Enzymatic Time-Temperature Indicator Prototype Developed by Immobilizing Laccase on Electrospun Fibers to Predict Lactic Acid Bacterial Growth in Milk during Storage
Abstract
1. Introduction
2. Material and Methods
2.1. Immobilization of Laccase on Electrospun Fibers
2.2. Morphology of Chitosan/Polyvinyl Alcohol/Tetraethyl Orthosilicate Tetraethylorthosilicate/Polypropylene/Glutaraldehyde/Laccase (ceCPTL)
2.3. Coloration
2.3.1. Color Measurement
2.3.2. Absorbance of Coloration
2.4. Kinetic Evaluation
2.5. Simulation of Milk Quality Change
2.6. Response of the Laccase Time–Temperature Indicator (TTI) Prototype and Lactic Acid Bacteria (LAB) Growth in Milk
2.6.1. Isothermal Response Test
2.6.2. Dynamic Temperature Response Test
2.7. Statistical Analysis
3. Results and Discussion
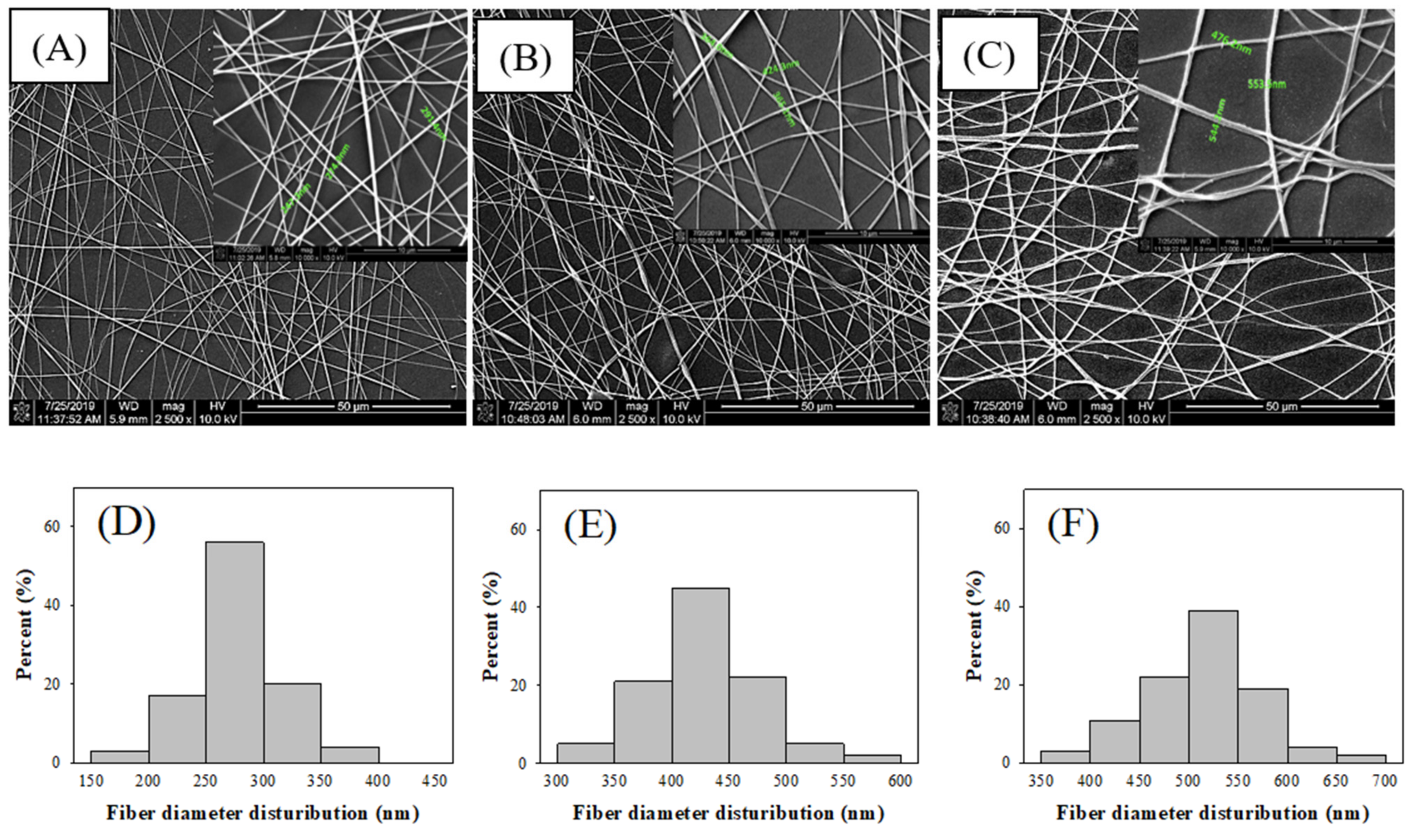
3.1. Immobilized Laccase on Electrospun Chitosan (CS) Fibers
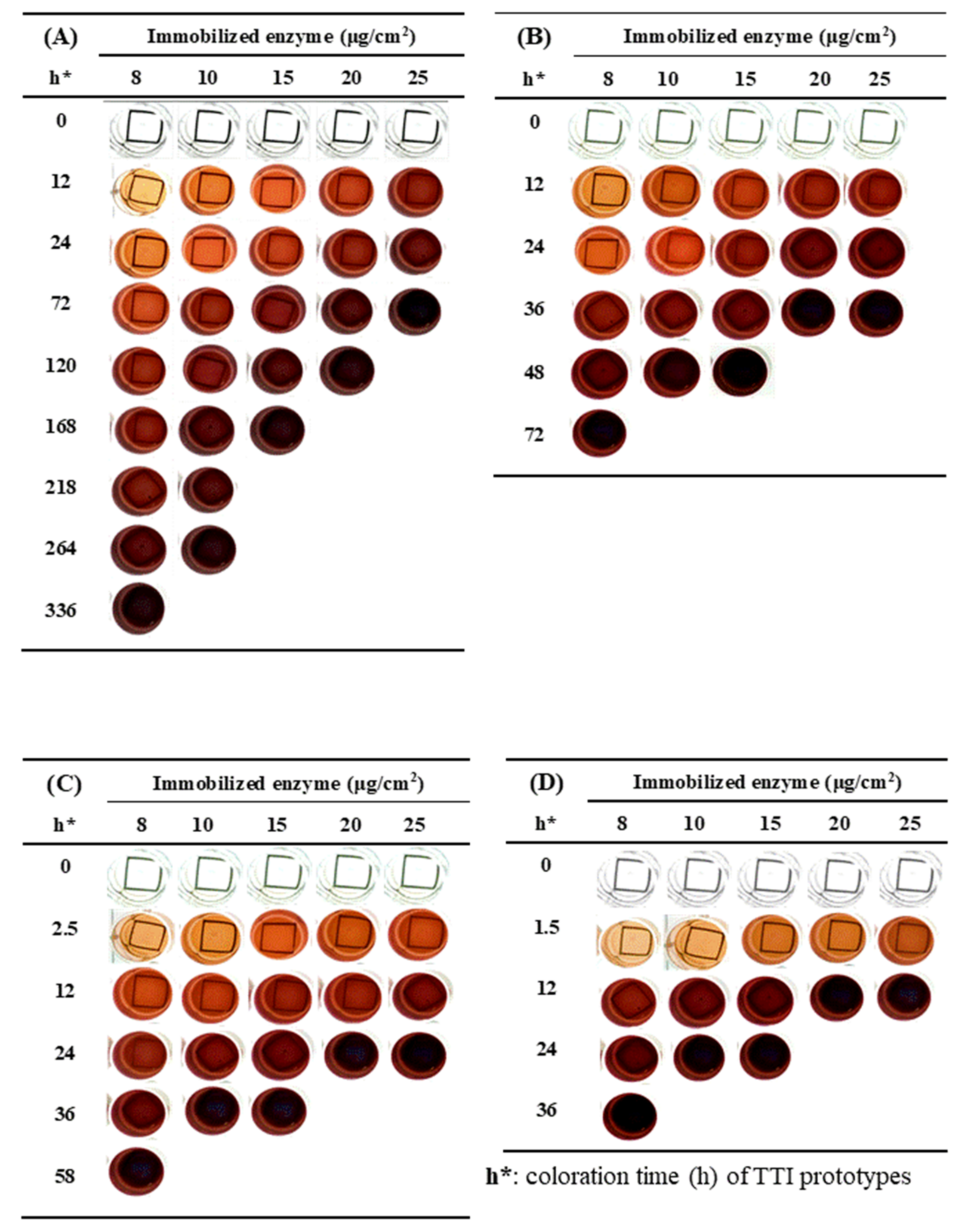
3.2. Coloration of the Laccase TTI Prototype
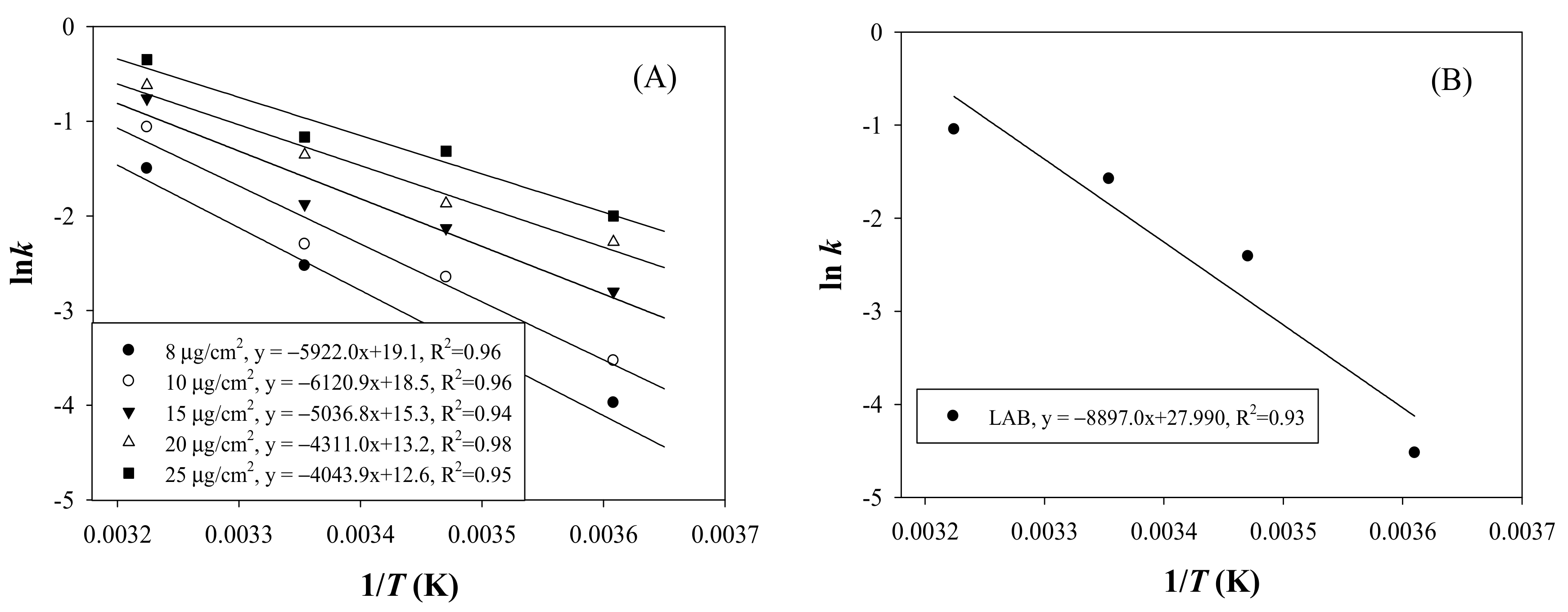
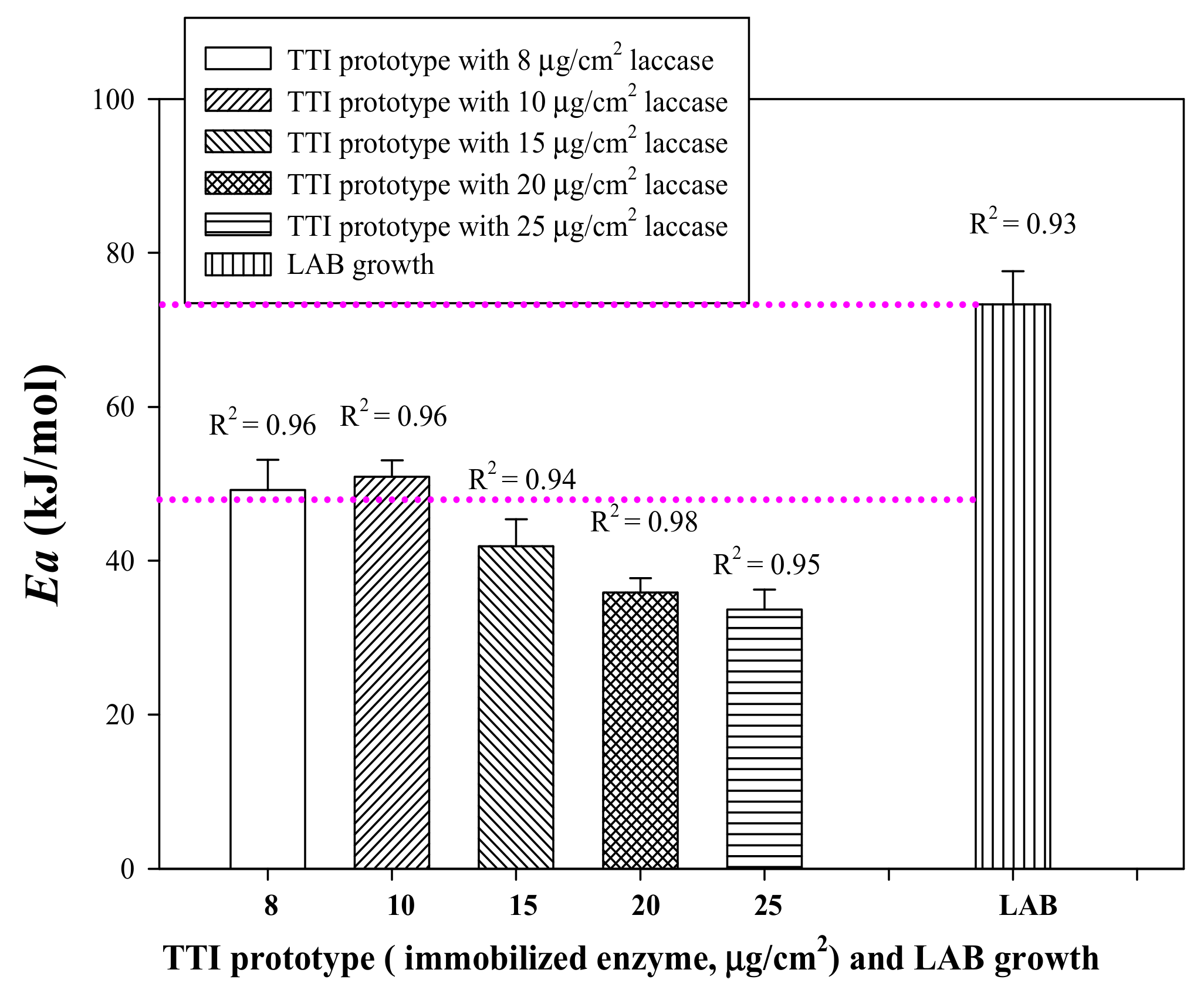
3.3. Arrhenius Activation Energy (Ea) of Laccase TTI Coloration and LAB Growth in Milk
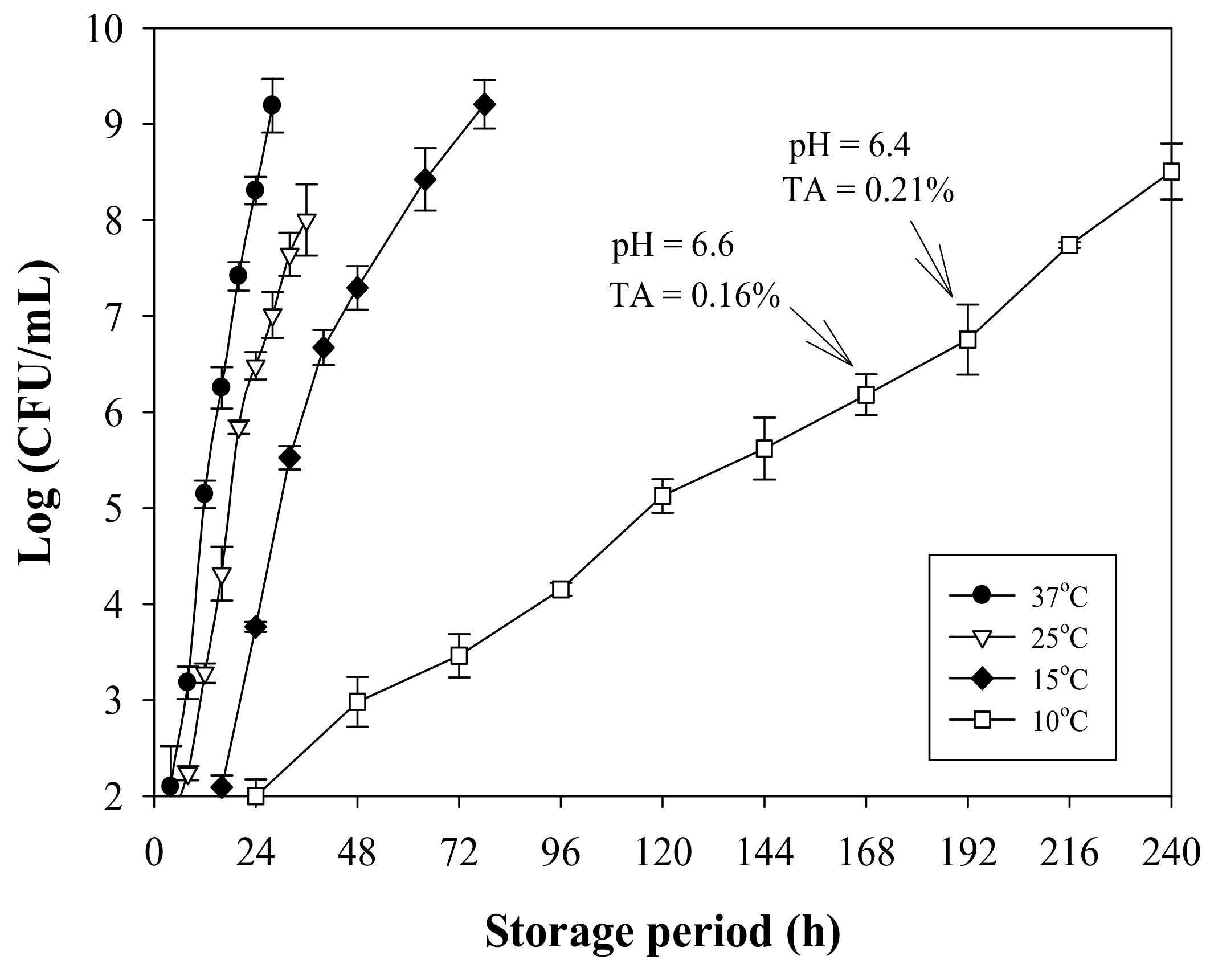
3.4. Milk Quality Change during Storage
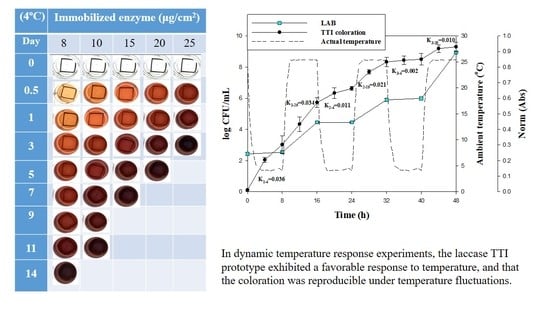
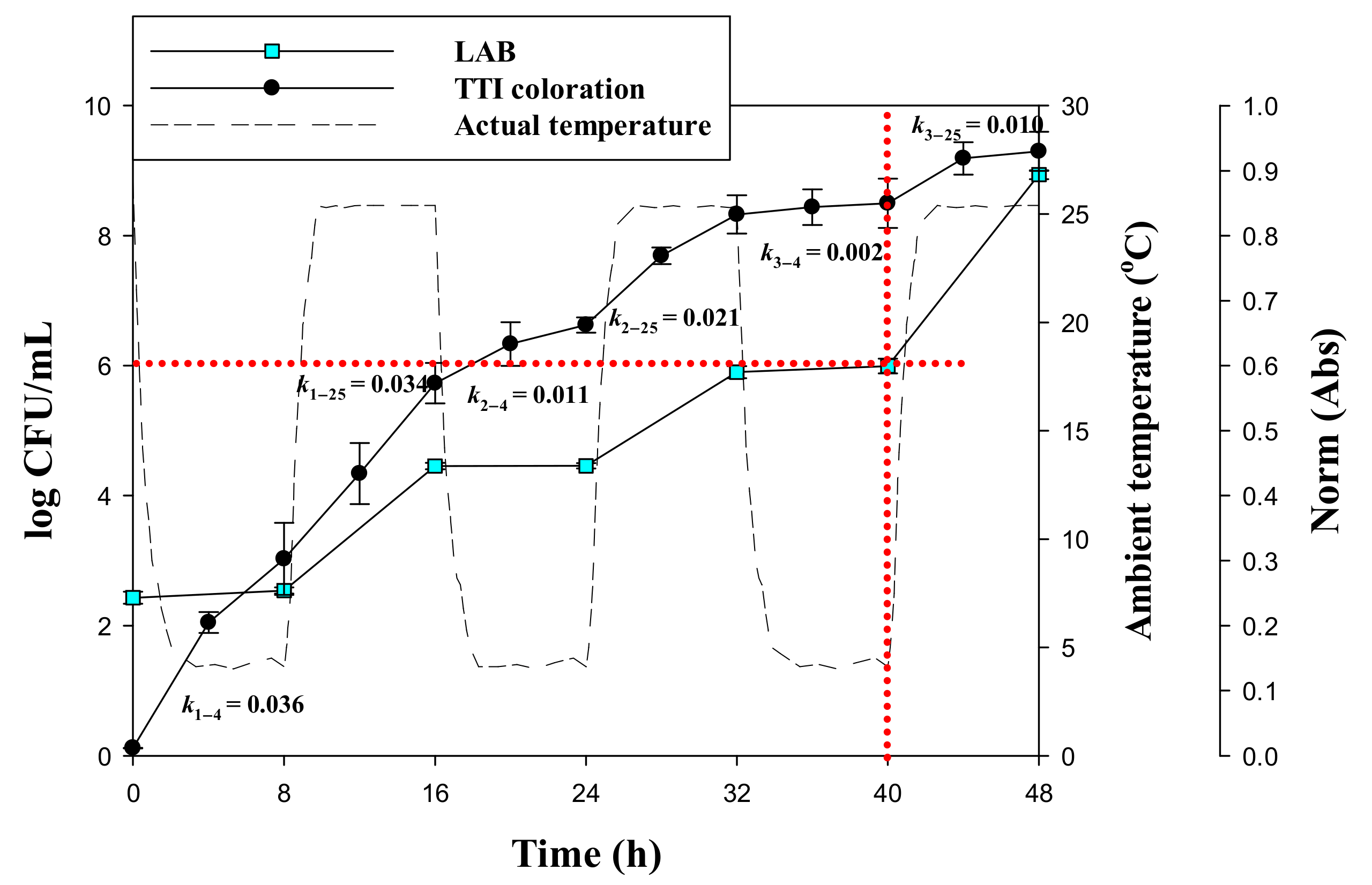
3.5. Response of Laccase TTI Prototype Coloration to Milk Quality Change
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- La Scalia, G.; Nasca, A.; Corona, O.; Settanni, L.; Micale, R. An Innovative Shelf Life Model Based on Smart Logistic Unit for an Efficient Management of the Perishable Food Supply Chain. J. Food Process. Eng. 2017, 40, e12311. [Google Scholar] [CrossRef]
- Singh, R.P.; Wells, J.H. Use of time-temperature indicators to monitor quality of frozen hamburger. Food Technol. 1985, 39, 42–50. [Google Scholar]
- Taoukis, P.; Labuza, T. Applicability of Time-Temperature Indicators as Shelf Life Monitors of Food Products. J. Food Sci. 1989, 54, 783–788. [Google Scholar] [CrossRef]
- Kim, J.U.; Ghafoor, K.; Ahn, J.; Shin, S.; Lee, S.H.; Shahbaz, H.M.; Shin, H.H.; Kim, S.; Park, J. Kinetic modeling and characterization of a diffusion-based time-temperature indicator (TTI) for monitoring microbial quality of non-pasteurized angelica juice. LWT-Food Sci. Technol. 2016, 67, 143–150. [Google Scholar] [CrossRef]
- Kim, K.; Kim, E.; Lee, S.J. New enzymatic time–temperature integrator (TTI) that uses laccase. J. Food Eng. 2012, 113, 118–123. [Google Scholar] [CrossRef]
- Dwivedi, U.N.; Singh, P.; Pandey, V.P.; Kumar, A. Structure–function relationship among bacterial, fungal and plant laccases. J. Mol. Catal. B Enzym. 2011, 68, 117–128. [Google Scholar] [CrossRef]
- Jhuang, J.-R.; Lou, S.-N.; Lin, S.-B.; Chen, S.H.; Chen, L.-C.; Chen, H.-H. Immobilizing laccase on electrospun chitosan fiber to prepare time-temperature indicator for food quality monitoring. Innov. Food Sci. Emerg. Technol. 2020, 63, 102370. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.H. Bioinformatics and enzymatics investigation of Trametes laccase for optical biosensing application. J Mater. Sci. 2019, 54, 4970–4983. [Google Scholar] [CrossRef]
- Hsu, C.A.; Wen, T.N.; Su, Y.C.; Jiang, Z.B.; Chen, C.W.; Shyur, L.F. Biological degradation of anthroquinone and azo dyes by a novel laccase from Lentinus sp. Environ. Sci. Technol. 2012, 46, 5109–5117. [Google Scholar] [CrossRef]
- Li, S.-F.; Chen, J.-P.; Wu, W.-T. Electrospun polyacrylonitrile nanofibrous membranes for lipase immobilization. J. Mol. Catal. B Enzym. 2007, 47, 117–124. [Google Scholar] [CrossRef]
- Kim, M.; Lee, H.; Kim, M.; Park, Y. Coloration and Chromatic Sensing Behavior of Electrospun Cellulose Fibers with Curcumin. Nanomaterials 2021, 11, 222. [Google Scholar] [CrossRef]
- Fatarella, E.; Spinelli, D.; Ruzzante, M.; Pogni, R. Nylon 6 film and nanofiber carriers: Preparation and laccase immobilization performance. J. Mol. Catal. B Enzym. 2014, 102, 41–47. [Google Scholar] [CrossRef]
- Kumari, A.; Datta, S. Phospholipid bilayer functionalized membrane pores for enhanced efficiency of immobilized glucose oxidase enzyme. J. Membr. Sci. 2017, 539, 43–51. [Google Scholar] [CrossRef]
- Susanto, H.; Samsudin, A.M.; Faz, M.W.; Rani, M.P.H. Impact of post-treatment on the characteristics of electrospun poly (vinyl alcohol)/chitosan nanofibers. In Proceedings of the 3rd International Conference on Advanced Materials Science and Technology (ICAMST 2015), Semarang, Indonesia, 6–7 October 2015; Volume 1725, p. 20087. [Google Scholar] [CrossRef]
- Pirzada, T.; Arvidson, S.A.; Saquing, C.D.; Shah, S.S.; Khan, S.A. Hybrid carbon silica nanofibers through sol-gel electro-spinning. Langmuir 2014, 30, 15504–15513. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Ready-to-Eat Foods from Food Safety for Moms to be. 2018. Available online: https://www.fda.gov/food/people-risk-foodborne-illness/ready-eat-foods-food-safety-moms-be (accessed on 27 September 2018).
- Yang, Y.L. Indicator Bacteria. Kinmen County Health Bureau. 2013. Available online: https://phb.kinmen.gov.tw/cp.aspx?n=0FAF18998E49EA5F. (accessed on 1 August 2020).
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a colorimetric pH indicator based on bacterial cellulose nanofibers and red cabbage (Brassica oleraceae) extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef]
- Haugen, J.E.; Rudi, K.; Langsrud, S.; Bredholt, S. Application of gas-sensor array technology for detection and monitoring of growth of spoilage bacteria in milk: A model study. Anal. Chim. Acta 2006, 565, 10–16. [Google Scholar] [CrossRef]
- Cuecas, A.; Cruces, J.; Galisteo-López, J.F.; Peng, X.; Gonzalez, J.M. Cellular Viscosity in Prokaryotes and Thermal Stability of Low Molecular Weight Biomolecules. Biophys. J. 2016, 111, 875–882. [Google Scholar] [CrossRef]
- Penasa, M.; Toffanin, V.; Cologna, N.; Cassandro, M.; De Marchi, M. Effects of dairy factory, milk casein content and titratable acidity on coagulation properties in Trentingrana dairy industry. J. Dairy Res. 2016, 83, 242–248. [Google Scholar] [CrossRef]
- Tsironi, T.; Stamatiou, A.; Giannoglou, M.; Velliou, E.; Taoukis, P.S. Predictive modelling and selection of Time Temperature Integrators for monitoring the shelf life of modified atmosphere packed gilthead seabream fillets. LWT 2011, 44, 1156–1163. [Google Scholar] [CrossRef]
- Koloti, L.E.; Gule, N.P.; Arotiba, O.A.; Malinga, S.P. Laccase-immobilized dendritic nanofibrous membranes as a novel ap-proach towards the removal of bisphenol A. Environ. Technol. 2018, 39, 392–404. [Google Scholar] [CrossRef]
- Corradini, M.G. Shelf Life of Food Products: From Open Labeling to Real-Time Measurements. Annu. Rev. Food Sci. Technol. 2018, 9, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, H.R.; Lee, S.J. Guideline for proper usage of time temperature integrator (TTI) avoiding underestimation of food deterioration in terms of temperature dependency: A case with a microbial TTI and milk. Food Sci. Biotechnol. 2016, 25, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Ziyaina, M.; Govindan, B.N.; Rasco, B.; Coffey, T.; Sablani, S.S. Monitoring shelf life of pasteurized whole milk under refrig-erated storage conditions: Predictive models for quality loss. J. Food Sci. 2018, 83, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.S.; Kaufmann, V.; Kulozik, U.; Scherer, S.; Wenning, M. Microbial biodiversity, quality and shelf life of microfiltered and pasteurized extended shelf life (ESL) milk from Germany, Austria and Switzerland. Int. J. Food Microbiol. 2012, 154, 1–9. [Google Scholar] [CrossRef]
- McAuley, C.M.; Singh, T.K.; Haro-Maza, J.F.; Williams, R.; Buckow, R. Microbiological and physicochemical stability of raw, pasteurised or pulsed electric field-treated milk. Innov. Food Sci. Emerg. Technol. 2016, 38, 365–373. [Google Scholar] [CrossRef]
- Lin, Y.; Kelly, A.L.; O’ Mahony, J.A.; Guinee, T.P. Altering the physico-chemical and processing characteristics of high heat-treated skim milk by increasing the pH prior to heating and restoring after heating. Food Chem. 2018, 245, 1079–1086. [Google Scholar] [CrossRef]
- Kim, W.; Park, E.; Hong, K. Development of a time-temperature integrator system using Burkholderia cepacia lipase. Food Sci. Biotechnol. 2012, 21, 497–502. [Google Scholar] [CrossRef]
| Time (h) | L* | a* | b* | ΔE | OD470 |
|---|---|---|---|---|---|
| 0.00 | 101.50 ± 1.94 a | 0.04 ± 0.03 f | 0.11 ± 0.16 e | 65.67 ± 0.84 a | 0.033 ± 0.00 |
| 4.00 | 86.24 ± 1.76 b | 14.61 ± 0.24 de | 17.14 ± 2.02 a | 52.64 ± 1.25 b | 1.209 ± 0.07 |
| 6.00 | 67.48 ± 1.90 c | 21.65 ± 0.96 a | 12.93 ± 1.84 b | 34.75 ± 0.91 c | 1.909 ± 0.04 |
| 18.00 | 52.59 ± 1.02 d | 21.67 ± 1.42 a | 6.71 ± 1.84 c | 19.53 ± 0.96 d | 2.58 ± 0.09 |
| 22.00 | 49.63 ± 1.08 e | 20.54 ± 1.29 a | 4.25 ± 1.29 cd | 15.64 ± 0.64 e | 2.64 ± 0.09 |
| 26.00 | 45.73 ± 0.73 g | 18.84 ± 1.33 b | 2.17 ± 1.91 de | 10.60 ± 1.75 f | 2.81 ± 0.02 |
| 58.00 | 43.37 ± 1.07 gh | 18.08 ± 1.45 bc | 0.42 ± 1.55 de | 4.55 ± 1.52 g | 3.04 ± 0.06 |
| 82.00 | 41.47 ± 1.05 hi | 16.43 ± 0.97 cd | −0.78 ± 1.51 e | 3.58 ± 0.93 g | 3.19 ± 0.08 |
| 106.00 | 40.24 ± 1.26 hi | 15.56 ± 1.65 d | −0.84 ± 1.46 e | 3.47 ± 0.88 g | 3.27 ± 0.04 |
| 202.00 | 37.24 ± 1.03 j | 13.38 ± 1.17 d | −2.06 ± 1.16 e | 0.00 ± 0.87 h | 3.50 ± 0.00 |
| Immobilized Enzyme (μg/cm2) | Coloration Endpoint (h) | Prediction Error (%) | Time for LAB Count Reached 106 CFU/mL (h) 1 | |||
|---|---|---|---|---|---|---|
| Temperature (°C) | 8 | 10 | 8 | 10 | ||
| 10 | 190.8 ± 4.9 | 165.6 ± 3.7 | −13.7 | 1.3 | 167.8 ± 2.5 | |
| 15 | 57.9 ± 2.1 | 39.5 ± 3.4 | −34.0 | 8.6 | 43.2 ± 2.3 | |
| 25 | 34.1 ± 2.6 | 21.8 ± 1.5 | −43.9 | 8.0 | 23.7 ± 1.7 | |
| 37 | 27.5 ± 3.3 | 15.2 ± 2.4 | −76.2 | 2.6 | 15.6 ± 2.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, T.-Y.; Chen, S.-H.; Chen, L.-C.; Lin, S.-B.; Lou, S.-N.; Chen, Y.-H.; Chen, H.-H. Enzymatic Time-Temperature Indicator Prototype Developed by Immobilizing Laccase on Electrospun Fibers to Predict Lactic Acid Bacterial Growth in Milk during Storage. Nanomaterials 2021, 11, 1160. https://doi.org/10.3390/nano11051160
Tsai T-Y, Chen S-H, Chen L-C, Lin S-B, Lou S-N, Chen Y-H, Chen H-H. Enzymatic Time-Temperature Indicator Prototype Developed by Immobilizing Laccase on Electrospun Fibers to Predict Lactic Acid Bacterial Growth in Milk during Storage. Nanomaterials. 2021; 11(5):1160. https://doi.org/10.3390/nano11051160
Chicago/Turabian StyleTsai, Ting-Yu, Shih-Hsin Chen, Li-Chen Chen, Shih-Bin Lin, Shyi-Neng Lou, Yen-Hui Chen, and Hui-Huang Chen. 2021. "Enzymatic Time-Temperature Indicator Prototype Developed by Immobilizing Laccase on Electrospun Fibers to Predict Lactic Acid Bacterial Growth in Milk during Storage" Nanomaterials 11, no. 5: 1160. https://doi.org/10.3390/nano11051160
APA StyleTsai, T.-Y., Chen, S.-H., Chen, L.-C., Lin, S.-B., Lou, S.-N., Chen, Y.-H., & Chen, H.-H. (2021). Enzymatic Time-Temperature Indicator Prototype Developed by Immobilizing Laccase on Electrospun Fibers to Predict Lactic Acid Bacterial Growth in Milk during Storage. Nanomaterials, 11(5), 1160. https://doi.org/10.3390/nano11051160