Tailoring Mesoporous Titania Features by Ultrasound-Assisted Sol-Gel Technique: Effect of Surfactant/Titania Precursor Weight Ratio
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mesoporous Titania by Conventional Sol-Gel Method—A-Series
2.3. Preparation of Mesoporous Titania by Ultrasound-Assisted Sol-Gel Method—US-Series
2.4. Characterization of Prepared Mesoporous Titania
2.5. Photocatalytic Performance Evaluation
3. Results and Discussion
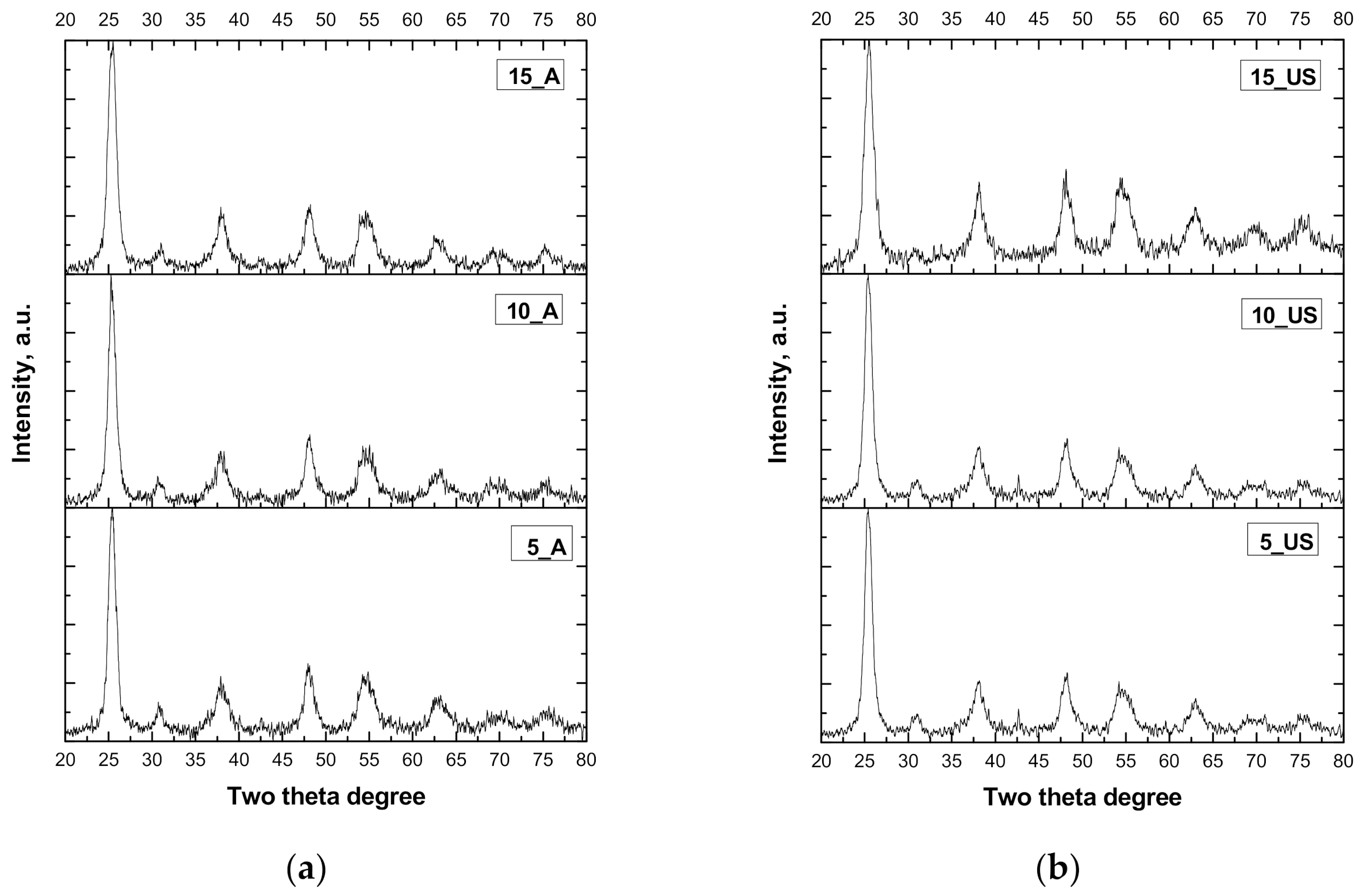
3.1. XRD Measurements
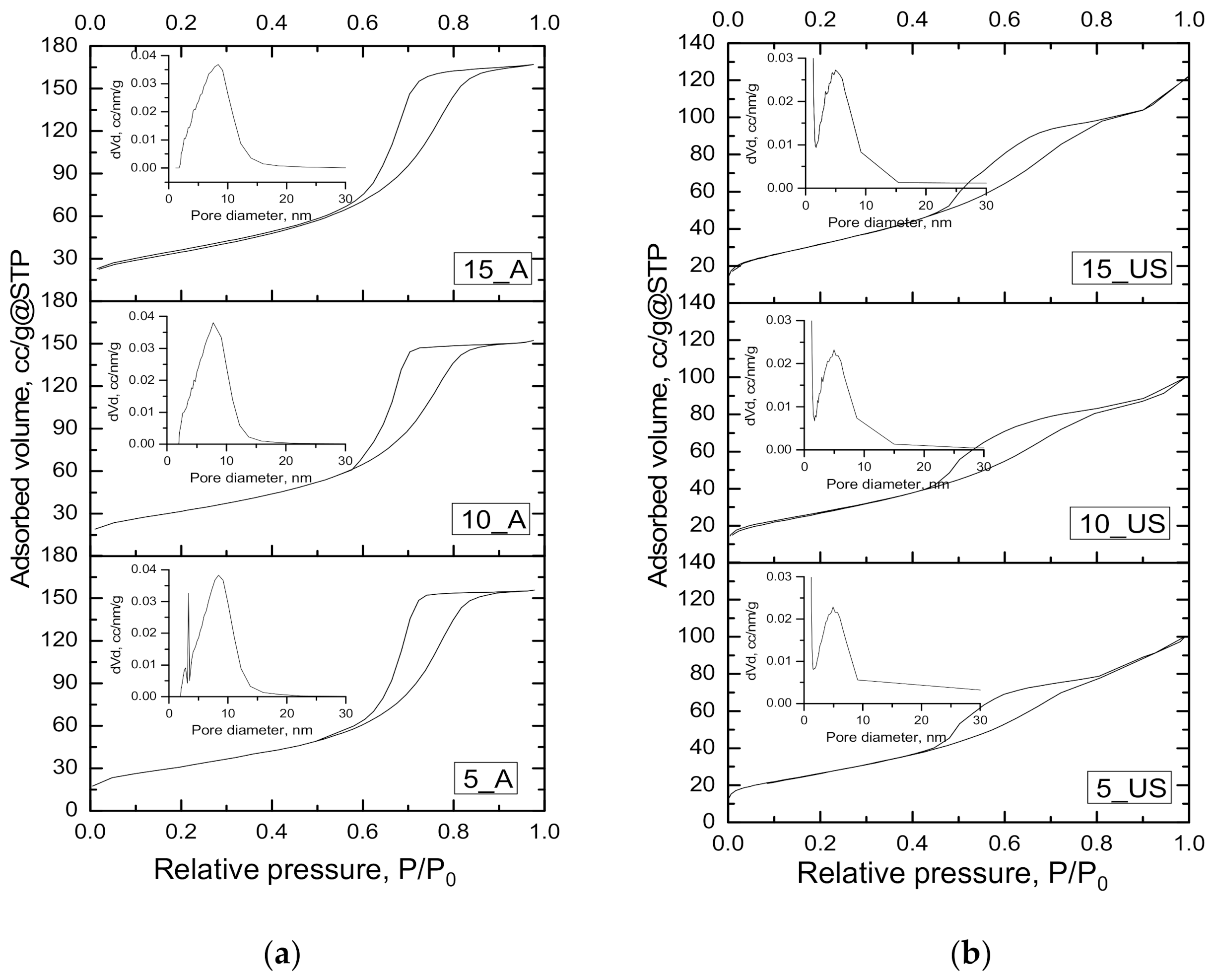
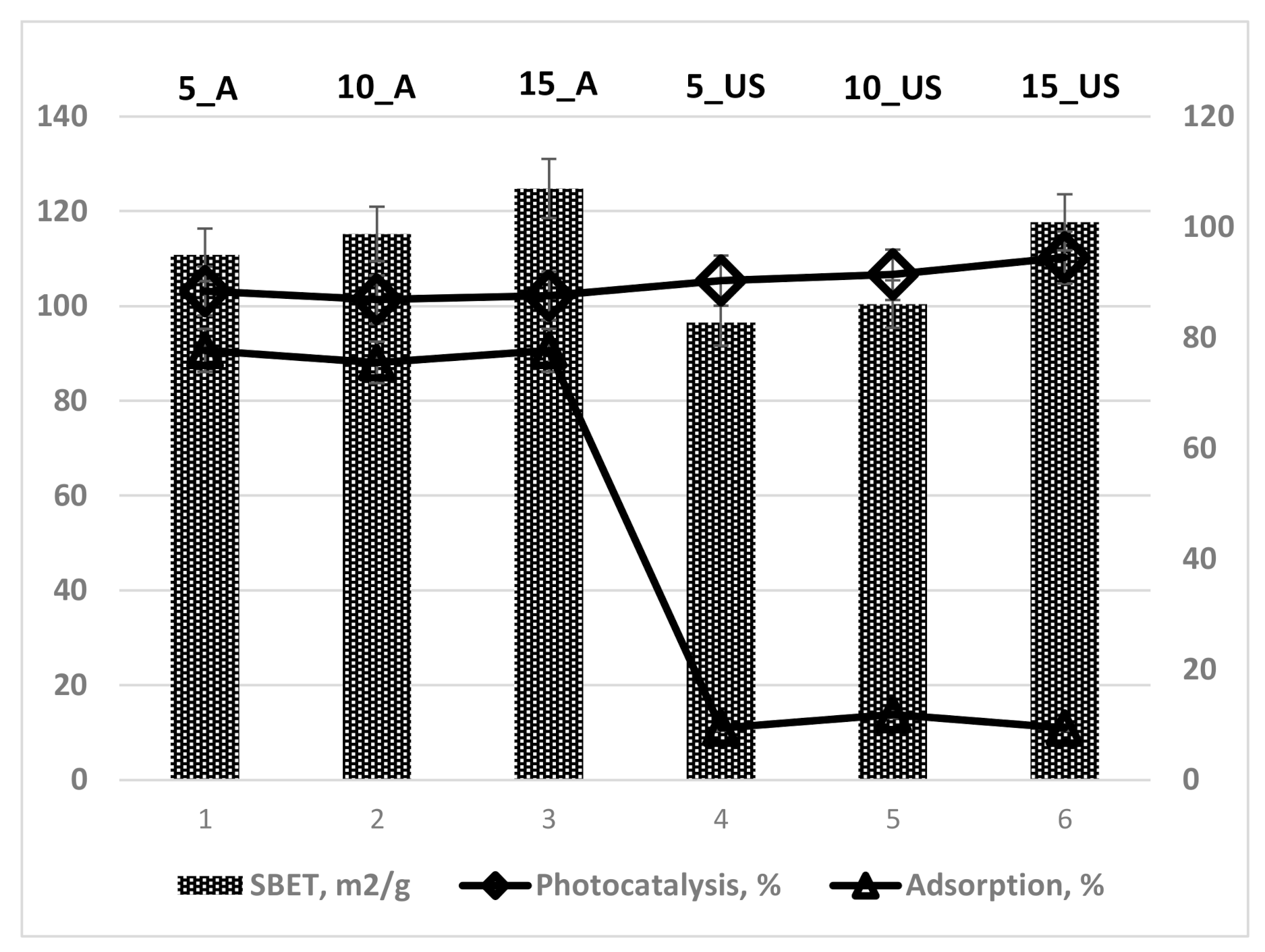
3.2. Textural Characteristics Investigation
3.3. Morphology Investigation by TEM
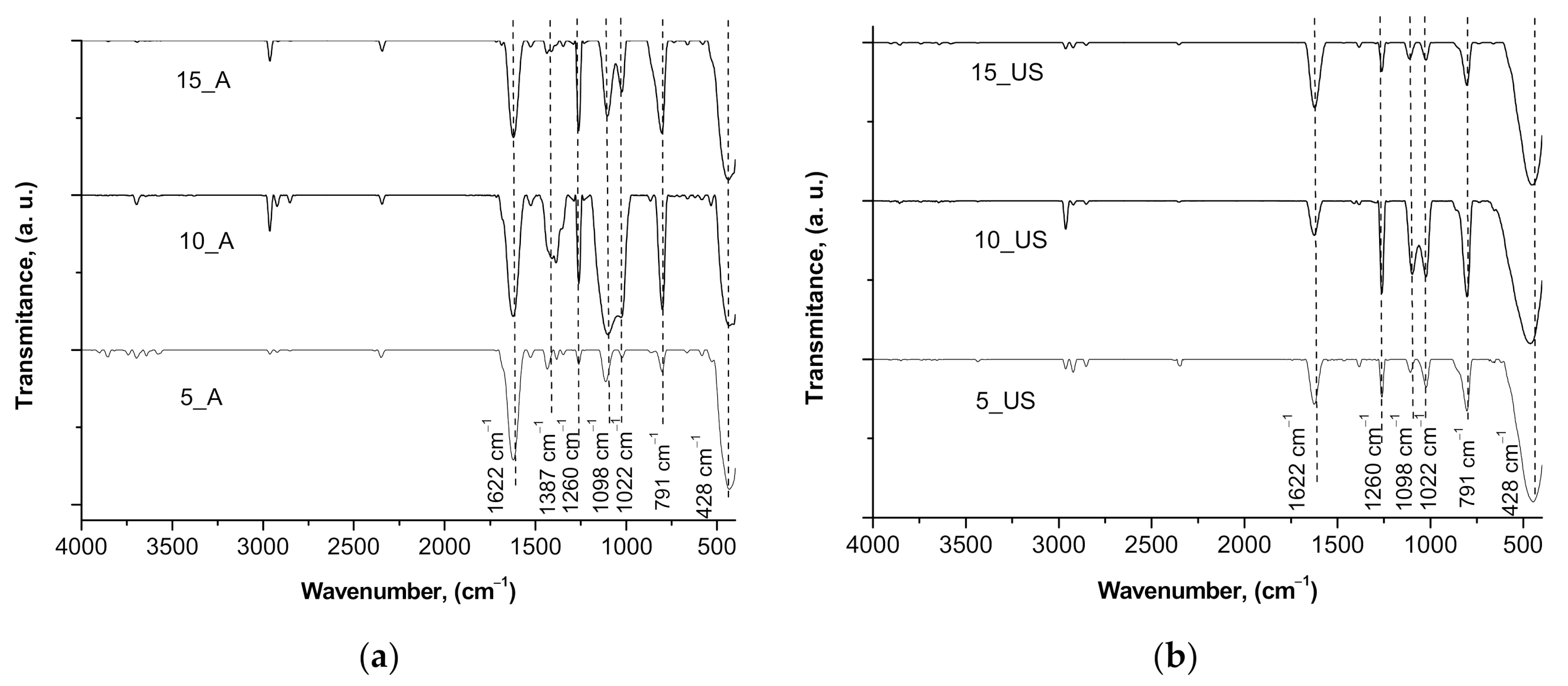
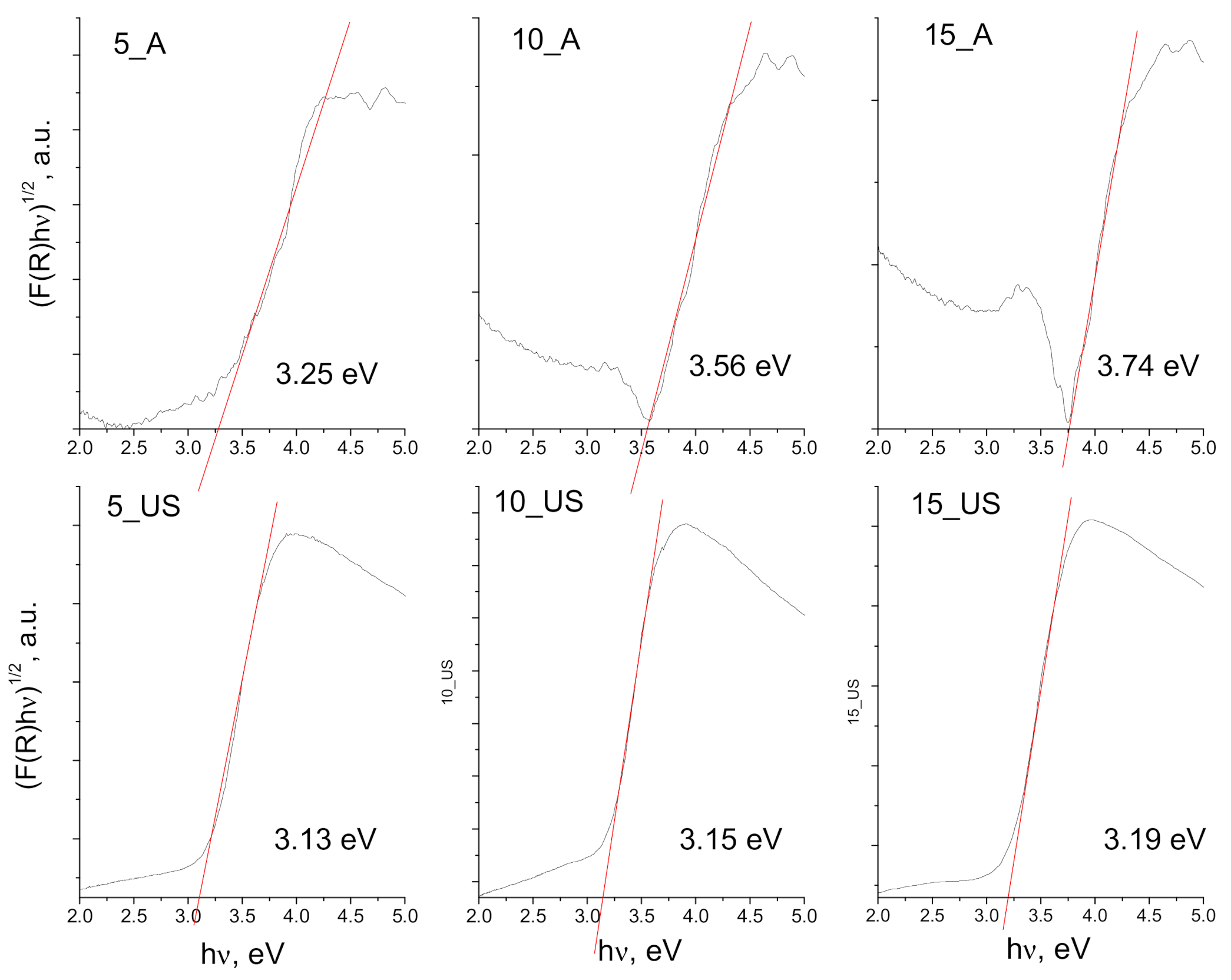
3.4. Surface Chemistry and Optical Properties of the Synthesized Titania Samples
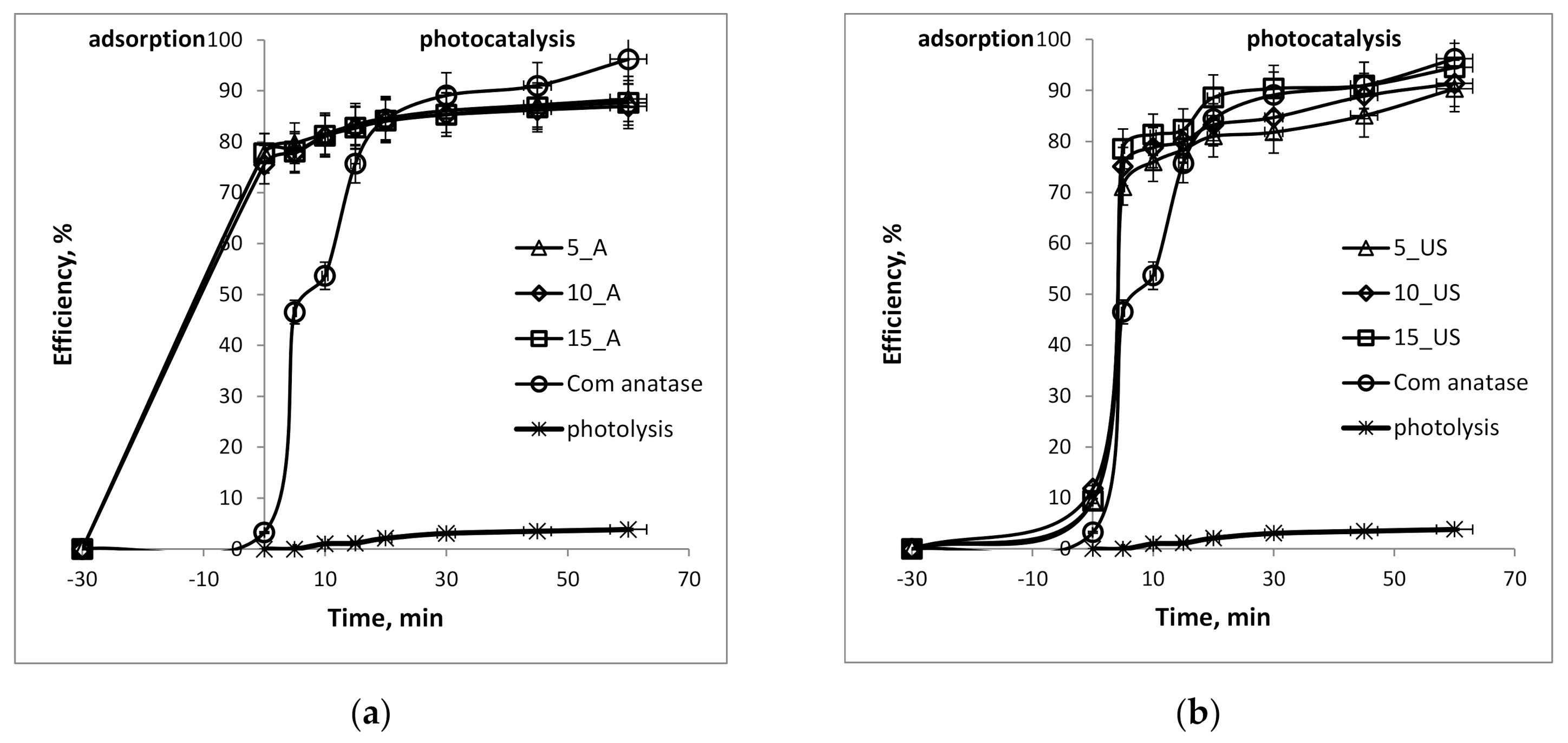
3.5. Photocatalytic Activity Investigation for Methylene Blue Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hozumi, A.; Cheng, D.F. Facile micropatterning of mesoporous titania film by low-energy electron beam irradiation. Mater. Chem. Phys. 2011, 129, 464–470. [Google Scholar] [CrossRef]
- Alahmadi, N.; Amin, M.; Mohamed, R. Corrigendum to “Facile synthesis of mesoporous Pt-doped, titania-silica nanocomposites as highly photoactive under visible light”. J. Mater. Res. Technol. 2021, 12, 1125. [Google Scholar] [CrossRef]
- Caraballo, R.M.; Onna, D.; Abdala, N.L.; Illia, G.J.S.; Hamer, M. Metalloporphyrins into mesoporous photonic crystals: Towards molecularly-tuned photonic sensing devices. Sens. Actuators B Chem. 2020, 309, 127712. [Google Scholar] [CrossRef]
- Morawski, F.D.M.; Xavier, B.B.; Virgili, A.H.; Caetano, K.D.S.; de Menezes, E.W.; Benvenutti, E.V.; Costa, T.M.H.; Arenas, L.T. A novel electrochemical platform based on mesoporous silica/titania and gold nanoparticles for simultaneous determination of norepinephrine and dopamine. Mater. Sci. Eng. C 2021, 120, 111646. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Ismail, A.A.; Bahnemann, D.W.; Kiel, T.A. Mesoporous TiO2 nanostructures: A route to minimize Pt loading on titania photocatalysts for hydrogen production. Phys. Chem. Chem. Phys. 2011, 13, 20155. [Google Scholar] [CrossRef] [Green Version]
- Luna, A.L.; Matter, F.; Schreck, M.; Wohlwend, J.; Tervoort, E.; Colbeau-Justin, C.; Niederberger, M. Monolithic metal-containing TiO2 aerogels assembled from crystalline pre-formed nanoparticles as efficient photocatalysts for H2 generation. Appl. Catal. B Environ. 2020, 267, 118660. [Google Scholar] [CrossRef]
- Skandan, G.; Singhal, A.; Gugotsi, Y. Nanomaterials Handbook; Taylor & Francis Inc.: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hamer, M.A.; Hendrickson, W.A.; Pryor, G.R. Organogel Particles. US Patent 6858666, 22 February 2005. [Google Scholar]
- Yu, J.C.; Yu, J.; Ho, W.; Zhang, L. Preparation of highly photocatalytic active nano-sized TiO2 particles via ultrasonic irradiation. Chem. Commun. 2001, 1942–1943. [Google Scholar] [CrossRef]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Chen, Z.; Li, Y.; Yu, Z.; Liu, Q.; Li, J.; Feng, C.; Zhang, D. Sonochemical synthesis of TiO2 nanoparticles on graphene for use as photocatalyst. Ultrason. Sonochem. 2011, 18, 1082–1090. [Google Scholar] [CrossRef]
- Mahu, E.; Ignat, M.; Cojocaru, C.; Samoila, P.; Coromelci, C.; Asaftei, I.; Harabagiu, V. Development of Porous Titania Structure with Improved Photocatalytic Activity: Response Surface Modeling and Multi-Objective Optimization. Nanomaterials 2020, 10, 998. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Li, L.; Chen, X.; Yao, Y.; Ru, Y.; Sun, Y.; Hua, W.; Zhuang, G.; Zhao, D.; Yan, S.; et al. Mesoporous anatase crystal-silica nanocomposites with large intrawall mesopores presenting quite excellent photocatalytic performances. Appl. Catal. B Environ. 2019, 246, 284–295. [Google Scholar] [CrossRef]
- Leodopoulos, C.; Doulia, D.; Gimouhopoulos, K. Adsorption of Cationic Dyes onto Bentonite. Sep. Purif. Rev. 2014, 44, 74–107. [Google Scholar] [CrossRef]
- Chenchana, A.; Nemamcha, A.; Moumeni, H.; Doña Rodríguez, J.M.; Araña, J.; Navío, J.A.; González Díaz, O.; Pulido Melián, E. Photodegradation of 2,4-dichlorophenoxyacetic acid over TiO2(B)/anatase nanobelts and Au-TiO2(B)/anatase nanobelts. Appl. Surf. Sci. 2019, 467–468, 1076–1087. [Google Scholar] [CrossRef]
- Varnagiris, S.; Medvids, A.; Lelis, M.; Milcius, D.; Antuzevics, A. Black carbon-doped TiO2 films: Synthesis, characterization and photocatalysis. J. Photochem. Photobiol. A Chem. 2019, 382, 111941. [Google Scholar] [CrossRef]
- Liu, H.; Wang, M.; Wang, Y.; Liang, Y.; Cao, W.; Su, Y. Ionic liquid-templated synthesis of mesoporous CeO2–TiO2 nanoparticles and their enhanced photocatalytic activities under UV or visible light. J. Photochem. Photobiol. A Chem. 2011, 223, 157–164. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- ISO. Determination of the Specific Surface Area of Solids by Gas Adsorption-BET Method; ISO 9277:2010. ISO, 2010; p. 24. Available online: https://www.iso.org/standard/44941.html (accessed on 10 May 2021).
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Braslavsky, S.E.; Braun, A.M.; Cassano, A.E.; Emeline, A.V.; Litter, M.I.; Palmisano, L.; Parmon, V.N.; Serpone, N. Glossary of terms used in photocatalysis and radiation catalysis (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 931–1014. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, K.; Mizusawa, M. X-ray Diffraction Imaging of Anatase and Rutile. Anal. Chem. 2010, 82, 3519–3522. [Google Scholar] [CrossRef]
- Bellardita, M.; Di Paola, A.; Megna, B.; Palmisano, L. Absolute crystallinity and photocatalytic activity of brookite TiO2 samples. Appl. Catal. B Environ. 2017, 201, 150–158. [Google Scholar] [CrossRef]
- Warren, B.E.; Averbach, B.L. The Separation of Cold-Work Distortion and Particle Size Broadening in X-ray Patterns. J. Appl. Phys. 1952, 23, 497. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Local structure modification and phase transformation of TiO2 nanoparticles initiated by oxygen defects, grain size, and annealing temperature. Int. Nano Lett. 2013, 3, 55. [Google Scholar] [CrossRef]
- Sotomayor, F.; Cychosz, K.; Thommes, M. Characterization of Micro/Mesoporous Materials by Physisorption: Concepts and Case Studies. Acc. Mater. Surf. Res. 2018, 2, 34–50. [Google Scholar]
- Rouquerol, J.; Rouquerol, F.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: London, UK, 1998. [Google Scholar]
- Grosman, A.; Ortega, C. Capillary Condensation in Porous Materials. Hysteresis and Interaction without Pore Blocking/Percolation Process. Langmuir 2008, 24, 3977–3986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benkacem, T.; Agoudjil, N. Synthesis of Mesoporous Titania with Surfactant and its Characterization. Am. J. Appl. Sci. 2008, 5. [Google Scholar] [CrossRef]
- Sener, S.; Erdemoglu, M.; Asiltirk, M.; Sayilkan, H. The effect of silane modificationon the adsorptive properties of natural pyrophyllite and synthetic titanium-based powders prepared by the sol–gel process. Turkish J. Chem. 2005, 29, 487–495. [Google Scholar]
- Soler-Illia, G.J.D.A.A.; Louis, A.A.; Sanchez, C. Synthesis and Characterization of Mesostructured Titania-Based Materials through Evaporation-Induced Self-Assembly. Chem. Mater. 2002, 14, 750–759. [Google Scholar] [CrossRef]
- Yu, J.C.; Zhang, L.; Zheng, A.Z.; Zhao, J. Synthesis and Characterization of Phosphated Mesoporous Titanium Dioxide with High Photocatalytic Activity. Chem. Mater. 2003, 15, 2280–2286. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; de Coss, C.C.R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.C.; Zhu, A.X.E.; Du, G.T. Dependence of the excitonic transition energies and mosaicity on residual strain in ZnO thin films. Appl. Phys. Lett. 2002, 80, 941–943. [Google Scholar] [CrossRef]

| Sample Label | Used Molar Ratio of Reactants | ||||
|---|---|---|---|---|---|
| Conventional Stirring | Ultrasound- Assisted | TTIP, ×10−2 | F-127, ×10−4 | H2O | Iso-C3H7OH |
| 5_A | 5_US | 4.9 | 3.3 | 2.3 | 1 |
| 10_A | 10_US | 4.9 | 6.6 | 2.3 | 1 |
| 15_A | 15_US | 4.9 | 9.9 | 2.3 | 1 |
| Sample | DScherrer (nm) | DW–H (nm) | δ, ×10−2 (nm−2) | ε (×10−3) | Crystallinity, % |
|---|---|---|---|---|---|
| 5_A | 9.6 ± 0.5 | 7.8 ± 0.8 | 1.077 | −0.9 | 82.5 |
| 10_A | 9.4 ± 0.5 | 8.0 ± 0.8 | 1.138 | −0.8 | 83.9 |
| 15_A | 8.6 ± 0.5 | 6.5 ± 0.8 | 1.349 | −2.3 | 83.0 |
| 5_US | 9.5 ± 0.5 | 7.2 ± 1.1 | 1.100 | −1.6 | 92.1 |
| 10_US | 8.8 ± 0.5 | 7.0 ± 1.1 | 1.306 | −1.4 | 92.4 |
| 15_US | 8.5 ± 0.5 | 5.1 ± 1.1 | 1.400 | −3 | 93.7 |
| Sample | SBET, m2/g | Dpore(BJH ads), nm | Dpore(BJH des), nm | Vt, cm3/g |
|---|---|---|---|---|
| 5_A | 110.8 | 6.82 | 6.90 | 0.241 |
| 10_A | 115.2 | 6.47 | 6.42 | 0.235 |
| 15_A | 124.8 | 6.55 | 6.45 | 0.259 |
| 5_US | 96.5 | 4.95 | 3.90; 4.43 | 0.151 |
| 10_US | 100.4 | 5.01 | 3.90; 4.45 | 0.141 |
| 15_US | 117.7 | 5.14 | 3.89; 4.72 | 0.190 |
| Sample | Adsorption, % | Photocatalysis, % | qe, mg/g | Apparent Constant Rate, 10−4 s−1 | Photonic Efficiency, % |
|---|---|---|---|---|---|
| 5_A | 77.7 | 88.4 | 55.3 | 4 | 11.9 |
| 10_A | 75.5 | 86.9 | 54.4 | 4 | 15.1 |
| 15_A | 77.7 | 87.6 | 54.8 | 4 | 13.5 |
| Com anatase | 3.3 | 96.2 | 60.1 | 12 | 19.7 |
| 5_US | 9.4 | 90.3 | 56.5 | 10 | 20.3 |
| 10_US | 11.8 | 91.4 | 57.1 | 12 | 19.7 |
| 15_US | 9.4 | 94.5 | 59.1 | 19 | 12.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahu, E.; Coromelci, C.G.; Lutic, D.; Asaftei, I.V.; Sacarescu, L.; Harabagiu, V.; Ignat, M. Tailoring Mesoporous Titania Features by Ultrasound-Assisted Sol-Gel Technique: Effect of Surfactant/Titania Precursor Weight Ratio. Nanomaterials 2021, 11, 1263. https://doi.org/10.3390/nano11051263
Mahu E, Coromelci CG, Lutic D, Asaftei IV, Sacarescu L, Harabagiu V, Ignat M. Tailoring Mesoporous Titania Features by Ultrasound-Assisted Sol-Gel Technique: Effect of Surfactant/Titania Precursor Weight Ratio. Nanomaterials. 2021; 11(5):1263. https://doi.org/10.3390/nano11051263
Chicago/Turabian StyleMahu, Elvira, Cristina Giorgiana Coromelci, Doina Lutic, Iuliean Vasile Asaftei, Liviu Sacarescu, Valeria Harabagiu, and Maria Ignat. 2021. "Tailoring Mesoporous Titania Features by Ultrasound-Assisted Sol-Gel Technique: Effect of Surfactant/Titania Precursor Weight Ratio" Nanomaterials 11, no. 5: 1263. https://doi.org/10.3390/nano11051263
APA StyleMahu, E., Coromelci, C. G., Lutic, D., Asaftei, I. V., Sacarescu, L., Harabagiu, V., & Ignat, M. (2021). Tailoring Mesoporous Titania Features by Ultrasound-Assisted Sol-Gel Technique: Effect of Surfactant/Titania Precursor Weight Ratio. Nanomaterials, 11(5), 1263. https://doi.org/10.3390/nano11051263







