.K.; Garbarczyk, J.E.; Gorzkowska, I.; Wasiucionek, M.; Nowinski, J.L.; Gierlotka, S.; Jozwiak, P. Correlation between electrical properties and microstructure of nanocrystallized V2O5-P2O5 glasses. J. Power Sources 2009, 194, 73–80. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Pawliszak, L.; Michalski, P.P.; Wasiucionek, M.; Garbarczyk, J.E. Highly conductive 90V2O5·10P2O5 nanocrystalline cathode materials for lithium-ion batteries. Procedia Eng. 2014, 98, 28–35. [Google Scholar] [CrossRef][Green Version]
- Pietrzak, T.K.; Garbarczyk, J.E.; Wasiucionek, M.; Gorzkowska, I.; Nowinski, J.L.; Gierlotka, S. Electrical properties vs. microstructure of nanocrystallized V2O5–P2O5 glasses—An extended temperature range study. Solid State Ionics 2011, 192, 210–214. [Google Scholar] [CrossRef]
- Patete, J.M.; Scofield, M.E.; Volkov, V.; Koenigsmann, C.; Zhang, Y.; Marschilok, A.C.; Wang, X.; Bai, J.; Han, J.; Wang, L.; et al. Ambient synthesis, characterization, and electrochemical activity of LiFePO4 nanomaterials derived from iron phosphate intermediates. Nano Res. 2015, 8, 2573–2594. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Wasiucionek, M.; Nowiński, J.L.; Garbarczyk, J.E. Isothermal nanocrystallization of vanadate–phosphate glasses. Solid State Ionics 2013, 251, 78–82. [Google Scholar] [CrossRef]
- Clavaguera-Mora, M.T.; Clavaguera, N.; Crespo, D.; Pradell, T. Crystallisation kinetics and microstructure development in metallic systems. Prog. Mater. Sci. 2002, 2002 47, 559–619. [Google Scholar] [CrossRef]
- Ho Lam, R.S.; Rogers, M.A. Experimental validation of the modified Avrami model for non-isothermal crystallization conditions. CrystEngComm 2011, 13, 866–875. [Google Scholar] [CrossRef]
- Sun, L.; Huang, X.; Wang, L.; Janotti, A. Disentangling the role of small polarons and oxygen vacancies in CeO2. Phys. Rev. B 2017, 95, 1–8. [Google Scholar] [CrossRef]
- Austin, I.G.; Mott, N.F. Polarons in crystalline and non-crystalline materials. Adv. Phys. 1969, 18, 41–102. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamya, K.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Prosini, P.P. Iron Phosphate Materials as Cathodes for Lithium Batteries; Springer: London, UK, 2011; ISBN 9780857297457. [Google Scholar]
- Pietrzak, T.K.; Wasiucionek, M.; Gorzkowska, I.; Nowiński, J.L.; Garbarczyk, J.E. Novel vanadium-doped olivine-like nanomaterials with high electronic conductivity. Solid State Ionics 2013, 251, 40–46. [Google Scholar] [CrossRef]
- Garbarczyk, J.E.; Pietrzak, T.K.; Wasiucionek, M.; Kaleta, A.; Dorau, A.; Nowiński, J.L. High electronic conductivity in nanostructured materials based on lithium-iron-vanadate-phosphate glasses. Solid State Ionics 2015, 272, 53–59. [Google Scholar] [CrossRef]
- Hirose, K.; Honma, T.; Doi, Y.; Hinatsu, Y.; Komatsu, T. Mössbauer analysis of Fe ion state in lithium iron phosphate glasses and their glass-ceramics with olivine-type LiFePO4 crystals. Solid State Commun. 2008, 146, 273–277. [Google Scholar] [CrossRef]
- Kaleta, A.; Dłużewski, P.; Wasiucionek, M.; Pietrzak, T.K.; Nowiński, J.L.; Michalski, P.P.; Garbarczyk, J.E. TEM studies on thermally nanocrystallized vanadium-containing glassy analogs of LiFePO4 olivine. Mater. Charact. 2017, 127, 214–221. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Wasiucionek, M.; Michalski, P.P.; Kaleta, A.; Garbarczyk, J.E. Highly conductive cathode materials for Li-ion batteries prepared by thermal nanocrystallization of selected oxide glasses. Mater. Sci. Eng. B 2016, 213, 140–147. [Google Scholar] [CrossRef]
- Frąckiewicz, J.E.; Pietrzak, T.K.; Wasiucionek, M.; Garbarczyk, J.E. Synthesis and characterization of highly-conducting nanocrystallized Li(Fe1–xMnx)0.88V0.08PO4 cathode materials (x = 0.25, 0.5, 0.75). ECS Trans. 2017, 80, 325–330. [Google Scholar] [CrossRef]
- Michalski, P.P.; Pietrzak, T.K.; Nowiński, J.L.; Wasiucionek, M.; Garbarczyk, J.E. Novel nanocrystalline mixed conductors based on LiFeBO3 glass. Solid State Ionics 2017, 302, 40–44. [Google Scholar] [CrossRef]
- Abouimrane, A.; Armand, M.; Ravet, N. Carbon Nano-Painting: Application to non-Phosphate Oxyanions, e.g. Borates. Proc. Electrochem. Soc. 2003, 20, 15–22. [Google Scholar]
- Michalski, P.P.; Gołębiewska, A.; Trébosc, J.; Lafon, O.; Pietrzak, T.K.; Ryl, J.; Nowiński, J.L.; Wasiucionek, M.; Garbarczyk, J.E. Properties of LiMnBO3 glasses and nanostructured glass-ceramics. Solid State Ionics 2019, 334, 88–94. [Google Scholar] [CrossRef]
- Jarocka, A.; Michalski, P.P.; Ryl, J.; Wasiucionek, M.; Garbarczyk, J.E.; Pietrzak, T.K. Synthesis, thermal, structural and electrical properties of vanadium-doped lithium-manganese-borate glass and nanocomposites. Ionics 2020, 26, 1275–1283. [Google Scholar] [CrossRef]
- El-Desoky, M.M. Giant electrical conductivity enhancement in BaO-V2O5-Bi2O3 glass by nanocrystallization. Mater. Chem. Phys. 2010, 119, 389–394. [Google Scholar] [CrossRef]
- El-Desoky, M.M.; Ibrahim, F.A.; Mostafa, A.G.; Hassaan, M.Y. Effect of nanocrystallization on the electrical conductivity enhancement and Mössbauer hyperfine parameters of iron based glasses. Mater. Res. Bull. 2010, 45, 1122–1126. [Google Scholar] [CrossRef]
- Al-Assiri, M.S.; El-Desoky, M.M. Nanocrystallization as a method of improvement of electrical properties of Fe2O3–PbO2–TeO2 glasses. J. Mater. Sci. Mater. Electron. 2014, 25, 3703–3711. [Google Scholar] [CrossRef]
- Al-Assiri, M.S.; El-Desoky, M.M. Correlation between nanostructural and electrical properties of barium titanate-based glass-ceramic nano-composites. J. Alloys Compd. 2011, 509, 8937–8943. [Google Scholar] [CrossRef]
- Bahgat, A.A.; Heikal, S.; Mahdy, I.A.; Abd-Rabo, A.S.; Abdel Ghany, A. Cyclic electrical conductivity in BaTiO3–PbTiO3–V2O5 glass-ceramic nanocomposite. Phys. B Condens. Matter 2014, 447, 23–29. [Google Scholar] [CrossRef]
- Renka, S.; Klaser, T.; Burazer, S.; Mošner, P.; Kalenda, P.; Šantić, A.; Moguš-Milanković, A. High electronically conductive tungsten phosphate glass-ceramics. Nanomaterials 2020, 10, 2515. [Google Scholar] [CrossRef] [PubMed]
- Pavić, L.; Skoko, Ž.; Gajović, A.; Su, D.; Moguš-Milanković, A. Electrical transport in iron phosphate glass-ceramics. J. Non. Cryst. Solids 2018, 502, 44–53. [Google Scholar] [CrossRef]
- Burley, G. Polymorphism of Silver Iodide. Am. Mineral. 1963, 48, 1266–1276. [Google Scholar]
- Drache, M.; Roussel, P.; Wignacourt, J.-P. Structures and Oxide Mobility in Bi−Ln−O Materials: Heritage of Bi2O3. Chem. Rev. 2007, 107, 80–96. [Google Scholar] [CrossRef]
- Lee, J.S.; Adams, S.; Maier, J. Defect chemistry and transport characteristics of beta-AgI. J. Phys. Chem. Solids 2000, 61, 1607–1622. [Google Scholar]
- Saito, T.; Torata, N.; Tatsumisago, M.; Minami, T. Microstructure of Superionic Composites Composed of Frozen a-AgI and AgI-Based Glasses Prepared by Rapid Quenching. J. Phys. Chem. 1995, 99, 10691–10693. [Google Scholar] [CrossRef]
- Funke, K.; Banhatti, R.D.D.; Grabowski, P.; Nowinski, J.; Wrobel, W.; Dinnebier, R.; Magdysyuk, O. Low-temperature α-AgI confined in glass: Structure and dynamics. Solid State Ionics 2015, 271, 2–9. [Google Scholar] [CrossRef]
- Makiura, R.; Yonemura, T.; Yamada, T.; Yamauchi, M.; Ikeda, R.; Kitagawa, H.; Kato, K.; Takata, M. Size-controlled stabilization of the superionic phase to room temperature in polymer-coated AgI nanoparticles. Nat. Mater. 2009, 8, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Terabe, K.; Hasegawa, T.; Aono, M.; Iyi, N. Anomalous phase transition and ionic conductivity of AgI nanowire grown using porous alumina template. J. Appl. Phys. 2007, 102, 1–5. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef]
- Sammes, N.M.; Tompsett, G.A.; Näfe, H.; Aldinger, F. Bismuth based oxide electrolytes— structure and ionic conductivity. J. Eur. Ceram. Soc. 1999, 19, 1801–1826. [Google Scholar] [CrossRef]
- Borowska-Centkowska, A.; Leszczyńska, M.; Wróbel, W.; Małys, M.; Kryński, M.; Hull, S.; Krok, F.; Abrahams, I. Structure and conductivity in tungsten doped δ-Bi3YO6. Solid State Ionics 2017, 308, 61–67. [Google Scholar] [CrossRef]
- Abrahams, I.; Liu, X.; Hull, S.; Norberg, S.T.; Krok, F.; Kozanecka-Szmigiel, A.; Islam, M.S.; Stokes, S.J. A combined total scattering and simulation approach to analyzing defect structure in Bi3YO6. Chem. Mater. 2010, 22, 4435–4445. [Google Scholar] [CrossRef]
- Borowska-Centkowska, A.; Liu, X.; Krynski, M.; Leszczynska, M.; Wrobel, W.; Malys, M.; Hull, S.; Norberg, S.T.; Krok, F.; Abrahams, I. Defect structure in δ-Bi5PbY2O11.5. RSC Adv. 2019, 9, 9640–9653. [Google Scholar] [CrossRef]
- Ahila, M.; Dhanalakshmi, J.; Selvakumari, J.C.; Padiyan, D.P. Heat treatment effect on crystal structure and design of highly sensitive room temperature CO2 gas sensors using anodic Bi2O3 nanoporous formed in a citric acid electrolyte. Mater. Res. Express 2016, 3, 105025. [Google Scholar] [CrossRef]
- Dapčević, A.; Poleti, D.; Rogan, J.; Radojković, A.; Radović, M.; Branković, G. A new electrolyte based on Tm3+-doped δ-Bi2O3-type phase with enhanced conductivity. Solid State Ionics 2015, 280, 18–23. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Switzer, J.A.; Shumsky, M.D.; Bohannan, E.W. Electrodeposited ceramic single crystals. Science 1999, 284, 293–296. [Google Scholar] [PubMed]
- Gomez, C.L.; Depablos-Rivera, O.; Medina, J.C.; Silva-Bermudez, P.; Muhl, S.; Zeinert, A.; Rodil, S.E. Stabilization of the delta-phase in Bi2O3 thin films. Solid State Ionics 2014, 255, 147–152. [Google Scholar] [CrossRef]
- Sanna, S.; Esposito, V.; Andreasen, J.W.; Hjelm, J.; Zhang, W.; Kasama, T.; Simonsen, S.B.; Christensen, M.; Linderoth, S.; Pryds, N. Enhancement of the chemical stability in confined δ-Bi2O3. Nat. Mater. 2015, 14, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, T.K.; Garbarczyk, J.E.; Wasiucionek, M. Stabilization of superionic δ–Bi2O3 phase at room temperature by thermal nanocrystallization of bismuth oxide glasses. Solid State Ionics 2018, 323, 78–84. [Google Scholar] [CrossRef]
- Shelby, J.E.; Lopes, M. Introduction to Glass Science and Technology, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2005; ISBN 978-0-85404-639-3. [Google Scholar]
- Pietrzak, T.K. Multi-device software for impedance spectroscopy measurements with stabilization in low and high temperature ranges working under Linux environment. Ionics 2019, 25, 2445–2452. [Google Scholar] [CrossRef]
- Kežionis, A.; Kazlauskas, S.; Petrulionis, D.; Orliukas, A.F. Broadband Method for the Determination of Small Sample ’ s Electrical and Dielectric Properties at High Temperatures. IEEE Trans. Microw. Theory Tech. 2014, 62, 2456–2461. [Google Scholar] [CrossRef]
- Kežionis, A.; Kazlauskas, S. High temperature ultrabroadband impedance spectrometer. In Proceedings of the International Workshop on Impedance Spectroscopy—IWIS 2013, Chemnitz, Germany, 25–27 September 2013; pp. 32–33. [Google Scholar]
- Takahashi, T.; Iwahara, H. Oxide ion conductors based on bismuthsesquioxide. Mater. Res. Bull. 1978, 13, 1447–1453. [Google Scholar] [CrossRef]



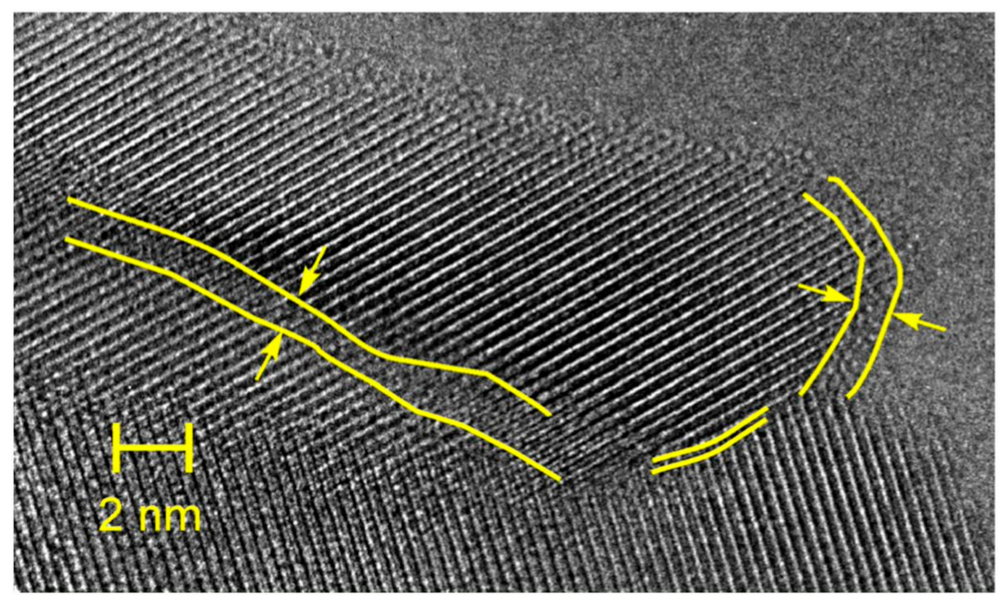
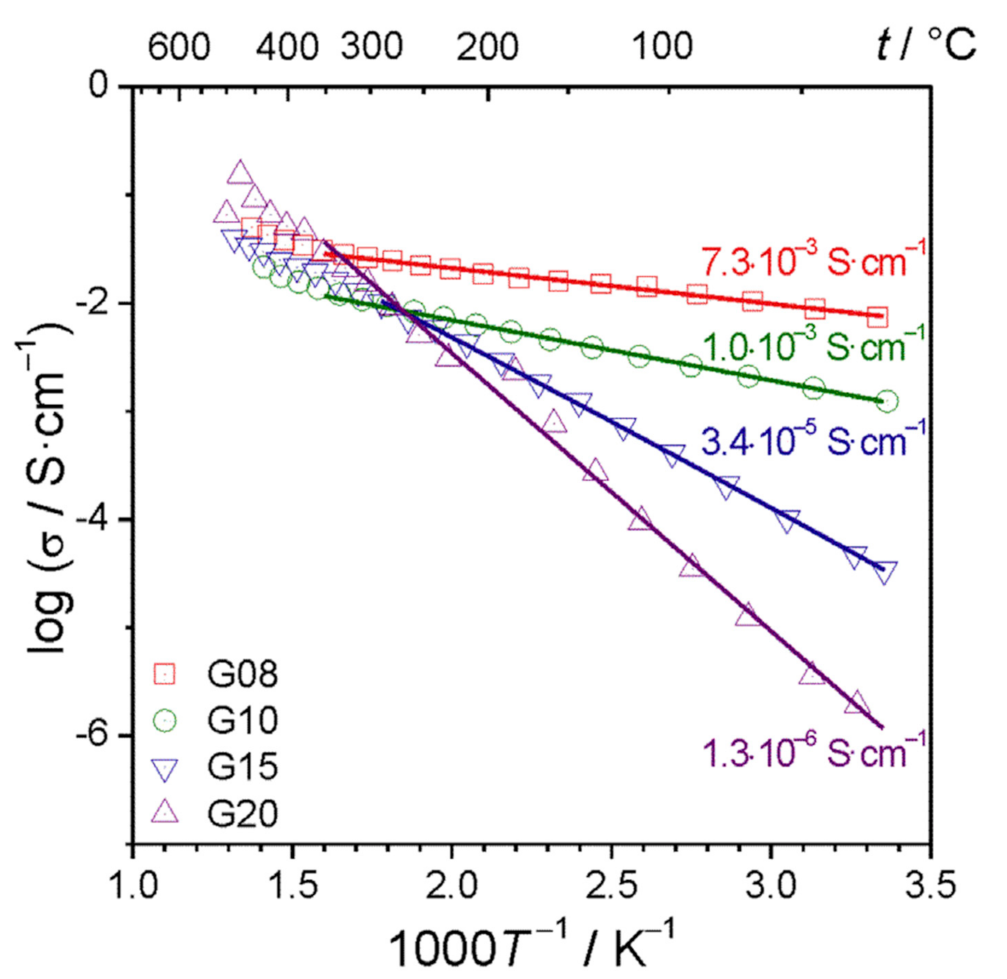
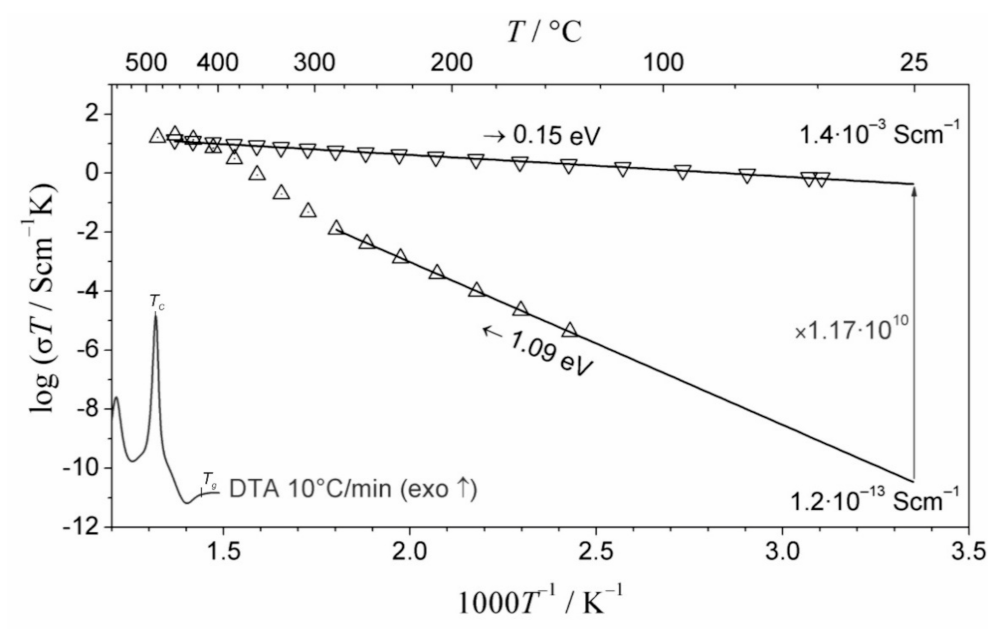
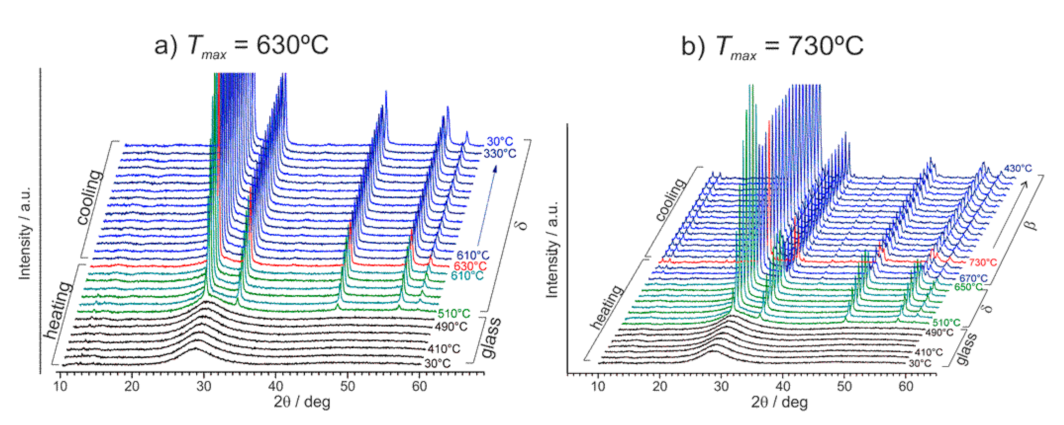
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrzak, T.K.; Wasiucionek, M.; Garbarczyk, J.E. Towards Higher Electric Conductivity and Wider Phase Stability Range via Nanostructured Glass-Ceramics Processing. Nanomaterials 2021, 11, 1321. https://doi.org/10.3390/nano11051321
Pietrzak TK, Wasiucionek M, Garbarczyk JE. Towards Higher Electric Conductivity and Wider Phase Stability Range via Nanostructured Glass-Ceramics Processing. Nanomaterials. 2021; 11(5):1321. https://doi.org/10.3390/nano11051321
Chicago/Turabian StylePietrzak, Tomasz K., Marek Wasiucionek, and Jerzy E. Garbarczyk. 2021. "Towards Higher Electric Conductivity and Wider Phase Stability Range via Nanostructured Glass-Ceramics Processing" Nanomaterials 11, no. 5: 1321. https://doi.org/10.3390/nano11051321
APA StylePietrzak, T. K., Wasiucionek, M., & Garbarczyk, J. E. (2021). Towards Higher Electric Conductivity and Wider Phase Stability Range via Nanostructured Glass-Ceramics Processing. Nanomaterials, 11(5), 1321. https://doi.org/10.3390/nano11051321