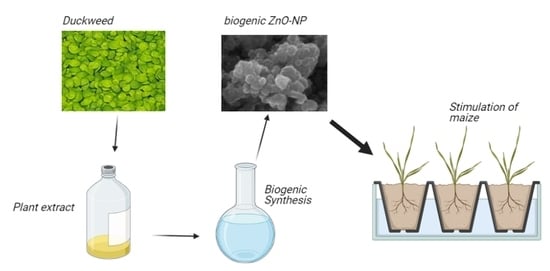
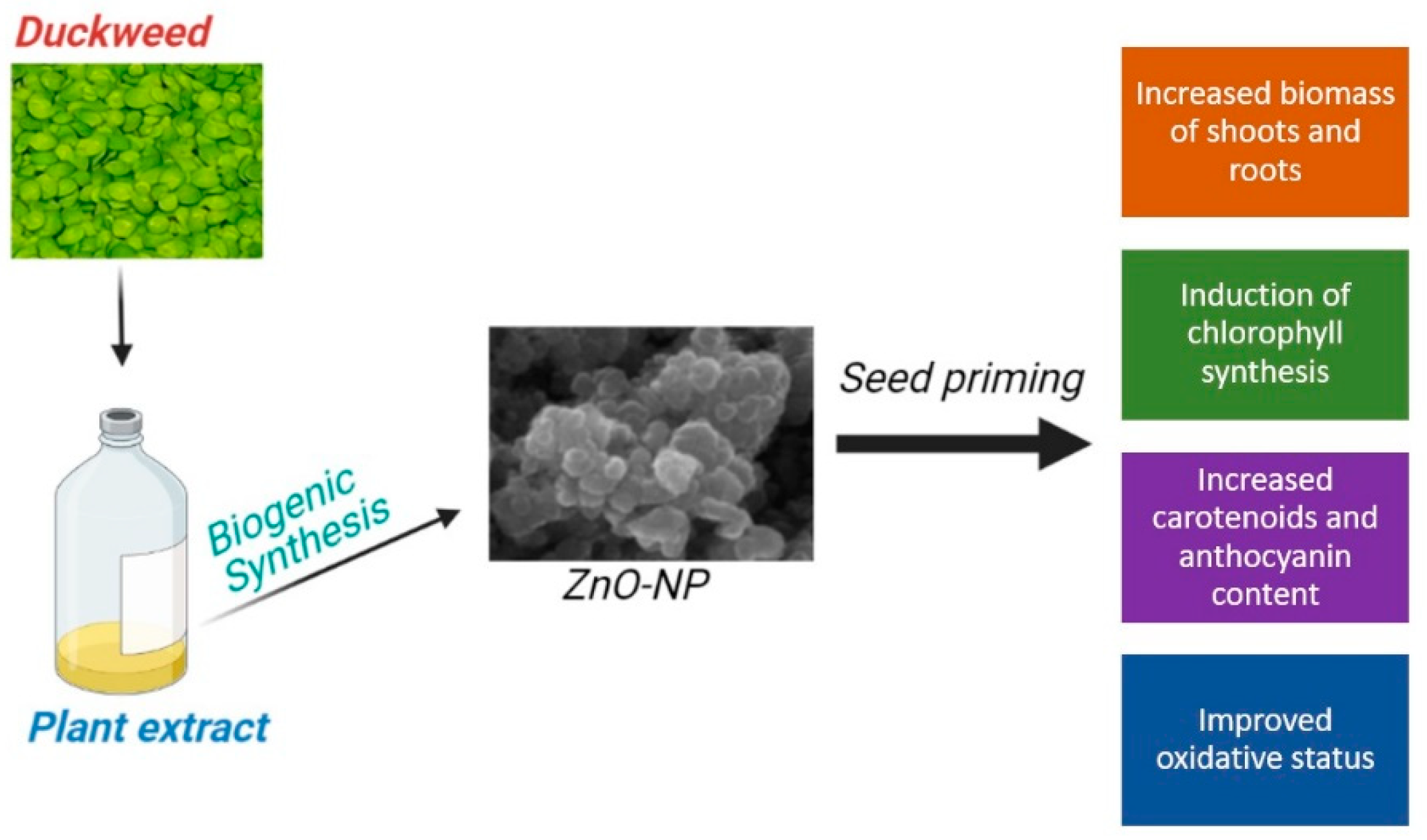
Biogenic ZnO Nanoparticles Synthesized Using a Novel Plant Extract: Application to Enhance Physiological and Biochemical Traits in Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Duckweed Growth Conditions
2.3. Preparation and Phytochemical Characterization of the Duckweed Extract
2.4. Synthesis of ZnO-NPs Using the Plant Extract (Biogenic ZnO-NPs) or Not (Non-Biogenic ZnO-NPs)
2.5. XRD, Fe-SEM, TEM and UV-vis Characterization of the ZnO-NPs Obtained
2.6. Maize Growth Condition and Treatments with Biogenic ZnO-NPs
2.7. Determination of Growth, Photosynthetic Pigments, Carotenoids and Anthocyanins of Maize Plants Treated with Biogenic ZnO-NPs
2.8. Malondialdehyde Content (MDA) in Treated Maize
2.9. Statistical Analysis
3. Results
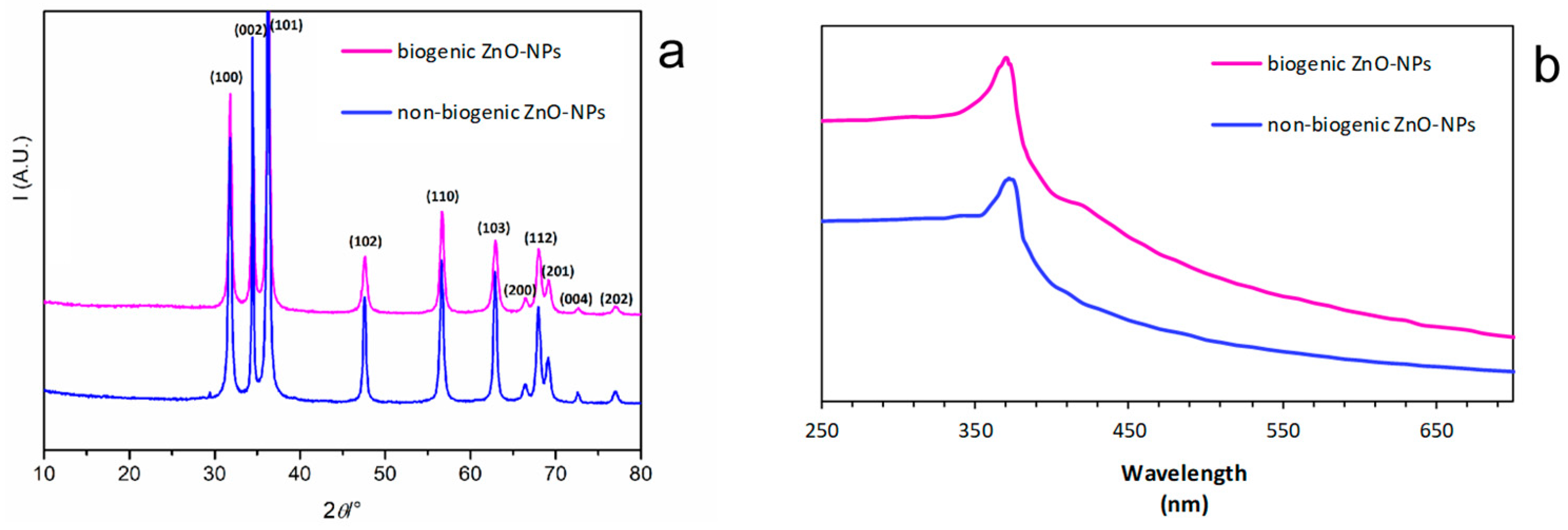
3.1. XRD Analysis of ZnO-NPs Synthesized with or without Duckweed Extract
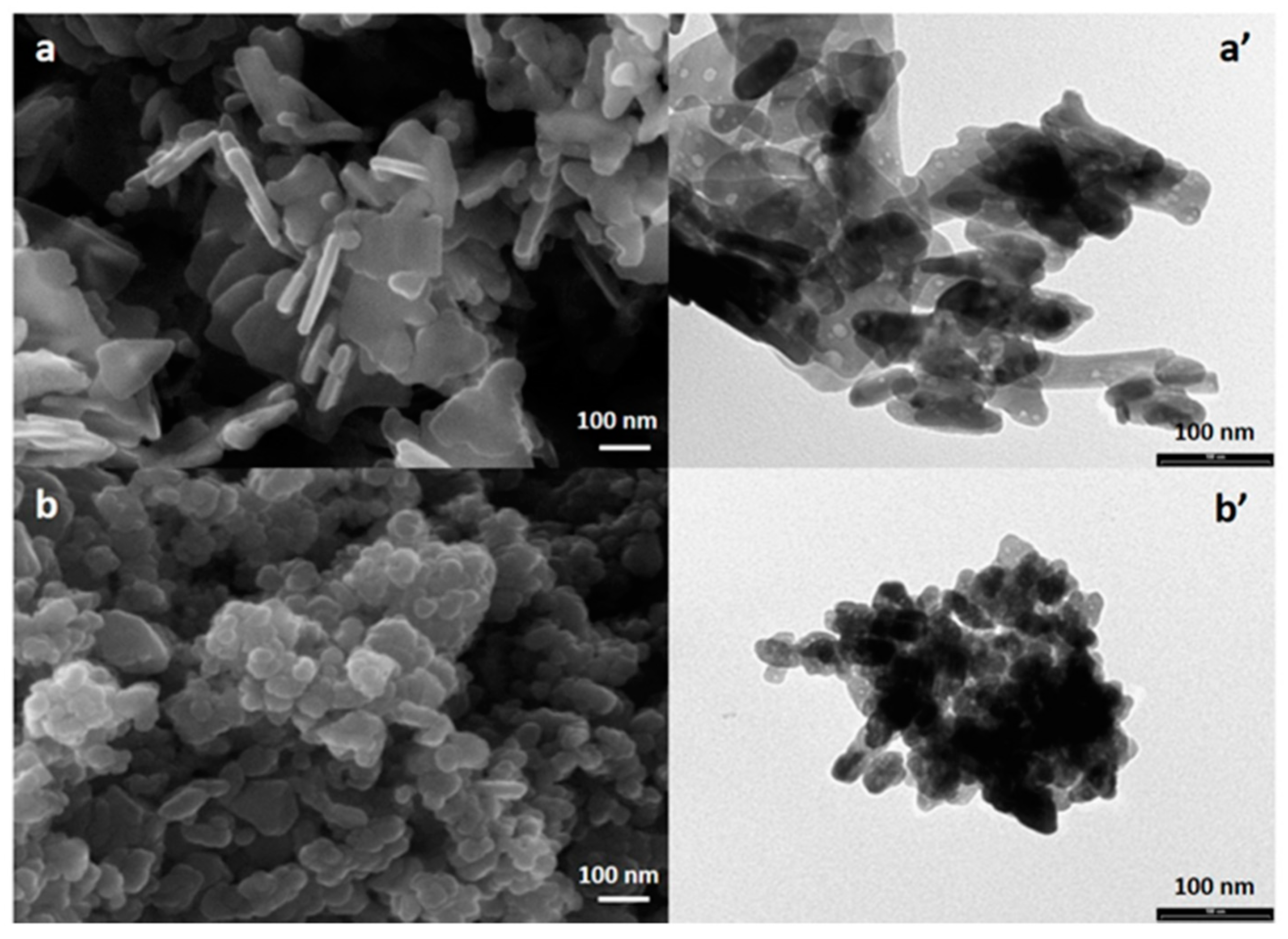
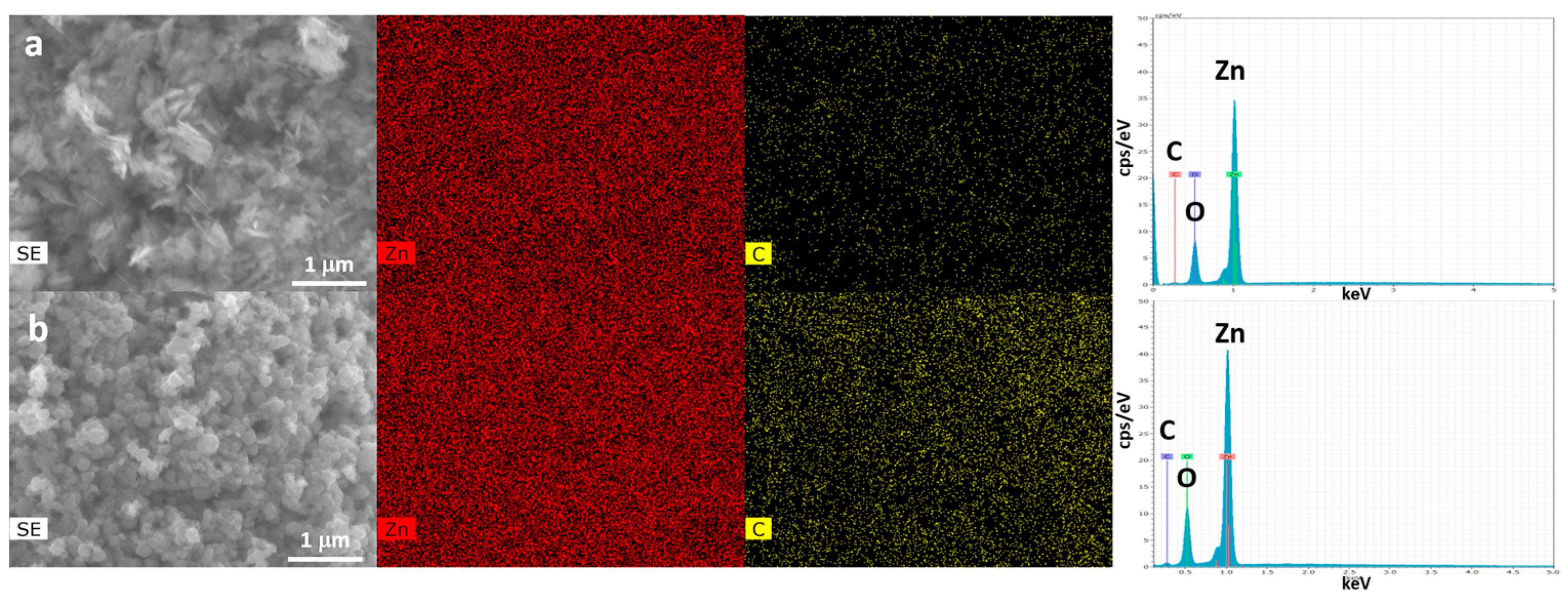
3.2. FE-SEM, EDX and TEM Analyses of ZnO-NPs Synthesized with or without Duckweed Extract
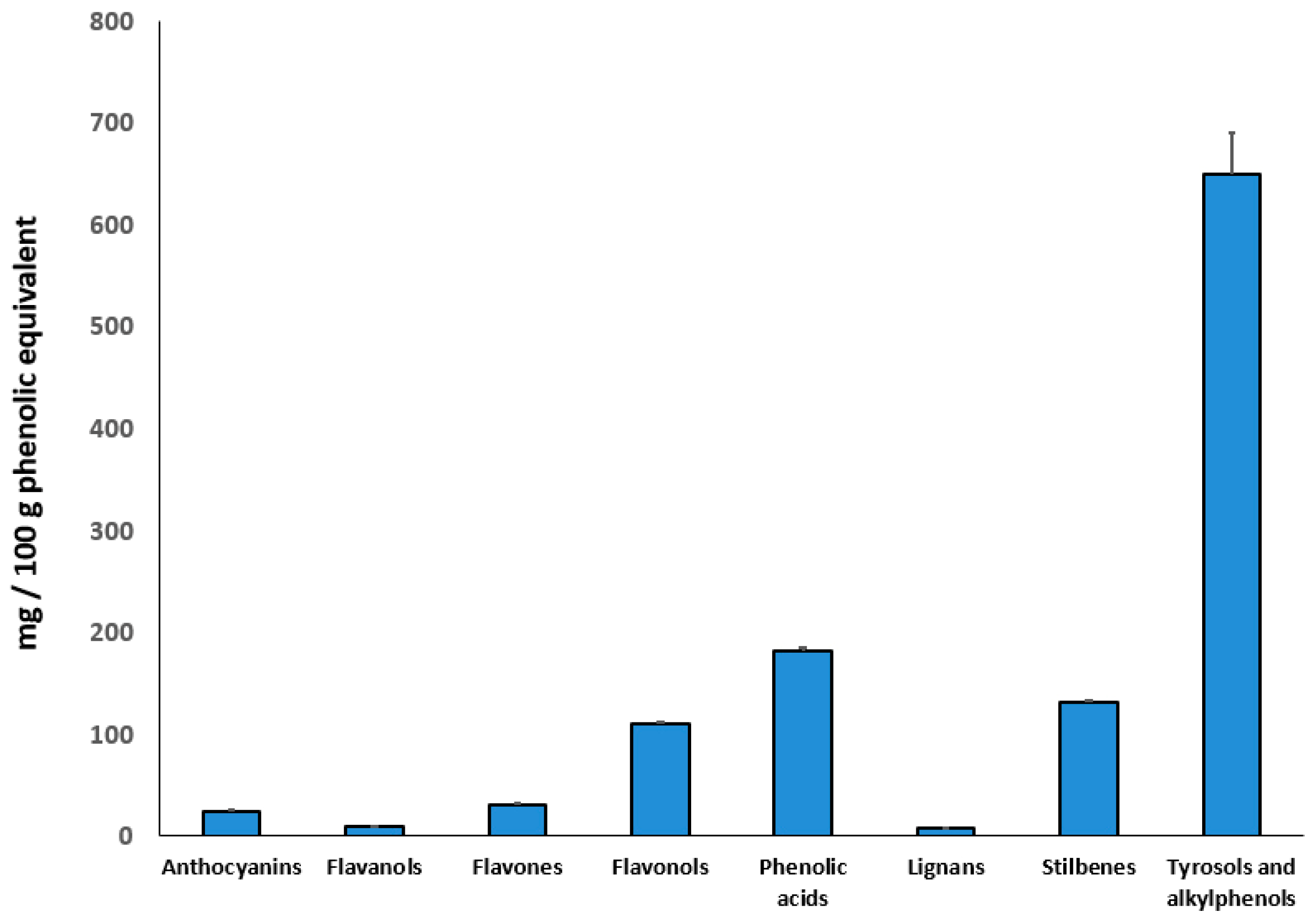
3.3. Duckweed Phenolic Characterization
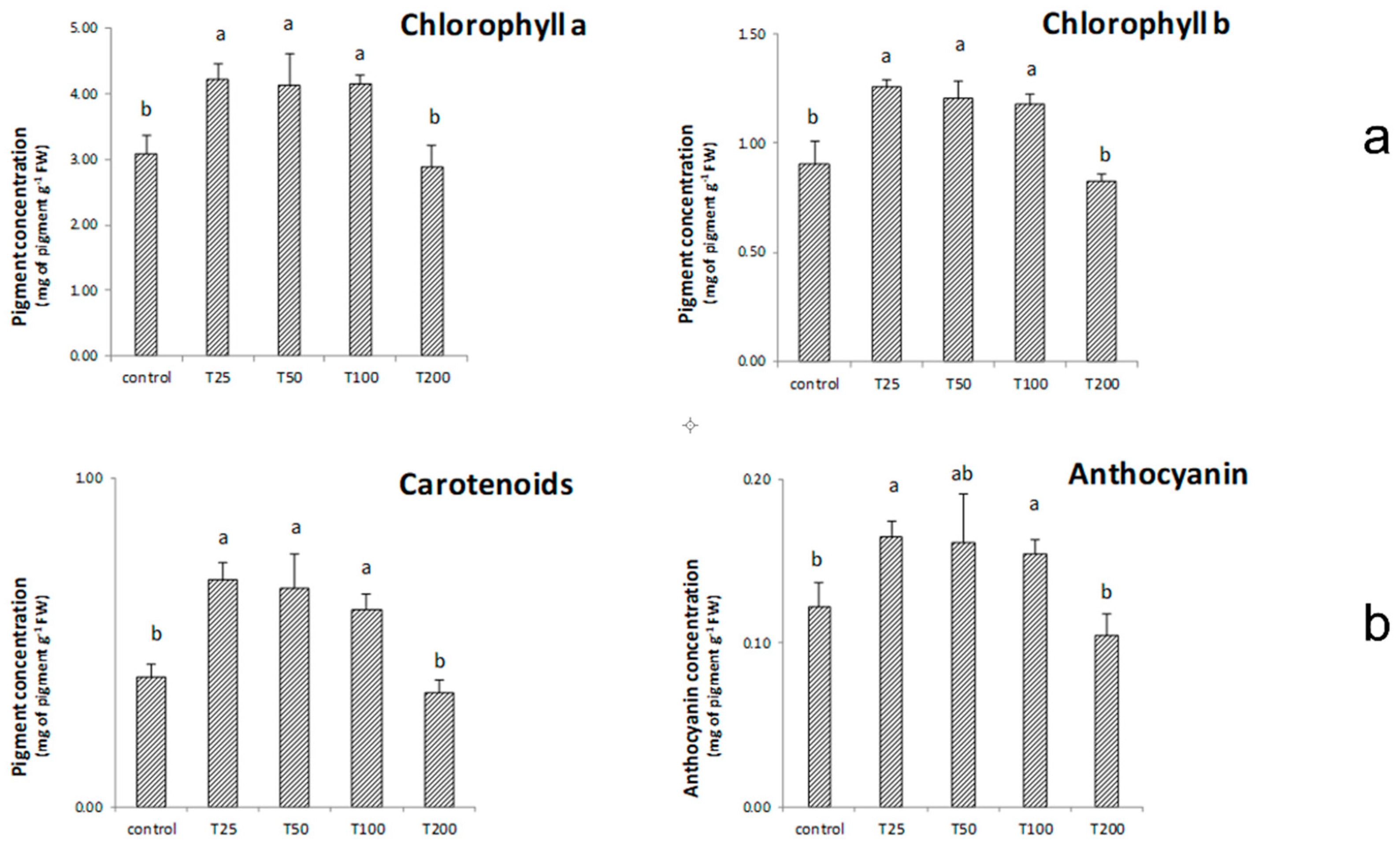
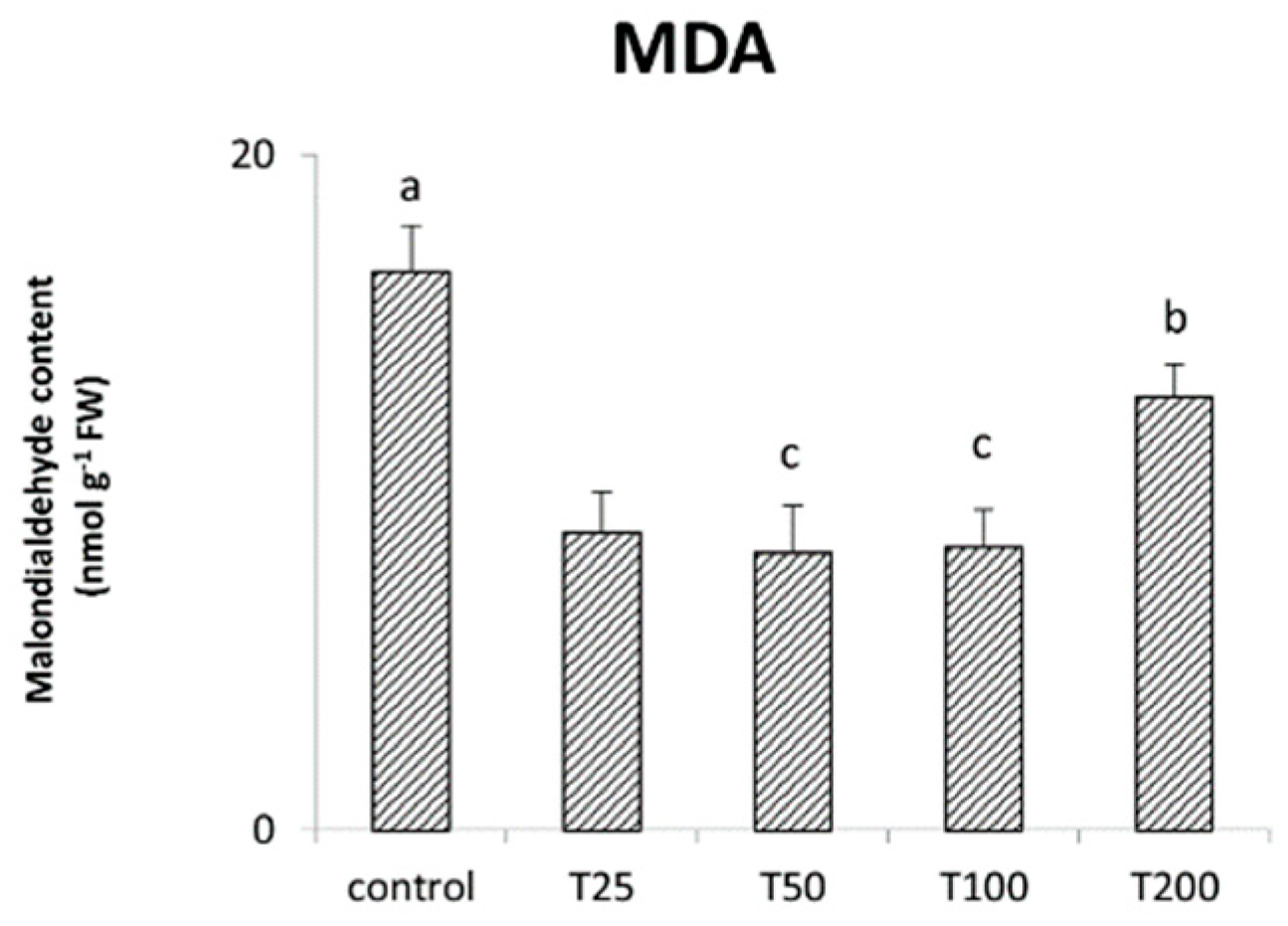
3.4. Effect of ZnO-NPs Synthesized Using Duckweed Extra on Maize Growth, Chlorophyll Content, Carotenoids, Anthocyanin and MDA
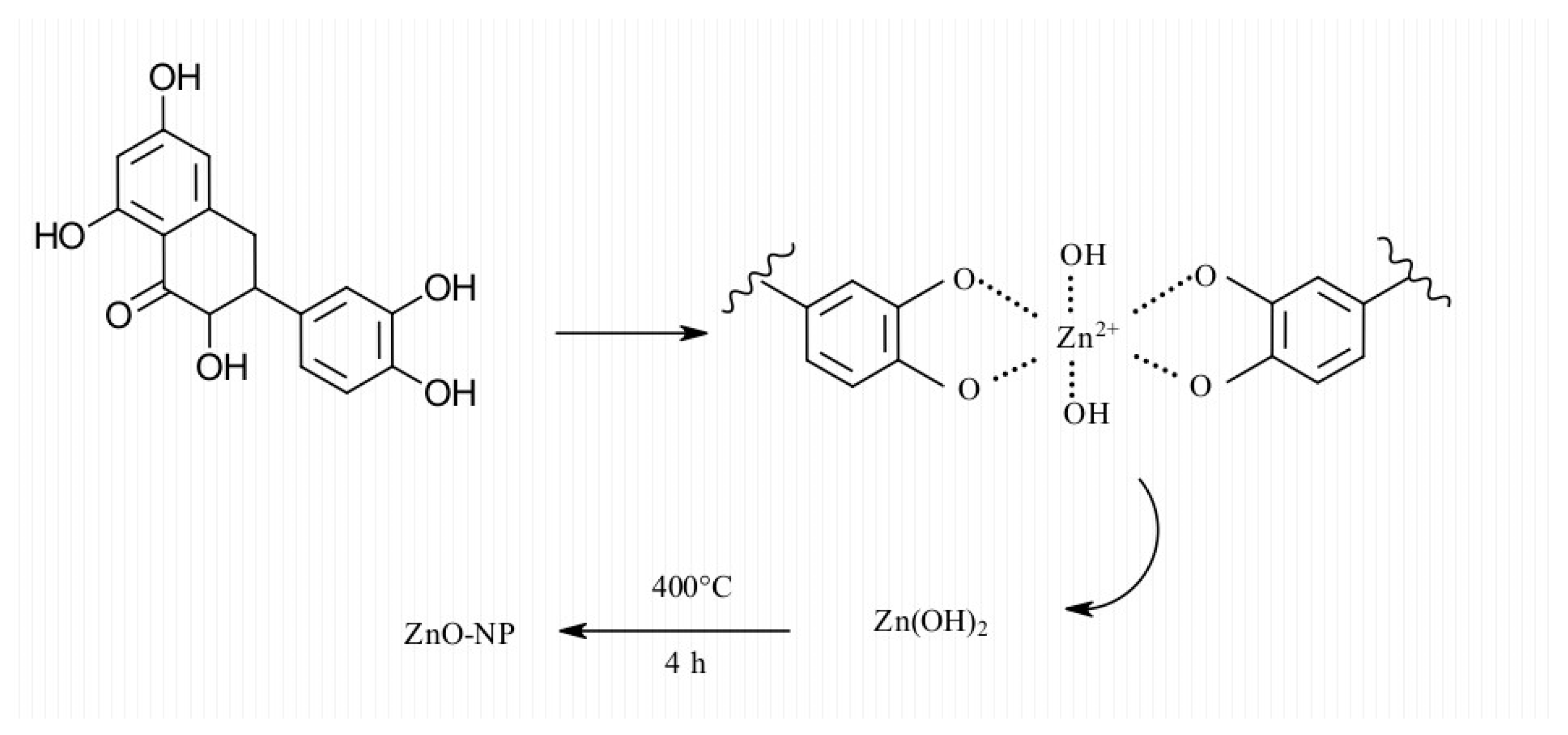
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Sanchez, A.; Santana, R.V.; Mariniello, L. Effect of Mesoporous Silica Nanoparticles on the Physicochemical Properties of Pectin Packaging Material for Strawberry Wrapping. Nanomaterials 2020, 10, 52. [Google Scholar] [CrossRef]
- Ahmed, S.; Chaudhry, S.A.; Ikram, S. A Review on Biogenic Synthesis of ZnO Nanoparticles Using Plant Extracts and Microbes: A Prospect towards Green Chemistry. J. Photochem. Photobiol. B 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Matussin, S.; Harunsani, M.H.; Tan, A.L.; Khan, M.M. Plant-Extract-Mediated SnO2 Nanoparticles: Synthesis and Applications. ACS Sustain. Chem. Eng. 2020, 8, 3040–3054. [Google Scholar] [CrossRef]
- Kasivelu, G.; Selvaraj, T.; Malaichamy, K.; Kathickeyan, D.; Shkolnik, D.; Chaturvedi, S. Nano-Micronutrients [γ-Fe2O3(Iron) and ZnO (Zinc)]: Green Preparation, Characterization, Agro-Morphological Characteristics and Crop Productivity Studies in Two Crops (Rice and Maize). New J. Chem. 2020, 44, 11373–11383. [Google Scholar] [CrossRef]
- Bilesky-José, N.; Maruyama, C.; Germano-Costa, T.; Campos, E.; Carvalho, L.; Grillo, R.; Fraceto, L.F.; De Lima, R. Biogenic α-Fe2O3 Nanoparticles Enhance the Biological Activity of Trichoderma against the Plant Pathogen Sclerotinia sclerotiorum. ACS Sustain. Chem. Eng. 2021, 9, 1669–1683. [Google Scholar] [CrossRef]
- Mazumder, J.A.; Khan, E.; Perwez, M.; Gupta, M.; Kumar, S.; Raza, K.; Sardar, M. Exposure of Biosynthesized Nanoscale ZnO to Brassica juncea Crop Plant: Morphological, Biochemical and Molecular Aspects. Sci. Rep. 2020, 10, 8531. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Wang, R.; Wang, R.; Zhang, P.; Ju, Q.; Xu, J. Physiological, Transcriptomic, and Metabolomic Analyses Reveal Zinc Oxide Nanoparticles Modulate Plant Growth in Tomato. Environ. Sci. Nano 2020, 7, 3587–3604. [Google Scholar] [CrossRef]
- Hessien, M.; Da’na, E.; Taha, A. Phytoextract Assisted Hydrothermal Synthesis of ZnO–NiO Nanocomposites Using Neem Leaves Extract. Ceram. Int. 2021, 47, 811–816. [Google Scholar] [CrossRef]
- Karnan, T.; Selvakumar, S.A.S. Biosynthesis of ZnO Nanoparticles Using Rambutan (Nephelium LappaceumL.) Peel Extract and Their Photocatalytic Activity on Methyl Orange Dye. J. Mol. Struct. 2016, 1125, 358–365. [Google Scholar] [CrossRef]
- Ambika, S.; Sundrarajan, M. Antibacterial Behaviour of Vitex negundo Extract Assisted ZnO Nanoparticles against Pathogenic Bacteria. J. Photochem. Photobiol. B 2015, 146, 52–57. [Google Scholar] [CrossRef]
- Ye, Y.; Cota-Ruiz, K.; Hernández-Viezcas, J.A.; Valdés, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese Nanoparticles Control Salinity-Modulated Molecular Responses in Capsicum Annuum L. Through Priming: A Sustainable Approach for Agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Sharma, D.; Sabela, M.I.; Kanchi, S.; Mdluli, P.S.; Singh, G.; Stenström, T.A.; Bisetty, K. Biosynthesis of ZnO Nanoparticles Using Jacaranda mimosifolia Flowers Extract: Synergistic Antibacterial Activity and Molecular Simulated Facet Specific Adsorption Studies. J. Photochem. Photobiol. B 2016, 162, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Q.; Jiang, J.; Mao, Y. Morphology-Tunable Synthesis of ZnO Nanoforest and Its Photoelectrochemical Performance. Nanoscale 2014, 6, 8769–8780. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Green-Synthesized Nanoparticles Enhanced Seedling Growth, Yield, and Quality of Onion (Allium Cepa L.). ACS Sustain. Chem. Eng. 2019, 7, 14580–14590. [Google Scholar] [CrossRef]
- Dao, T.N.T.; Seshadri Reddy, A.; Zhao, F.; Liu, H.; Koo, B.; Moniruzzaman, M.; Kim, J.; Shin, Y. Green Synthesis-Based Magnetic Diatoms for Biological Applications. ACS Sustain. Chem. Eng. 2021. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K.; Asrar, M.; Alyemeni, M.N.; Wijaya, L.; Ali, S. Foliar Exposure of Zinc Oxide Nanoparticles Improved the Growth of Wheat (Triticum Aestivum L.) and Decreased Cadmium Concentration in Grains under Simultaneous Cd and Water Deficient Stress. Ecotoxicol. Environ. Saf. 2021, 208, 111627. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; García-Pérez, V.I.; Álvarez-Pérez, M.A.; Arenas-Alatorre, J.Á. Green Synthesis of ZnO Nanoparticles Using a Dysphania ambrosioides Extract. Structural Characterization and Antibacterial Properties. Mater. Sci. Eng. C 2021, 118, 111540. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Mohamad, R.; Bahadoran, A.; Bayat, S.; Rahim, R.A.; Ariff, A.; Saad, W.Z. Effect of Annealing Temperature on Antimicrobial and Structural Properties of Bio-Synthesized Zinc Oxide Nanoparticles Using Flower Extract of Anchusa italica. J. Photochem. Photobiol. B 2016, 161, 441–449. [Google Scholar] [CrossRef]
- Dobrucka, R.; Długaszewska, J. Biosynthesis and Antibacterial Activity of ZnO Nanoparticles Using Trifolium pratense Flower Extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef]
- Fowsiya, J.; Madhumitha, G.; Al-Dhabi, N.A.; Arasu, M.V. Photocatalytic Degradation of Congo Red Using Carissa edulis Extract Capped Zinc Oxide Nanoparticles. J. Photochem. Photobiol. B 2016, 162, 395–401. [Google Scholar] [CrossRef]
- Soto-Robles, C.A.; Nava, O.; Cornejo, L.; Lugo-Medina, E.; Vilchis-Nestor, A.R.; Castro-Beltrán, A.; Luque, P.A. Biosynthesis, Characterization and Photocatalytic Activity of ZnO Nanoparticles Using Extracts of Justicia spicigera for the Degradation of Methylene Blue. J. Mol. Struct. 2021, 1225, 129101. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S.; Alok, A.; Upadhyay, S.K.; Rawat, M.; Tsang, D.C.W.; Bolan, N.; Kim, K.-H. The Potential of Green Synthesized Zinc Oxide Nanoparticles as Nutrient Source for Plant Growth. J. Clean. Prod. 2019, 214, 1061–1070. [Google Scholar] [CrossRef]
- Panfili, I.; Bartucca, M.L.; Ballerini, E.; Del Buono, D. Combination of Aquatic Species and Safeners Improves the Remediation of Copper Polluted Water. Sci. Total Environ. 2017, 601–602, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Artamonova, A.A.; Baldov, D.A.; Mirzeabasov, O.A.; Rasskazova, M.M.; Synzynys, B.I.; Shatalova, R.O. Bioassay of Radioactive Water Contamination Using Duckweed Lemna minor and Spirodella polyrrhiza: Experimental Justification. J. Phys. Conf. Ser. 2020, 1701, 012011. [Google Scholar] [CrossRef]
- Panfili, I.; Bartucca, M.L.; Del Buono, D. The Treatment of Duckweed with a Plant Biostimulant or a Safener Improves the Plant Capacity to Clean Water Polluted by Terbuthylazine. Sci. Total Environ. 2019, 646, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Ubuza, L.J.A.; Padero, P.C.S.; Nacalaban, C.M.N.; Tolentino, J.T.; Alcoran, D.C.; Tolentino, J.C.; Ido, A.L.; Mabayo, V.I.F.; Arazo, R.O. Assessment of the Potential of Duckweed (Lemna minor L.) in Treating Lead-Contaminated Water through Phytoremediation in Stationary and Recirculated Set-Ups. Environ. Eng. Res. 2020, 25, 977–982. [Google Scholar] [CrossRef]
- Khellaf, N.; Zerdaoui, M. Growth Response of the Duckweed Lemna gibba L. to Copper and Nickel Phytoaccumulation. Ecotoxicology 2010, 19, 1363–1368. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Wang, X.; Fang, Y.; Zhang, Y.; Huang, M.; Zhao, H. The Influence of Different Plant Hormones on Biomass and Starch Accumulation of Duckweed: A Renewable Feedstock for Bioethanol Production. Renew. Energy 2019, 138, 659–665. [Google Scholar] [CrossRef]
- Khan, M.A.; Wani, G.A.; Majid, H.; Farooq, F.U.; Reshi, Z.A.; Husaini, A.M.; Shah, M.A. Differential Bioaccumulation of Select Heavy Metals from Wastewater by Lemna minor. Bull. Environ. Contam. Toxicol. 2020, 105, 777–783. [Google Scholar] [CrossRef]
- Pagliuso, D.; Palacios Jara, C.E.; Grandis, A.; Lam, E.; Pena Ferreira, M.J.; Buckeridge, M.S. Flavonoids from Duckweeds: Potential Applications in the Human Diet. RSC Adv. 2020, 10, 44981–44988. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Chiodelli, G.; Giuberti, G.; Gallo, A.; Masoero, F.; Trevisan, M. Phenolic Profile and Fermentation Patterns of Different Commercial Gluten-Free Pasta during in Vitro Large Intestine Fermentation. Food Res. Int. 2017, 97, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Mimmo, T.; Tiziani, R.; Valentinuzzi, F.; Lucini, L.; Nicoletto, C.; Sambo, P.; Scampicchio, M.; Pii, Y.; Cesco, S. Selenium Biofortification in Fragaria × Ananassa: Implications on Strawberry Fruits Quality, Content of Bioactive Health Beneficial Compounds and Metabolomic Profile. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Arriola, N.D.A.; Chater, P.I.; Wilcox, M.; Lucini, L.; Rocchetti, G.; Dalmina, M.; Pearson, J.P.; de Mello Castanho Amboni, R.D. Encapsulation of Stevia rebaudiana Bertoni Aqueous Crude Extracts by Ionic Gelation—Effects of Alginate Blends and Gelling Solutions on the Polyphenolic Profile. Food Chem. 2019, 275, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Railean-Plugaru, V.; Pomastowski, P.; Buszewski, B. Phytochemical Investigation of Medicago Sativa L. Extract and Its Potential as a Safe Source for the Synthesis of ZnO Nanoparticles: The Proposed Mechanism of Formation and Antimicrobial Activity. Phytochem. Lett. 2019, 31, 170–180. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.C.; Tiwari, M.; Sharma, N.; et al. Enhanced Plant Growth Promoting Role of Phycomolecules Coated Zinc Oxide Nanoparticles with P Supplementation in Cotton (Gossypium Hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Panfili, I.; Bartucca, M.L.; Marrollo, G.; Povero, G.; Del Buono, D. Application of a Plant Biostimulant To Improve Maize (Zea mays) Tolerance to Metolachlor. J. Agric. Food Chem. 2019, 67, 12164–12171. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Alzahrani, H.S. The Potential Mitigation Effect of ZnO Nanoparticles on [Abelmoschus Esculentus L. Moench] Metabolism under Salt Stress Conditions. Saudi J. Biol. Sci. 2020, 27, 3132–3137. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of Nanomaterials in Agricultural Production and Crop Protection: A Review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.; Mahdavi, M.; Abdolmohammadi, S. Preparation, Characterization, and Antimicrobial Activities of ZnO Nanoparticles/Cellulose Nanocrystal Nanocomposites. BioResources 2013, 8, 1841–1851. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and Their Enhanced Antibacterial Property. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Trouillas, P.; Marsal, P.; Siri, D.; Lazzaroni, R.; Duroux, J.-L. A DFT Study of the Reactivity of OH Groups in Quercetin and Taxifolin Antioxidants: The Specificity of the 3-OH Site. Food Chem. 2006, 97, 679–688. [Google Scholar] [CrossRef]
- Huang, J.; Zhan, G.; Zheng, B.; Sun, D.; Lu, F.; Lin, Y.; Chen, H.; Zheng, Z.; Zheng, Y.; Li, Q. Biogenic Silver Nanoparticles by Cacumen Platycladi Extract: Synthesis, Formation Mechanism, and Antibacterial Activity. Ind. Eng. Chem. Res. 2011, 50, 9095–9106. [Google Scholar] [CrossRef]
- Mirgorod, Y.A.; Borodina, V.G.; Borsch, N.A. Investigation of Interaction between Silver Ions and Rutin in Water by Physical Methods. Biophys. Russ. Fed. 2013, 58, 743–747. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.C.; Yadav, S.K. Syzygium cumini Leaf and Seed Extract Mediated Biosynthesis of Silver Nanoparticles and Their Characterization. J. Chem. Technol. Biotechnol. 2010, 85, 1301–1309. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Plant-Mediated Synthesis of Silver and Gold Nanoparticles and Their Applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of Plant-Mediated Synthesis of Silver Nanoparticles—A Review on Biomolecules Involved, Characterisation and Antibacterial Activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef]
- Poynton, H.C.; Lazorchak, J.M.; Impellitteri, C.A.; Smith, M.E.; Rogers, K.; Patra, M.; Hammer, K.A.; Allen, H.J.; Vulpe, C.D. Differential Gene Expression in Daphnia magna Suggests Distinct Modes of Action and Bioavailability for Zno Nanoparticles and Zn Ions. Environ. Sci. Technol. 2011, 45, 762–768. [Google Scholar] [CrossRef]
- Adhikari, S.; Adhikari, A.; Ghosh, S.; Roy, D.; Azahar, I.; Basuli, D.; Hossain, Z. Assessment of ZnO-NPs Toxicity in Maize: An Integrative MicroRNAomic Approach. Chemosphere 2020, 249, 126197. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.-C.; Braam, J.; Alvarez, P.J.J. Developmental Phytotoxicity of Metal Oxide Nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 2010, 29, 669–675. [Google Scholar] [CrossRef]
- Changlian, P.; Zhifang, L.; Guizhu, L.; Shaowei, C. The Anti-Photooxidation of Anthocyanins-Rich Leaves of a Purple Rice Cultivar. Sci. China Ser. C Life Sci. 2006, 49, 543–551. [Google Scholar] [CrossRef]
- Reinbothe, S.; Bartsch, S.; Rossig, C.; Davis, M.Y.; Yuan, S.; Reinbothe, C.; Gray, J. A Protochlorophyllide (Pchlide) a Oxygenase for Plant Viability. Front. Plant Sci. 2019, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Salama, D.M.; Osman, S.A.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A.; Shaaban, E.A. Effect of Zinc Oxide Nanoparticles on the Growth, Genomic DNA, Production and the Quality of Common Dry Bean (Phaseolus Vulgaris). Biocatal. Agric. Biotechnol. 2019, 18, 101083. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Chen, S.; Li, Q.; Wang, W.; Hou, C.; Gao, X.; Wang, L.; Wang, S. Zinc Oxide Nanoparticles Affect Biomass Accumulation and Photosynthesis in Arabidopsis. Front. Plant Sci. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Bartucca, M.L.; Guiducci, M.; Falcinelli, B.; Del Buono, D.; Benincasa, P. Blue:Red LED Light Proportion Affects Vegetative Parameters, Pigment Content, and Oxidative Status of Einkorn (Triticum Monococcum L. Ssp. Monococcum) Wheatgrass. J. Agric. Food Chem. 2020, 68, 8757–8763. [Google Scholar] [CrossRef]
- Zoufan, P.; Azad, Z.; Rahnama Ghahfarokhie, A.; Kolahi, M. Modification of Oxidative Stress through Changes in Some Indicators Related to Phenolic Metabolism in Malva Parviflora Exposed to Cadmium. Ecotoxicol. Environ. Saf. 2020, 187, 109811. [Google Scholar] [CrossRef]
- Ibrahim, A.B.M.; Mahmoud, G.A. Chemical- vs. Sonochemical-assisted Synthesis of ZnO Nanoparticles from a New Zinc Complex for Improvement of Carotene Biosynthesis from Rhodotorula Toruloides MH023518. Appl. Organomet. Chem. 2020. [Google Scholar] [CrossRef]
- Nanou, K.; Roukas, T.; Papadakis, E. Oxidative Stress and Morphological Changes in Blakeslea trispora Induced by Enhanced Aeration during Carotene Production in a Bubble Column Reactor. Biochem. Eng. J. 2011, 54, 172–177. [Google Scholar] [CrossRef]
- Dobrikova, A.G.; Apostolova, E.L.; Hanć, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Adamakis, I.-D.S.; Moustakas, M. Cadmium Toxicity in Salvia sclarea L.: An Integrative Response of Element Uptake, Oxidative Stress Markers, Leaf Structure and Photosynthesis. Ecotoxicol. Environ. Saf. 2021, 209, 111851. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Torres, K.A.; Vazquez-Rodriguez, S.; la Cruz, A.M.; Sepulveda-Guzman, S.; Benavides, R.; Lopez-Gonzalez, R.; Torres-Martínez, L.M. Influence of the Morphology of ZnO Nanomaterials on Photooxidation of Polypropylene/ZnO Composites. Mater. Sci. Semicond. Process. 2017, 68, 217–225. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum Extract Biostimulants and Their Role in Enhancing Tolerance to Drought Stress in Tomato Plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.; Asrar, Z.; Pourseyedi, S.; Nadernejad, N. Investigation of ZnO Nanoparticles on Proline, Anthocyanin Contents and Photosynthetic Pigments and Lipid Peroxidation in the Soybean. IET Nanobiotechnol. 2019, 13, 66–70. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate Material Delivery to Plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Kanchana, U.S.; Mathew, T.V. Surface Functionalization of ZnO Nanoparticles with Functionalized Bovine Serum Albumin as a Biocompatible Photochemical and Antimicrobial Agent. Surf. Interfaces 2021, 24. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Buono, D.; Di Michele, A.; Costantino, F.; Trevisan, M.; Lucini, L. Biogenic ZnO Nanoparticles Synthesized Using a Novel Plant Extract: Application to Enhance Physiological and Biochemical Traits in Maize. Nanomaterials 2021, 11, 1270. https://doi.org/10.3390/nano11051270
Del Buono D, Di Michele A, Costantino F, Trevisan M, Lucini L. Biogenic ZnO Nanoparticles Synthesized Using a Novel Plant Extract: Application to Enhance Physiological and Biochemical Traits in Maize. Nanomaterials. 2021; 11(5):1270. https://doi.org/10.3390/nano11051270
Chicago/Turabian StyleDel Buono, Daniele, Alessandro Di Michele, Ferdinando Costantino, Marco Trevisan, and Luigi Lucini. 2021. "Biogenic ZnO Nanoparticles Synthesized Using a Novel Plant Extract: Application to Enhance Physiological and Biochemical Traits in Maize" Nanomaterials 11, no. 5: 1270. https://doi.org/10.3390/nano11051270
APA StyleDel Buono, D., Di Michele, A., Costantino, F., Trevisan, M., & Lucini, L. (2021). Biogenic ZnO Nanoparticles Synthesized Using a Novel Plant Extract: Application to Enhance Physiological and Biochemical Traits in Maize. Nanomaterials, 11(5), 1270. https://doi.org/10.3390/nano11051270