Accidental Impurities in Epitaxial Pb(Zr0.2Ti0.8)O3 Thin Films Grown by Pulsed Laser Deposition and Their Impact on the Macroscopic Electric Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Pure Target
2.2. Deposition of Thin Films
2.3. Characterization Methods
3. Results
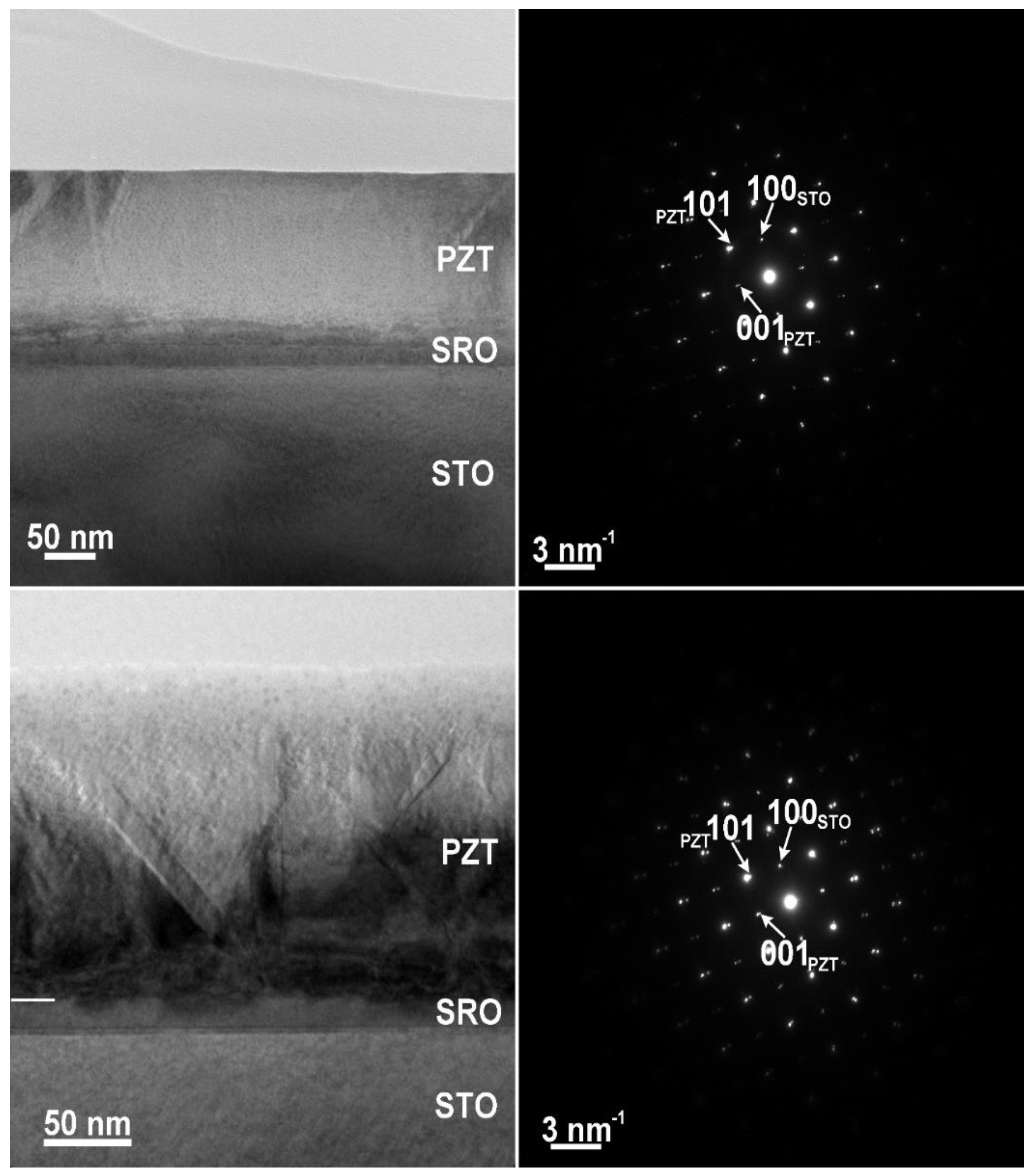
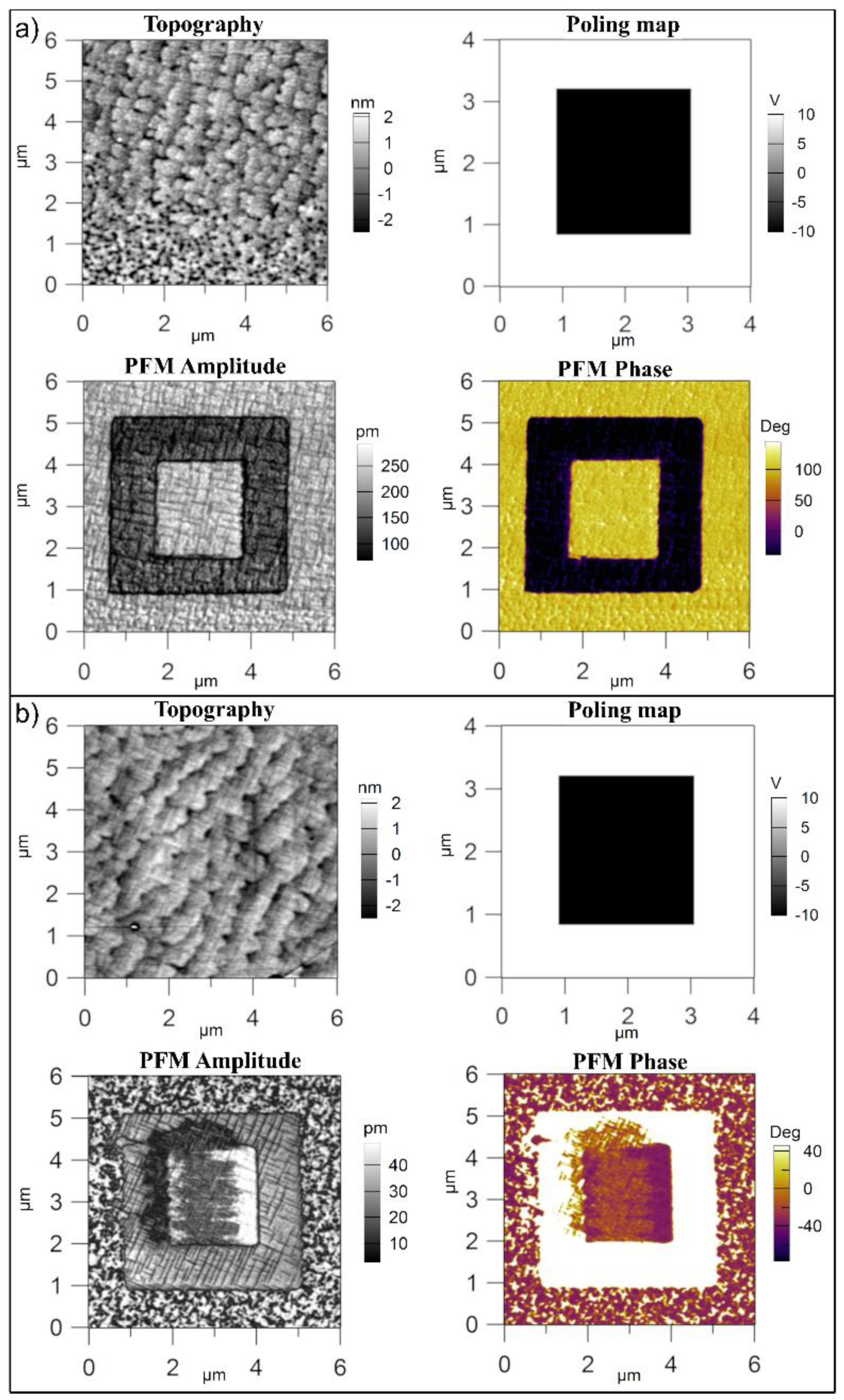
3.1. Structural Analysis
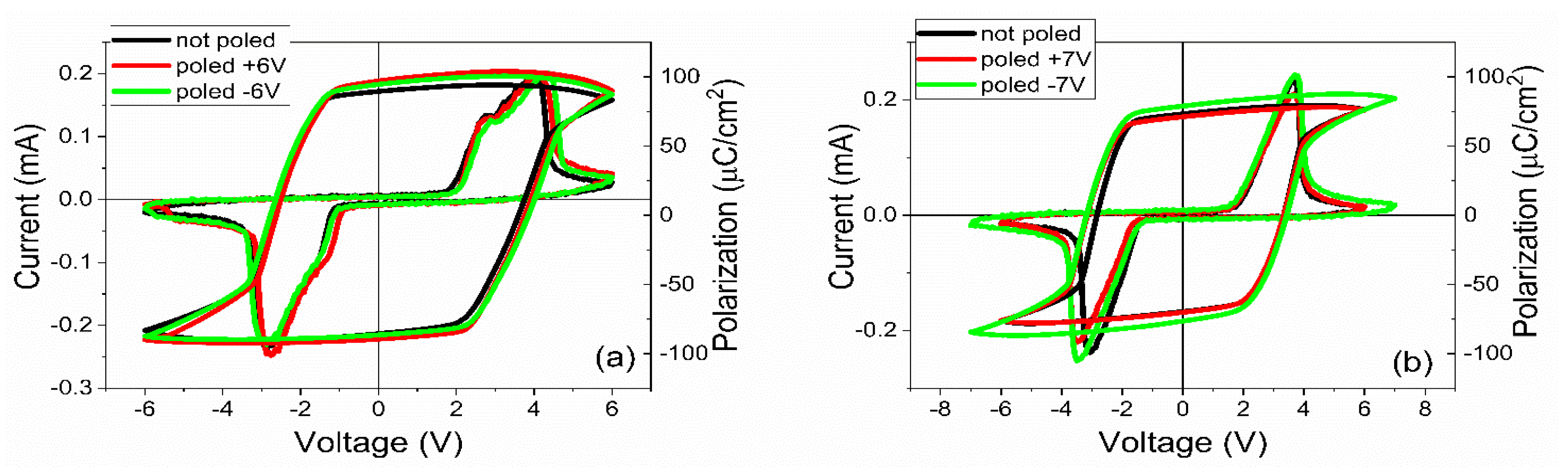
3.2. Electrical Characterization
- -
- The polarization values were around 75–80 µC/cm2 for PZT deposited from pure target (PZT-IH) and about 85–90 µC/cm2 for the film deposited from commercial target (PZT-CO). This is in agreement with the findings of the XRD analysis, showing a larger value for the out-of-plane lattice constant in the case of PZT-CO compared to PZT-IH.
- -
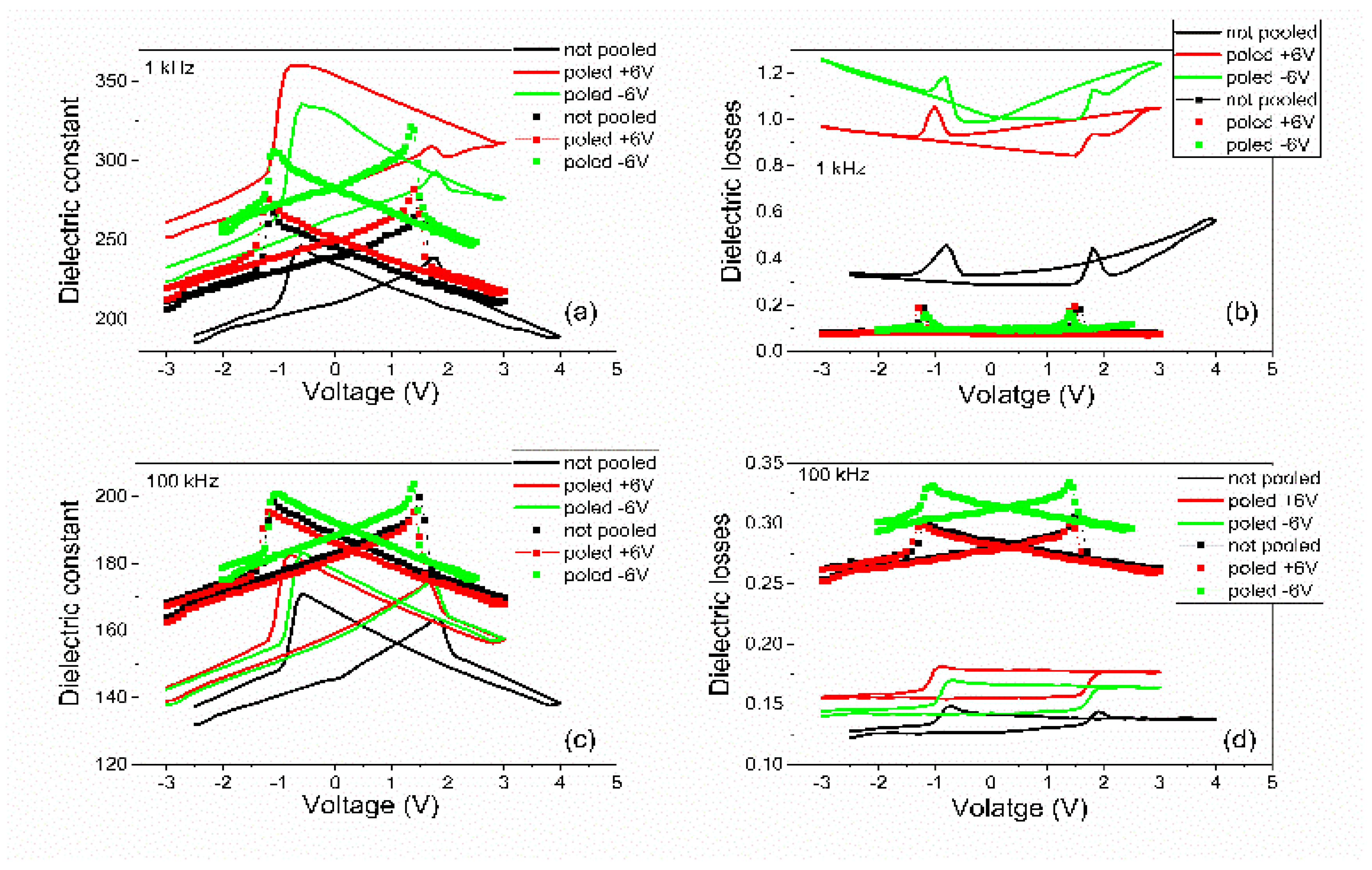
- The dielectric constant had different values depending on the polarization state. However, the dispersion of the values seemed to be larger for the PZT film deposited from the commercial target. One can notice that, in the case of PZT-CO, the complete butterfly shape at 1 kHz was obtained only for the contact that was not poled initially. For the other two cases, with upward or downward poling, there were large differences between the magnitudes of the dielectric constant when the voltage was swept up or down. Complete butterfly shapes were obtained at 100 kHz for both samples. An internal electric field was present, as suggested by the fact the C–V characteristics were shifted towards positive voltages for both PZT-IH and PZT-CO. This field was oriented towards the top electrode in all cases, in agreement with the polarization hysteresis loops presented in Figure 4 and showing also a shift towards the positive voltage. This fact suggests that the origin of the internal field might be the same and related to the presence of the strained layer just above the substrate, at the beginning of the epitaxial growth. In fact, the presence of strained and more relaxed layers in both PZT films suggests the presence of a strain gradient, which can lead to the presence of a flexoelectric internal field [36,37,38,39].
- -
- The dielectric losses at 1 kHz were much larger for PZT-CO compared to PZT-IH, suggesting a larger dc conduction (larger leakage current). As in the case of the dielectric constant, the magnitude of the losses depended significantly on the polarization orientation and state (poled or not poled). At 100 kHz, the losses were larger for PZT-IH. However, it is beyond the scope of this study to investigate in detail the dependence of the dielectric properties on the polarization state and orientation, as well as on frequency. One can just assume that the impurity atoms were introducing levels in the gap that could be filled or not depending on polarization orientation (especially near the electrode interfaces, where the band bending occurs), and that could influence the frequency response depending on their presence in the sample.
- -
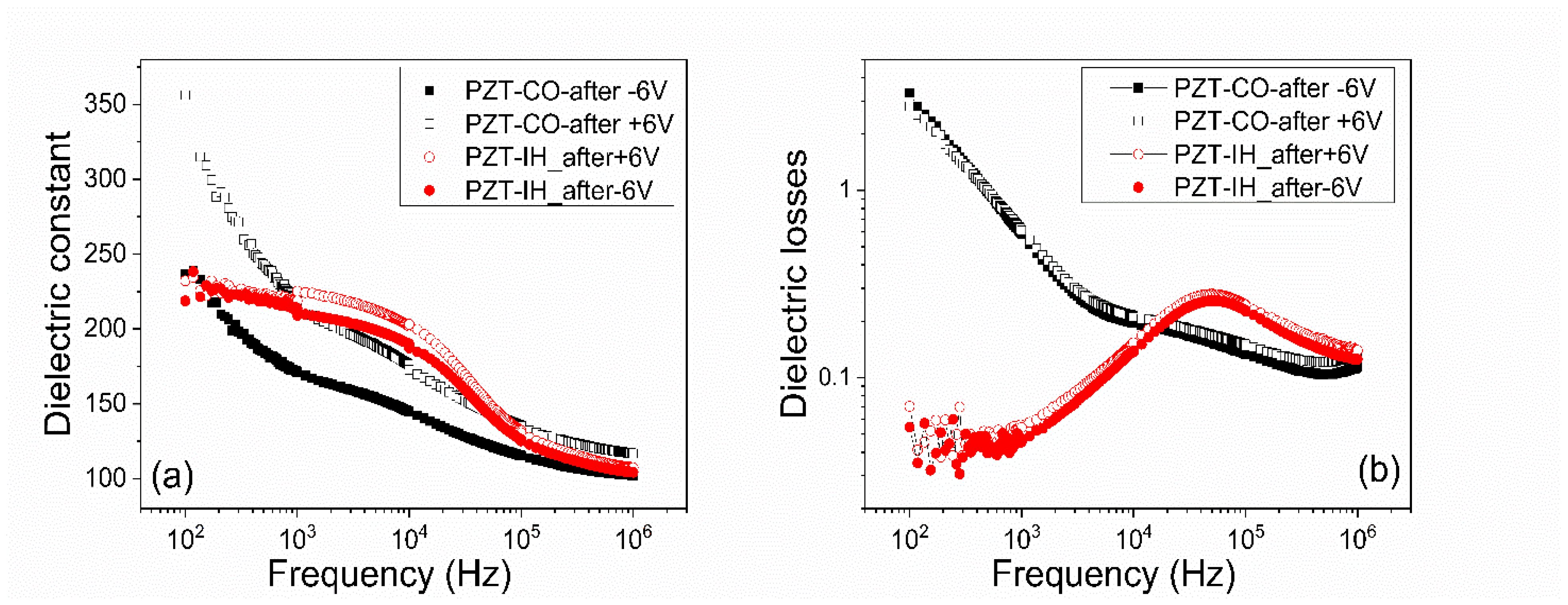
- The frequency dependence of the dielectric constant (Figure 6a) showed a continuous decrease of value for the PZT film deposited from the commercial target, while the film deposited from the pure target (PZT-IH) showed a relatively constant dielectric constant up to about 10 kHz, and then a decrease towards a value comparable to that of the PZT-CO sample. The results were in agreement with those obtained from C–V measurements. The losses for PZT-CO were very large at low frequencies, also in agreement with the results presented in Figure 5b, suggesting high dc leakage current. The losses for PZT-IH showed a relaxation peak at around 50–60 kHz. The losses for PZT-IH became larger than for PZT-CO at frequencies above 10 kHz, in agreement with the results presented in Figure 5c. The differences can be attributed to different content of impurities, triggering different distribution of energy levels in the gap. However, it is beyond the scope of the present study to identify the nature of the impurities and the position of the associated energy levels in the gap.
- -
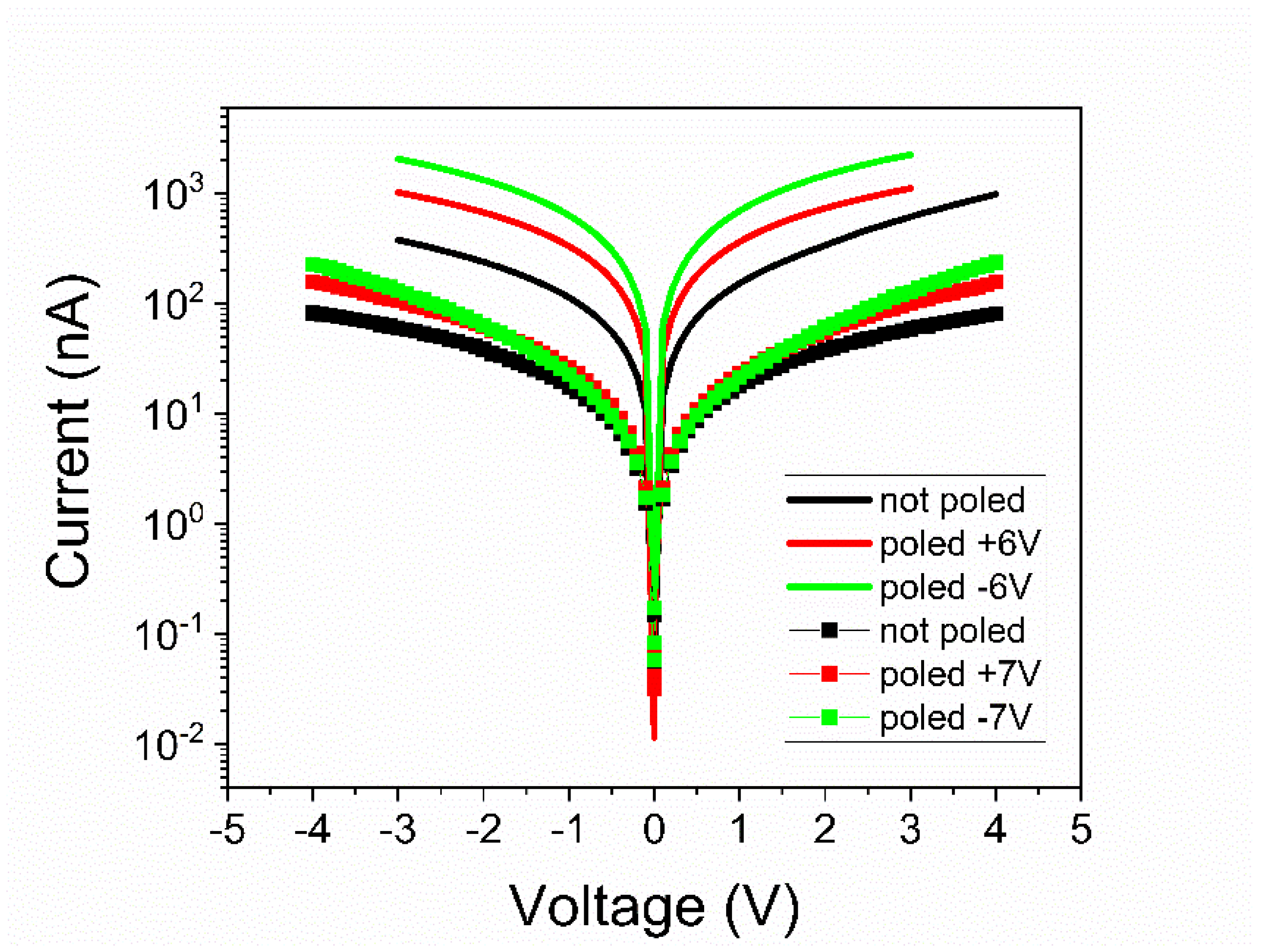
- The magnitude of the leakage current decreased significantly for the film grown from the pure target, statistically with more than one order of magnitude. Statistics performed on several electrodes, randomly selected, revealed that the leakage current, could have, sometimes, a significant dependence on the polarization state, more precisely on the polarization orientation, as presented in Figure 7. However, in most cases, the difference was small or absent. Statistically it was also observed that the current magnitude was larger after poling the samples compared to currents measured on not poled samples (as grown).
- -
- The potential barriers for the PZT film deposited from commercial target were rather similar, no matter the initial polarization state. The difference for positive and negative branches of the I–V characteristics was larger only for the as-deposited sample, and this was because there might have been defects that could migrate under the applied electric field, affecting the interface properties. Once the polarization state was clearly defined and there were no longer migrating defects, the potential barrier stabilized at around 0.11–0.13 eV for both voltage polarities. It has to be mentioned that the error in estimating the potential barrier was of about 0.015 eV; thus, the values presented in Table 2 are within these errors.
- -
- The results were similar for the film deposited from the pure target; the as deposited films had larger barriers, but these dropped to about 0.140–0.155 eV once the ferroelectric polarization had a clear orientation in the film. Nevertheless, the potential barriers were constantly higher compared to those obtained for the film deposited from the commercial target. The difference was between 0.020 eV and 0.070 eV depending on the polarization state. On average, the potential barriers were with about 0.04–0.05 eV higher for the film deposited from the pure target. Assuming that the Schottky emission was the dominant conduction mechanism around room temperature, the results are that this difference in the height of the potential barriers at the electrode interfaces could produce a difference of at least one order of magnitude between the leakage currents, with a lower value in the film deposited from the pure target. This is in good agreement with the experimental results presented in Figure 7.
- -
- One can note that the heights of potential barriers were comparable to previous reports in the literature [26]. One can argue that these values were considerably lower than those extracted from X-ray photoelectron spectroscopy (XPS) measurements, which were around 1 eV [43]. However, if one considers the polarization contribution to the reduction of the potential barrier [42], the estimated values increased to about 0.6 eV (a value obtained when using a static dielectric constant of about 200, an average polarization value of about 80 µC/cm2, and an optical dielectric constant of about 6.5 [42] (see also Supplemental Information), a value that started to be comparable to the values extracted from XPS. The issue for discussion is which value should be used for the static dielectric constant, the one extracted from capacitance measurements (see Figure 5) or the intrinsic value, estimated to be between 30 and 40 [44]. If a value of 40 is used for the static dielectric constant, then the height of the potential barrier becomes about 0.8–0.9 eV, much closer to the values estimated from XPS data. In any case, the topic of the dielectric constant in ferroelectrics is beyond the scope of the present article.
3.3. Chemical Analysis
- -
- If one adds the estimated quantities for all trace elements, one obtains about the same total, around 130–140 ppm weight, although the declared purity of the commercial target is 99.9% by weight and the purity of the starting metal oxides for the pure target is at least 99.99% by weight. This result suggests that the commercial target may contain other impurities that could not be detected by the method used to assess the target purity.
- -
- There are isovalent impurities, such as Ba, Ca, Cd, Sr, Hf, Si, and Sn, which can replace Pb or Ti/Zr without introducing free carriers in the sample.
- -
- Aside from Pb and Ti that are present in the matrix, some traces of Pb and Ti were detected in the targets, but it is not clear if these traces are related to some unreacted PbO and TiO2 quantities.
- -
- The other elements found in the targets are of different valance compared to Pb and Ti/Zr and can replace these elements in the PZT matrix if they fulfill the Goldschmidt tolerance factor [46], or the newly defined tolerance factor in [47]. In any case, these heterovalent impurities can act as donors or acceptors in PZT, thus affecting the density of the free carriers in the sample. Eliminating the isovalent elements, then the amount of heterovalent elements, those that can introduce free carriers in the PZT layer, is about 95 ppm in the commercial target and about 19 ppm in the pure target manufactured in house from as purse as possible precursor oxides. The difference can justify the larger leakage current in the film deposited from the commercial target, as well the larger polarization value due to a better compensation of the depolarization field. They can affect also the lattice constant, due to different ionic radii, and can introduce a multitude of energy levels in the PZT’s gap, affecting the position of the Fermi level and the height of the potential barriers at the electrode interfaces.
4. Conclusions
- -
- The c lattice constant is with about 0.15% larger in the film deposited from the commercial target compared to the film deposited from the in-house made target, leading to a larger tetragonality and larger value of polarization, namely 85–90 µC/cm2 in PZT-CO compared to 75–80 µC/cm2 in PZT-IH.
- -
- The PZT-CO has an upward orientation of polarization, with the characteristic grid of 90 degree domain walls, while the PZT-IH shows a structure of mixed domains, 180 degree domains being also present. These differences in domain structure reflect in different values of the dielectric constant, which is larger in PZT-IH.
- -
- The leakage current is about one order of magnitude lower in PZT-IH, while the potential barriers at electrode interfaces are about 40–50 meV higher compared to PZT-CO.
- -
- Composition analysis revealed that the amount of heterovalent impurities is about two times larger in the commercial target, with impacts on the density of free carriers, on the position of the Fermi level, and on the magnitude of the leakage current.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Y. Ferroelectric Materials and Their Applications; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-1-4832-9095-9. [Google Scholar]
- Merchant, S. (Ed.) Ferroelectrics: Principles, Structure and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2014; ISBN 978-1-63321-962-5. [Google Scholar]
- Tagantsev, A.K.; Sherman, V.O.; Astafiev, K.F.; Venkatesh, J.; Setter, N. Ferroelectric Materials for Microwave Tunable Applications. J. Electroceramics 2003, 11, 5–66. [Google Scholar] [CrossRef]
- Ramesh, R.; Aggarwal, S.; Auciello, O. Science and Technology of Ferroelectric Films and Heterostructures for Non-Volatile Ferroelectric Memories. Mater. Sci. Eng. R Rep. 2001, 32, 191–236. [Google Scholar] [CrossRef]
- Rogalski, A.; Chrzanowski, K. Infrared Devices and Techniques (Revision). Metrol. Meas. Syst. 2014. [Google Scholar] [CrossRef]
- Muralt, P. Micromachined Infrared Detectors Based on Pyroelectric Thin Films. Rep. Prog. Phys. 2001, 64, 1339. [Google Scholar] [CrossRef]
- Bowen, C.R.; Taylor, J.; LeBoulbar, E.; Zabek, D.; Chauhan, A.; Vaish, R. Pyroelectric Materials and Devices for Energy Harvesting Applications. Energy Environ. Sci. 2014, 7, 3836–3856. [Google Scholar] [CrossRef]
- Scott, J.F. Ferroelectric Memories. In Springer Series in Advanced Microelectronics; Springer: Berlin/Heidelberg, Germany, 2000; Volume 3, ISBN 978-3-642-08565-9. [Google Scholar]
- Yuan, Y.; Xiao, Z.; Yang, B.; Huang, J. Arising Applications of Ferroelectric Materials in Photovoltaic Devices. J. Mater. Chem. A 2014, 2, 6027–6041. [Google Scholar] [CrossRef]
- Schwartz, R.W.; Schneller, T.; Waser, R. Chemical Solution Deposition of Electronic Oxide Films. Comptes Rendus Chim. 2004, 7, 433–461. [Google Scholar] [CrossRef]
- Dawber, M.; Rabe, K.M.; Scott, J.F. Physics of Thin-Film Ferroelectric Oxides. Rev. Mod. Phys. 2005, 77, 1083–1130. [Google Scholar] [CrossRef]
- Schwarzkopf, J.; Fornari, R. Epitaxial Growth of Ferroelectric Oxide Films. Prog. Cryst. Growth Charact. Mater. 2006, 52, 159–212. [Google Scholar] [CrossRef]
- Jaffe, B. Piezoelectric Ceramics, 1st ed.; Academic Press: London, UK; New York, NY, USA, 1971. [Google Scholar]
- Lin, E.L.; Posadas, A.B.; Zheng, L.; Wu, H.W.; Chen, P.-Y.; Coffey, B.M.; Lai, K.; Demkov, A.A.; Smith, D.J.; Ekerdt, J.G. Epitaxial Integration of Ferroelectric and Conductive Perovskites on Silicon. J. Vac. Sci. Technol. A 2020, 38, 022403. [Google Scholar] [CrossRef]
- Lyu, J.; Fina, I.; Bachelet, R.; Saint-Girons, G.; Estandía, S.; Gázquez, J.; Fontcuberta, J.; Sánchez, F. Enhanced Ferroelectricity in Epitaxial Hf0.5Zr0.5O2 Thin Films Integrated with Si (001) Using SrTiO3 Templates. Appl. Phys. Lett. 2019, 114, 222901. [Google Scholar] [CrossRef]
- Dubourdieu, C.; Bruley, J.; Arruda, T.M.; Posadas, A.; Jordan-Sweet, J.; Frank, M.M.; Cartier, E.; Frank, D.J.; Kalinin, S.V.; Demkov, A.A.; et al. Switching of Ferroelectric Polarization in Epitaxial BaTiO 3 Films on Silicon without a Conducting Bottom Electrode. Nat. Nanotechnol. 2013, 8, 748–754. [Google Scholar] [CrossRef]
- Eichel, R.-A.; Kungl, H.; Jakes, P. Defect Structure of Non-Stoichiometric and Aliovalently Doped Perovskite Oxides. Mater. Technol. 2013, 28, 241–246. [Google Scholar] [CrossRef]
- Lee, B.W.; Lee, E.J. Effects of Complex Doping on Microstructural and Electrical Properties of PZT Ceramics. J. Electroceram. 2006, 17, 597–602. [Google Scholar] [CrossRef]
- Guo, X.; Pithan, C.; Ohly, C.; Jia, C.-L.; Dornseiffer, J.; Haegel, F.-H.; Waser, R. Enhancement of P-Type Conductivity in Nanocrystalline BaTiO3 Ceramics. Appl. Phys. Lett. 2005, 86, 082110. [Google Scholar] [CrossRef]
- Nagano, D.; Funakubo, H.; Shinozaki, K.; Mizutani, N. Electrical Properties of Semiconductive Nb-Doped BaTiO3 Thin Films Prepared by Metal–Organic Chemical-Vapor Deposition. Appl. Phys. Lett. 1998, 72, 2017–2019. [Google Scholar] [CrossRef]
- Cross, J.S.; Kim, S.-H.; Wada, S.; Chatterjee, A. Characterization of Bi and Fe Co-Doped PZT Capacitors for FeRAM. Sci. Technol. Adv. Mater. 2010, 11, 044402. [Google Scholar] [CrossRef]
- Klissurska, R.D.; Brooks, K.G.; Reaney, I.M.; Pawlaczyk, C.; Kosec, M.; Setter, N. Effect of Nb Doping on the Microstructure of Sol—Gel-Derived PZT Thin Films. J. Am. Ceram. Soc. 1995, 78, 1513–1520. [Google Scholar] [CrossRef]
- Auslender, M.; Hava, S. Single-Crystal Silicon: Electrical and Optical Properties. In Springer Handbook of Electronic and Photonic Materials; Kasap, S., Capper, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; p. 1. ISBN 978-3-319-48933-9. [Google Scholar]
- Newman, R.C. Defects in Silicon. Rep. Prog. Phys. 1982, 45, 1163–1210. [Google Scholar] [CrossRef]
- Pintilie, I.; Pasuk, I.; Ibanescu, G.A.; Negrea, R.; Chirila, C.; Vasile, E.; Pintilie, L. The impact of the Pb(Zr,Ti)O3-ZnO interface quality on the hysteretic properties of a metal-ferroelectric-semiconductor structure. J. Appl. Phys. 2012, 112, 104103. [Google Scholar] [CrossRef]
- Pintilie, I.; Teodorescu, C.M.; Ghica, C.; Chirila, C.; Boni, A.G.; Hrib, L.; Pasuk, I.; Negrea, R.; Apostol, N.; Pintilie, L. Polarization-Control of the Potential Barrier at the Electrode Interfaces in Epitaxial Ferroelectric Thin Films. ACS Appl. Mater. Interfaces 2014, 6, 2929–2939. [Google Scholar] [CrossRef]
- Pintilie, L.; Ghica, C.; Teodorescu, C.M.; Pintilie, I.; Chirila, C.; Pasuk, I.; Trupina, L.; Hrib, L.; Boni, A.G.; Georgiana Apostol, N.; et al. Polarization Induced Self-Doping in Epitaxial Pb(Zr0.20Ti0.80)O3 Thin Films. Sci. Rep. 2015, 5, 14974. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, Y.; Ono, T.; Segawa, H.; Tominaga, K.; Okada, M. Preparation and Electrical Properties of MOCVD-deposited PZT Thin Films. J. Appl. Phys. 1991, 69, 8352–8357. [Google Scholar] [CrossRef]
- Janolin, P.-E.; Fraisse, B.; Le Marrec, F.; Dkhil, B. Partial Decoupling between Strain and Polarization in Mono-Oriented Pb(Zr0.2Ti0.8)O3 Thin Film. Appl. Phys. Lett. 2007, 90, 212904. [Google Scholar] [CrossRef]
- Jona, F.; Shirane, G. Ferroelectric Crystals; Dover Publications: New York, NY, USA, 1993; ISBN 978-0-486-67386-8. [Google Scholar]
- Morioka, H.; Yokoyama, S.; Oikawa, T.; Funakubo, H.; Saito, K. Spontaneous Polarization Change with Zr∕(Zr+Ti) Ratios in Perfectly Polar-Axis-Orientated Epitaxial Tetragonal Pb(Zr,Ti)O3 Films. Appl. Phys. Lett. 2004, 85, 3516–3518. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Fan, L.; Diéguez, O.; Cao, J.; Pan, Z.; Wang, Y.; Wang, J.; Kim, M.; Deng, S.; et al. Giant Polarization in Super-Tetragonal Thin Films through Interphase Strain. Science 2018, 361, 494–497. [Google Scholar] [CrossRef]
- Kwak, B.S.; Erbil, A.; Budai, J.D.; Chisholm, M.F.; Boatner, L.A.; Wilkens, B.J. Domain Formation and Strain Relaxation in Epitaxial Ferroelectric Heterostructures. Phys. Rev. B 1994, 49, 14865–14879. [Google Scholar] [CrossRef]
- Kwak, B.S.; Erbil, A.; Wilkens, B.J.; Budai, J.D.; Chisholm, M.F.; Boatner, L.A. Strain Relaxation by Domain Formation in Epitaxial Ferroelectric Thin Films. Phys. Rev. Lett. 1992, 68, 3733–3736. [Google Scholar] [CrossRef]
- Luo, Y.; Li, X.; Chang, L.; Gao, W.; Yuan, G.; Yin, J.; Liu, Z. Upward Ferroelectric Self-Poling in (001) Oriented PbZr0.2Ti0.8O3 Epitaxial Films with Compressive Strain. AIP Adv. 2013, 3, 122101. [Google Scholar] [CrossRef]
- Lee, D.; Yoon, A.; Jang, S.Y.; Yoon, J.-G.; Chung, J.-S.; Kim, M.; Scott, J.F.; Noh, T.W. Giant Flexoelectric Effect in Ferroelectric Epitaxial Thin Films. Phys. Rev. Lett. 2011, 107, 057602. [Google Scholar] [CrossRef]
- Chen, H.T.; Soh, A.K.; Ni, Y. Phase Field Modeling of Flexoelectric Effects in Ferroelectric Epitaxial Thin Films. Acta Mech. 2014, 225, 1323–1333. [Google Scholar] [CrossRef]
- Ma, W. A Study of Flexoelectric Coupling Associated Internal Electric Field and Stress in Thin Film Ferroelectrics. Phys. Satus Solidi (B) 2008, 245, 761–768. [Google Scholar] [CrossRef]
- Lu, H.; Bark, C.-W.; de los Ojos, D.E.; Alcala, J.; Eom, C.B.; Catalan, G.; Gruverman, A. Mechanical Writing of Ferroelectric Polarization. Science 2012, 336, 59–61. [Google Scholar] [CrossRef]
- Park, B.H.; Hyun, S.J.; Moon, C.R.; Choe, B.-D.; Lee, J.; Kim, C.Y.; Jo, W.; Noh, T.W. Imprint Failures and Asymmetric Electrical Properties Induced by Thermal Processes in Epitaxial Bi4Ti3O12 Thin Films. J. Appl. Phys. 1998, 84, 4428–4435. [Google Scholar] [CrossRef]
- Pintilie, L.; Alexe, M. Metal-Ferroelectric-Metal Heterostructures with Schottky Contacts. I. Influence of the Ferroelectric Properties. J. Appl. Phys. 2005, 98, 124103. [Google Scholar] [CrossRef]
- Pintilie, L.; Vrejoiu, I.; Hesse, D.; LeRhun, G.; Alexe, M. Ferroelectric Polarization-Leakage Current Relation in High Quality Epitaxial Pb(Zr,Ti)O3 Films. Phys. Rev. B 2007, 75, 104103. [Google Scholar] [CrossRef]
- Li, S.; Morasch, J.; Klein, A.; Chirila, C.; Pintilie, L.; Jia, L.; Ellmer, K.; Naderer, M.; Reichmann, K.; Gröting, M.; et al. Influence of Orbital Contributions to the Valence Band Alignment of Bi2O3, Fe2O3, BiFeO3, and Bi0.5Na0.5TiO3. Phys. Rev. B 2013, 88, 045428. [Google Scholar] [CrossRef]
- Pintilie, L.; Vrejoiu, I.; Hesse, D.; LeRhun, G.; Alexe, M. Extrinsic Contributions to the Apparent Thickness Dependence of the Dielectric Constant in Epitaxial PbZrTiO3 Thin Films. Phys. Rev. B 2007, 75, 224113. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New Tolerance Factor to Predict the Stability of Perovskite Oxides and Halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef]
- Chirila, C.; Boni, G.A.; Filip, L.D.; Husanu, M.; Neatu, S.; Istrate, C.M.; Rhun, G.L.; Vilquin, B.; Trupina, L.; Pasuk, I.; et al. Effect of Strain and Stoichiometry on the Ferroelectric and Pyroelectric Properties of the Epitaxial Pb(Zr0.2Ti0.8)O3 Films Deposited on Si Wafers. Mater. Sci. Eng. B 2021, 266, 115042. [Google Scholar] [CrossRef]

| Sample | Neff (m−3) | ||||
|---|---|---|---|---|---|
| 100 K | 300 K | ||||
| Positive Voltage | Negative Voltage | Positive Voltage | Negative Voltage | ||
| PZT-IH | AD | 9.093 × 1024 | 8.83 × 1024 | 9.9 × 1024 | 9.61 × 1024 |
| P+ | 8.16 × 1024 | 9.92 × 1024 | 1.013 × 1025 | 9.69 × 1024 | |
| P− | 8.89 × 1024 | 8.55 × 1024 | 1.0269 × 1025 | 9.84 × 1024 | |
| PZT-CO | AD | 7.05 × 1024 | 8 × 1024 | 6 × 1024 | 7.17 × 1024 |
| P+ | 7.35 × 1024 | 6.54 × 1024 | 9.58 × 1024 | 8.15 × 1024 | |
| P− | 9.58 × 1024 | 8.8 × 1024 | 8.17 × 1024 | 6.52 × 1024 | |
| Sample | |||
|---|---|---|---|
| Positive Voltage | Negative Voltage | ||
| PZT-IH | AD | 0.285 | 0.273 |
| P+ | 0.136 | 0.153 | |
| P− | 0.153 | 0.149 | |
| PZT-CO | AD | 0.113 | 0.209 |
| P+ | 0.118 | 0.114 | |
| P− | 0.127 | 0.121 | |
| Elements | Commercial Target (ppm Weight) | Pure Target (ppm Weight) | Valence State | Ionic Radii (pM) |
|---|---|---|---|---|
| Ag | <1 | ND | 1+/2+/3+ | 129/108/89 |
| Al | ND | 10 | 3+ | 67.5 |
| As | <4 | 11.5 | 3+/5+ | 72/68 |
| B | ND | 0.4 | 3+ | 41 |
| Ba | 2 | 1 | 4+ | 149 |
| Bi | <15 | ND | 3+/5+ | 117/90 |
| Ca | ND | 17.4 | 2+ | 114 |
| Cd | <3 | 17 | 2+ | 109 |
| Cl | <8 | ND | 1− | 167 |
| Co | <1 | ND | 2+/3+ | 79/68.5 |
| Cr | <1 | ND | 2+/3+/4+/5+/6+ | 87/75.5/69/63/58 |
| Cu | <2 | ND | 1+/2+ | 91/87 |
| Fe | <4 | 5.2 | 2+/3+ | 92/78.5 |
| Hf | <10 | ND | 4+ | 85 |
| K | 1 | 50.7 | 1+ | 152 |
| Mg | 2 | 1.1 | 2+ | 86 |
| Mn | <1 | 0.2 | 2+/3+ | 97/78.5 |
| N | <27 | ND | 3− | 132 |
| Na | <10 | 8 | 1+ | 116 |
| Ni | <4 | ND | 2+/3+/4+ | 83/70/62 |
| Pb | 1 | 6.9 | 2+/4+ | 133/91.5 |
| S | <2 | ND | 2− | 170 |
| Sb | <4 | ND | 3+/5+ | 90/74 |
| Se | <1 | ND | 4+/6+ | 64/56 |
| Si | <10 | 6 | 4+ | 54 |
| Sn | <4 | ND | 4+ | 83 |
| Sr | 2 | ND | 2+ | 132 |
| Te | <3 | ND | 4+/6+ | 111/70 |
| Ti | 2 | ND | 3+/4+ | 81/74.5 |
| V | ND | 2 | 2+/3+/4+/5+ | 93/78/72/68 |
| Zn | <3 | 2.3 | 2+ | 88 |
| Zr | ND | ND | 4+ | 86 |
| Commercial Target | ||
|---|---|---|
| Symbol | Element | Concentration (at/cm3) |
| Ag | Silver | 1.38 × 1019 |
| Al | Aluminum | 2.04 × 1019 |
| Bi | Bismuth | 3.09 × 1017 |
| Cr | Chromium | 2.29 × 1018 |
| Mg | Magnesium | 4.25 × 1018 |
| Mn | Manganese | 9.96 × 1018 |
| Ni | Nickel | 1.29 × 1018 |
| Si | Silicon | 9.47 × 1018 |
| Sr | Strontium | 3.5 × 1018 |
| Zn | Zinc | 1.33 × 1017 |
| Pure Target | ||
| Symbol | Element | Concentration (at/cm3) |
| Al | Aluminum | 1.12 × 1019 |
| Bi | Bismuth | 4.56 × 1017 |
| Cr | Chromium | 2.83 × 1017 |
| Mn | Manganese | 9.15 × 1018 |
| Ni | Nickel | 1.59 × 1018 |
| Si | Silicon | 7.64 × 1018 |
| Sr | Strontium | 3.16 × 1018 |
| Zn | Zinc | 9.14 × 1017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boni, G.A.; Chirila, C.F.; Stancu, V.; Amarande, L.; Pasuk, I.; Trupina, L.; Istrate, C.M.; Radu, C.; Tomulescu, A.; Neatu, S.; et al. Accidental Impurities in Epitaxial Pb(Zr0.2Ti0.8)O3 Thin Films Grown by Pulsed Laser Deposition and Their Impact on the Macroscopic Electric Properties. Nanomaterials 2021, 11, 1177. https://doi.org/10.3390/nano11051177
Boni GA, Chirila CF, Stancu V, Amarande L, Pasuk I, Trupina L, Istrate CM, Radu C, Tomulescu A, Neatu S, et al. Accidental Impurities in Epitaxial Pb(Zr0.2Ti0.8)O3 Thin Films Grown by Pulsed Laser Deposition and Their Impact on the Macroscopic Electric Properties. Nanomaterials. 2021; 11(5):1177. https://doi.org/10.3390/nano11051177
Chicago/Turabian StyleBoni, Georgia Andra, Cristina Florentina Chirila, Viorica Stancu, Luminita Amarande, Iuliana Pasuk, Lucian Trupina, Cosmin Marian Istrate, Cristian Radu, Andrei Tomulescu, Stefan Neatu, and et al. 2021. "Accidental Impurities in Epitaxial Pb(Zr0.2Ti0.8)O3 Thin Films Grown by Pulsed Laser Deposition and Their Impact on the Macroscopic Electric Properties" Nanomaterials 11, no. 5: 1177. https://doi.org/10.3390/nano11051177
APA StyleBoni, G. A., Chirila, C. F., Stancu, V., Amarande, L., Pasuk, I., Trupina, L., Istrate, C. M., Radu, C., Tomulescu, A., Neatu, S., Pintilie, I., & Pintilie, L. (2021). Accidental Impurities in Epitaxial Pb(Zr0.2Ti0.8)O3 Thin Films Grown by Pulsed Laser Deposition and Their Impact on the Macroscopic Electric Properties. Nanomaterials, 11(5), 1177. https://doi.org/10.3390/nano11051177









