Eruption of Bioengineered Teeth: A New Approach Based on a Polycaprolactone Biomembrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrospun Nanofibers
2.3. Morphological Characterization of Electrospun Nanofibers
2.4. Tooth Bioengineering
2.5. In Vivo Micro-Surgical Protocol
2.6. Microtomography
2.7. Histology
2.8. Statistical Analysis
3. Results
3.1. Characterization of the Biomembrane
3.2. Tooth Development
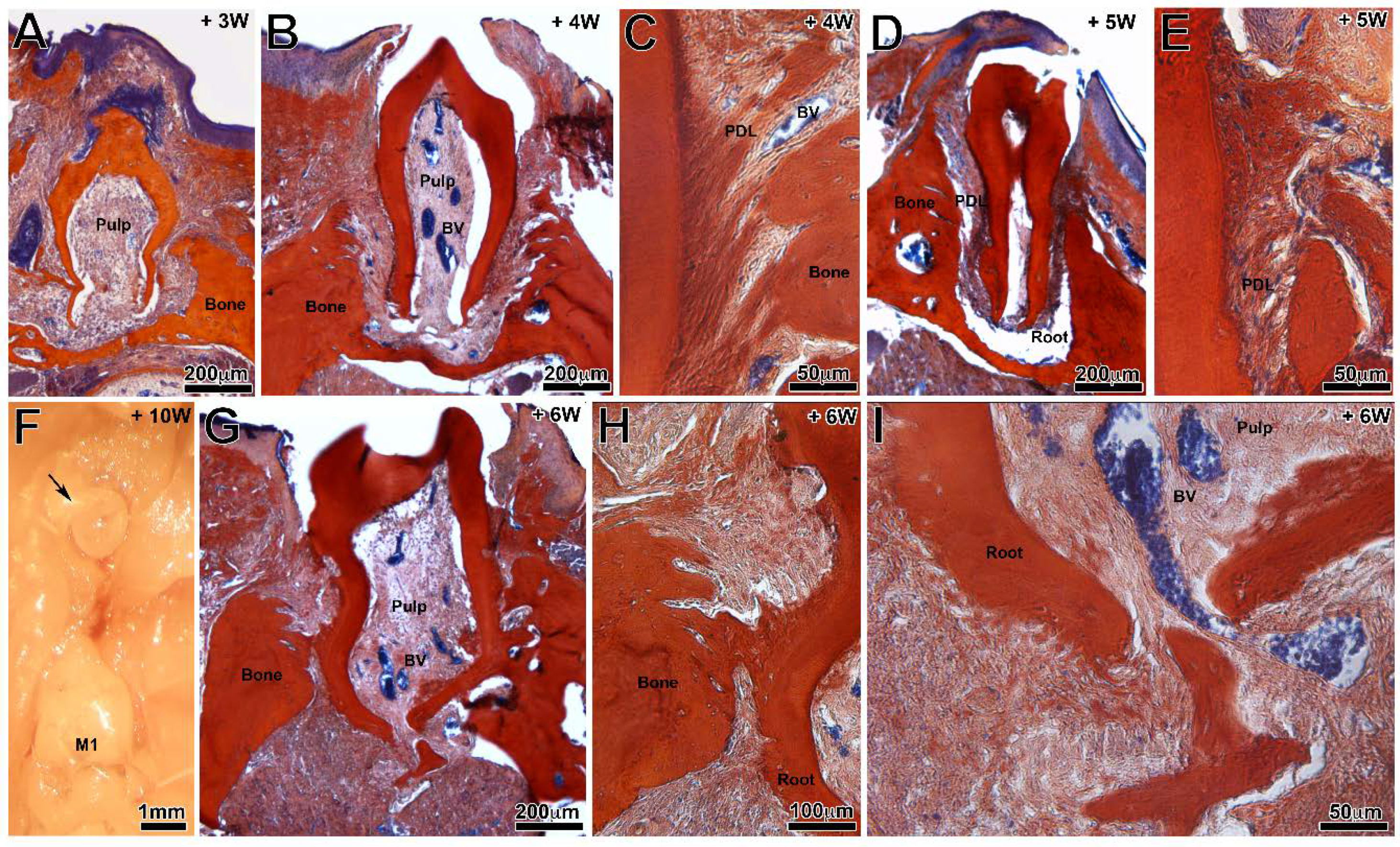
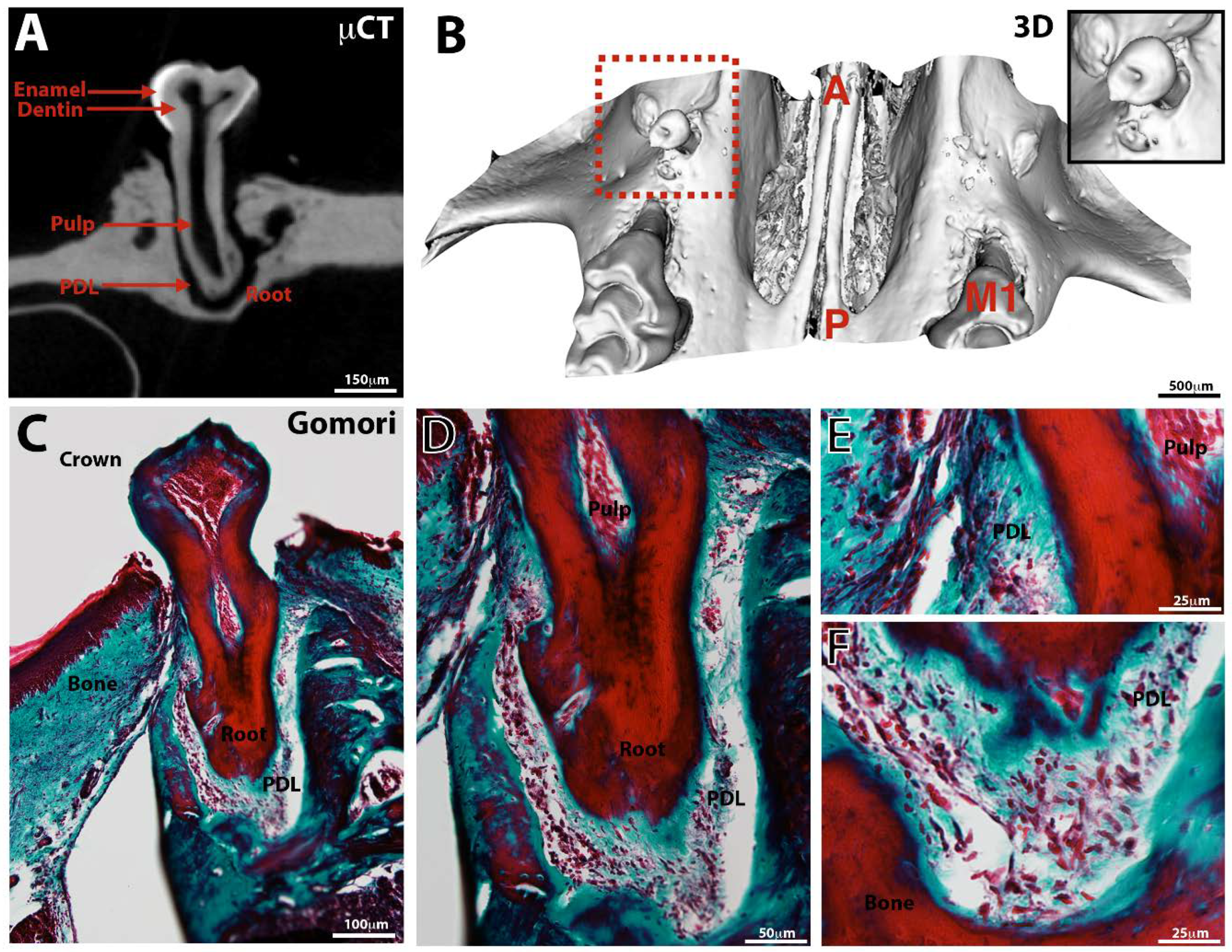
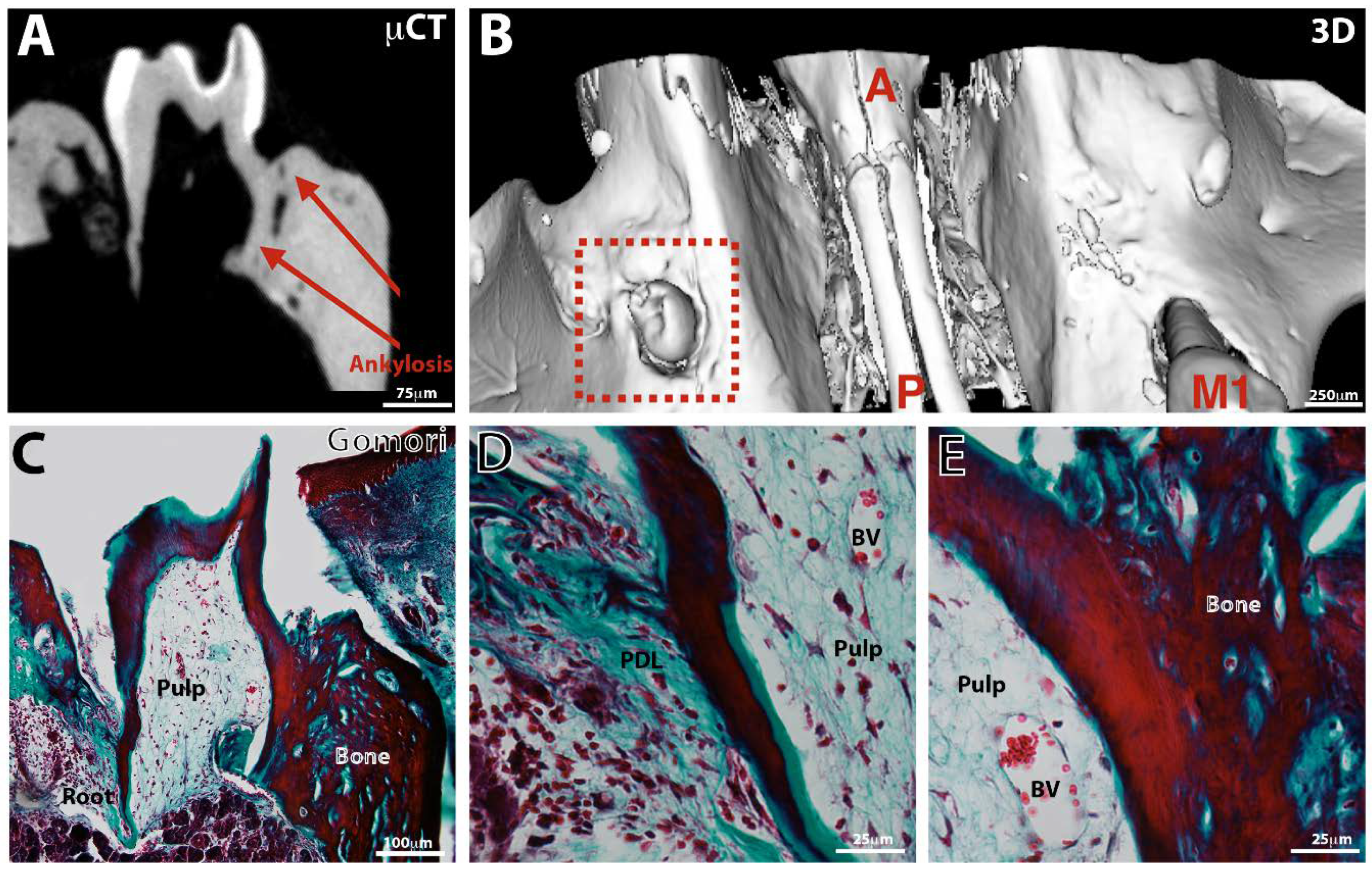
3.3. Tooth Eruption
4. Discussion
4.1. Tooth Germs Culture and Implantation in Jawbones
4.2. Long-Term Success Assessment
4.3. Comparison with Other Experimental Strategies for Bioengineered Tooth Eruption
4.4. Development Prospects for the Nanofibrous Membrane
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol 2000 2017, 73, 7–21. [Google Scholar] [CrossRef]
- Tenenbaum, H.; Bogen, O.; Séverac, F.; Elkaim, R.; Davideau, J.L.; Huck, O. Long-term prospective cohort study on dental implants: Clinical and microbiological parameters. Clin. Oral Implant. Res. 2017, 28, 86–94. [Google Scholar] [CrossRef]
- Gómez-de Diego, R.; Mang-de la Rosa, M.R.; Romero-Pérez, M.J.; Cutando-Soriano, A.; López-Valverde-Centeno, A. Indications and contraindications of dental implants in medically compromised patients: Update. Med. Oral Patol. Oral Cir. Bucal. 2014, 19, e483. [Google Scholar] [CrossRef] [PubMed]
- Bohner, L.; Hanisch, M.; Kleinheinz, J.; Jung, S. Dental implants in growing patients: A systematic review. Br. J. Oral Maxillofac. Surg. 2019, 57, 397–406. [Google Scholar] [CrossRef]
- Schnabl, D.; Grunert, I.; Schmuth, M.; Kapferer-Seebacher, I. Prosthetic rehabilitation of patients with hypohidrotic ectodermal dysplasia: A systematic review. J. Oral Rehabil. 2018, 45, 555–570. [Google Scholar] [CrossRef]
- Oshima, M.; Tsuji, T. Whole Tooth Regeneration as a Future Dental Treatment. Adv. Exp. Med. Biol. 2015, 881, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xie, L.; Yang, B.; Tian, W. Three-dimensional printing biotechnology for the regeneration of the tooth and tooth-supporting tissues. Biotechnol. Bioeng. 2019, 116, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Mizuno, M.; Imamura, A.; Ogawa, M.; Yasukawa, M.; Yamazaki, H.; Morita, R.; Ikeda, E.; Nakao, K.; Takano-Yamamoto, T.; et al. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS ONE 2011, 6, e21531. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Strub, M.; Petit, C.; Bugueno, I.M.; Bornert, F.; Clauss, F.; Huck, O.; Kuchler-Bopp, S.; Benkirane-Jessel, N. Periodontal Tissues, Maxillary Jaw Bone, and Tooth Regeneration Approaches: From Animal Models Analyses to Clinical Applications. Nanomaterials 2018, 16, 337. [Google Scholar] [CrossRef]
- Kuchler-Bopp, S.; Bagnard, D.; Van-der-Heyden, M.; Idoux-Gillet, Y.; Strub, M.; Gegout, H.; Lesot, H.; Benkirane-Jessel, N.; Keller, L. Semaphorin 3A receptor inhibitor as a novel therapeutic to promote innervation of bioengineered teeth. J. Tissue Eng. Reg. Med. 2018, 12, e2151–e2161. [Google Scholar] [CrossRef]
- Kuchler-Bopp, S.; Larrea, A.; Petry, L.; Idoux-Gillet, Y.; Sebastian, V.; Ferrandon, A.; Schwinté, P.; Arruebo, M.; Benkirane-Jessel, N. Promoting bioengineered tooth innervation using nanostructured and hybrid scaffolds. Acta Biomater. 2017, 50, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.C., Jr.; Schroeder, H.E. Tooth eruption: Theories and facts. Anat. Rec. 1996, 245, 374–393. [Google Scholar] [CrossRef]
- Lungová, V.; Radlanski, R.J.; Tucker, A.S.; Renz, H.; Míšek, I.; Matalová, E. Tooth-bone morphogenesis during postnatal stages of mouse first molar development. J. Anat. 2011, 218, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrota, D. Polycaprolactone as biomaterial for bone scaffold: Review of literature. J. Oral Biol. Craniofac. Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Morand, D.N.; Thoma, L.; Bugueno, I.M.; Aragon, J.; Irusta, S.; Keller, L.; Benkirane-Jessel, N.; Tenenbaum, H.; Huck, O. Synthesis of a Novel Electrospun Polycaprolactone Scaffold Functionalized with Ibuprofen for Periodontal Regeneration: An In Vitro andIn Vivo Study. Materials 2018, 11, 580. [Google Scholar] [CrossRef]
- Morand, D.N.; Huck, O.; Keller, L.; Jessel, N.; Tenenbaum, H.; Davideau, J.L. Active Nanofibrous Membrane Effects on Gingival Cell Inflammatory Response. Materials 2015, 8, 7217–7229. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Nazarnezhad, S.; Baino, F.; Kim, H.W.; Webster, T.J.; Kargozar, S. Electrospun Nanofibers for Improved Angiogenesis: Promises for Tissue Engineering Applications. Nanomaterials 2020, 10, 1609. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Monavari, M.; Koenen, B.; Boccaccini, A.R. Biomimetic biohybrid nanofibers containing bovine serum albumin as a bioactive moiety for wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111965. [Google Scholar] [CrossRef]
- Stutz, C.; Strub, M.; Clauss, F.; Huck, O.; Schulz, G.; Gegout, H.; Benkirane-Jessel, N.; Bornert, F.; Kuchler-Bopp, S. A new polycaprolactone-based biomembrane functionalized with BMP-2 and stem cells improves maxillary bone regeneration. Nanomaterials 2020, 10, 1774. [Google Scholar] [CrossRef]
- Cahill, D.R.; Marks, S.C. Tooth eruption: Evidence for the central role of the dental follicle. J. Oral Pathol. 1980, 9, 189–200. [Google Scholar] [CrossRef]
- Pan, K.; Sun, Q.; Zhang, J.; Ge, S.; Li, S.; Zhao, Y.; Yang, P. Multilineage differentiation of dental follicle cells and the roles of Runx2 over-expression in enhancing osteoblast/cementoblast-related gene expression in dental follicle cells. Cell Prolif. 2010, 43, 219–228. [Google Scholar] [CrossRef]
- Wise, G.E. Cellular and molecular basis of tooth eruption. Orthod. Craniofac. Res. 2009, 12, 67–73. [Google Scholar] [CrossRef]
- Zhou, T.; Pan, J.; Wu, P.; Huang, R.; Du, W.; Zhou, Y.; Wan, M.; Fan, Y.; Xu, X.; Zhou, X.; et al. Dental Follicle Cells: Roles in Development and Beyond. Stem Cells Int. 2019, 2019, 9159605. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Soto-Gutierrez, A.; Parekkadan, B.; Kitagawa, Y.; Tompkins, R.G.; Kobayashi, N.; Yarmush, M.L. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010, 19, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, B. Dental implants: A review. Morphologie 2016, 100, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Engelberg, I.; Kohn, J. Physico-mechanical properties of degradable polymers used in medical applications: A comparative study. Biomaterials 1991, 12, 292–304. [Google Scholar] [CrossRef]
- Grier, R.L.; Zhao, L.; Adams, C.E.; Wise, G.E. Secretion of CSF-1 and its inhibition in rat dental follicle cells: Implications for tooth eruption. Eur. J. Oral Sci. 1998, 106, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, M.; Bhattacharya, D.; Simoes-Costa, M. The molecular basis of neural crest axial identity. Dev. Biol. 2018, 444, S170–S180. [Google Scholar] [CrossRef] [PubMed]
- Cielinski, M.J.; Jolie, M.; Wise, G.E.; Marks, S.C., Jr. The contrasting effects of colonystimulating factor-1 and epidermal growth factor on tooth eruption in the rat. Connect. Tissue Res. 1995, 32, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Plakwicz, P.; Larsson, L.; Czochrowska, E.; Westerlund, A.; Ransjö, M. Study on site-specific expression of bone formation and resorption factors in human dental follicles. Eur. J. Oral Sci. 2018, 126, 439–448. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stutz, C.; Clauss, F.; Huck, O.; Schulz, G.; Benkirane-Jessel, N.; Bornert, F.; Kuchler-Bopp, S.; Strub, M. Eruption of Bioengineered Teeth: A New Approach Based on a Polycaprolactone Biomembrane. Nanomaterials 2021, 11, 1315. https://doi.org/10.3390/nano11051315
Stutz C, Clauss F, Huck O, Schulz G, Benkirane-Jessel N, Bornert F, Kuchler-Bopp S, Strub M. Eruption of Bioengineered Teeth: A New Approach Based on a Polycaprolactone Biomembrane. Nanomaterials. 2021; 11(5):1315. https://doi.org/10.3390/nano11051315
Chicago/Turabian StyleStutz, Céline, François Clauss, Olivier Huck, Georg Schulz, Nadia Benkirane-Jessel, Fabien Bornert, Sabine Kuchler-Bopp, and Marion Strub. 2021. "Eruption of Bioengineered Teeth: A New Approach Based on a Polycaprolactone Biomembrane" Nanomaterials 11, no. 5: 1315. https://doi.org/10.3390/nano11051315
APA StyleStutz, C., Clauss, F., Huck, O., Schulz, G., Benkirane-Jessel, N., Bornert, F., Kuchler-Bopp, S., & Strub, M. (2021). Eruption of Bioengineered Teeth: A New Approach Based on a Polycaprolactone Biomembrane. Nanomaterials, 11(5), 1315. https://doi.org/10.3390/nano11051315







