Combining Dextran Conjugates with Stimuli-Responsive and Folate-Targeting Activity: A New Class of Multifunctional Nanoparticles for Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
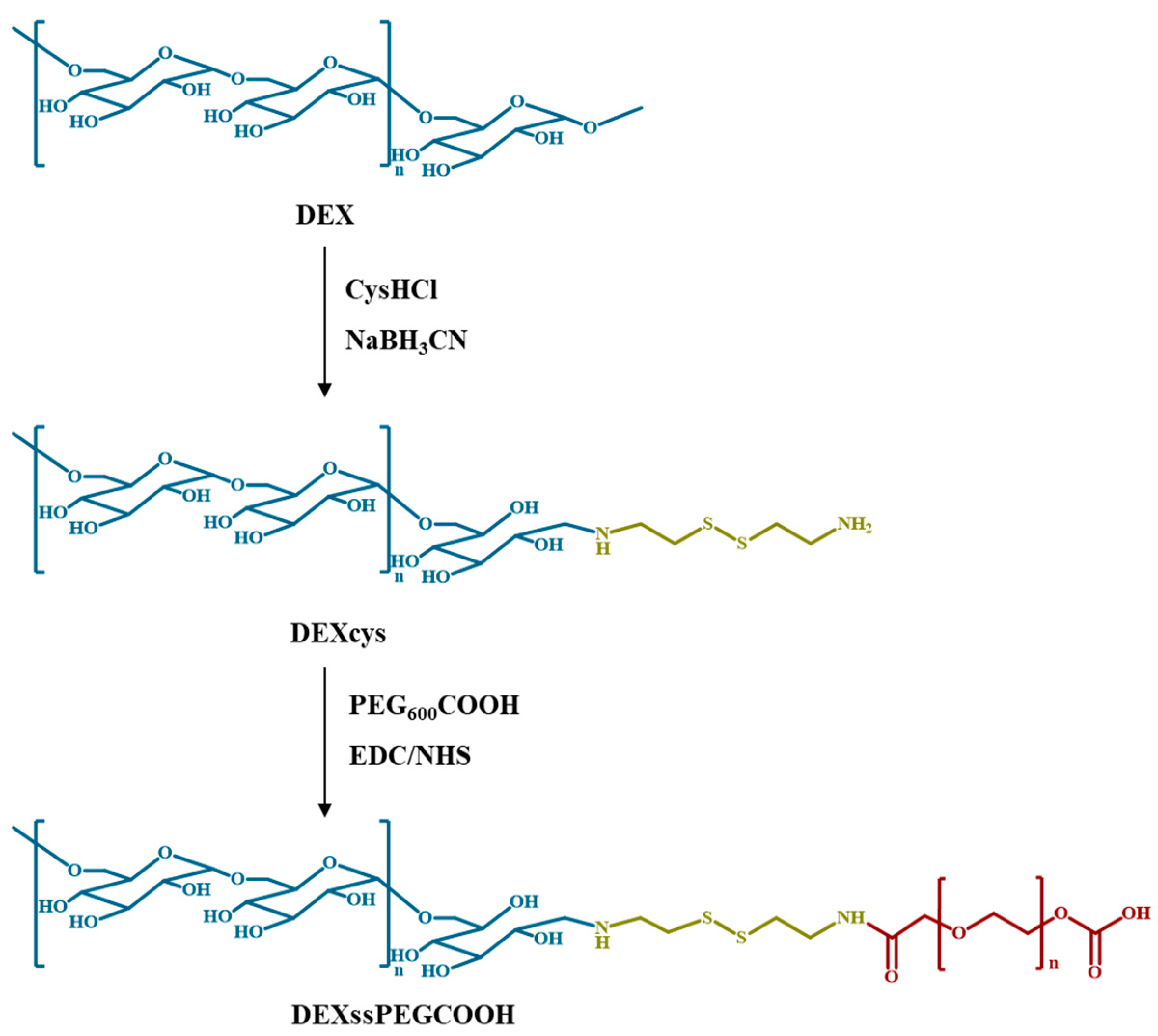
2.1. Synthesis of DEXcys
2.2. Synthesis of DEXssPEGCOOH Conjugate
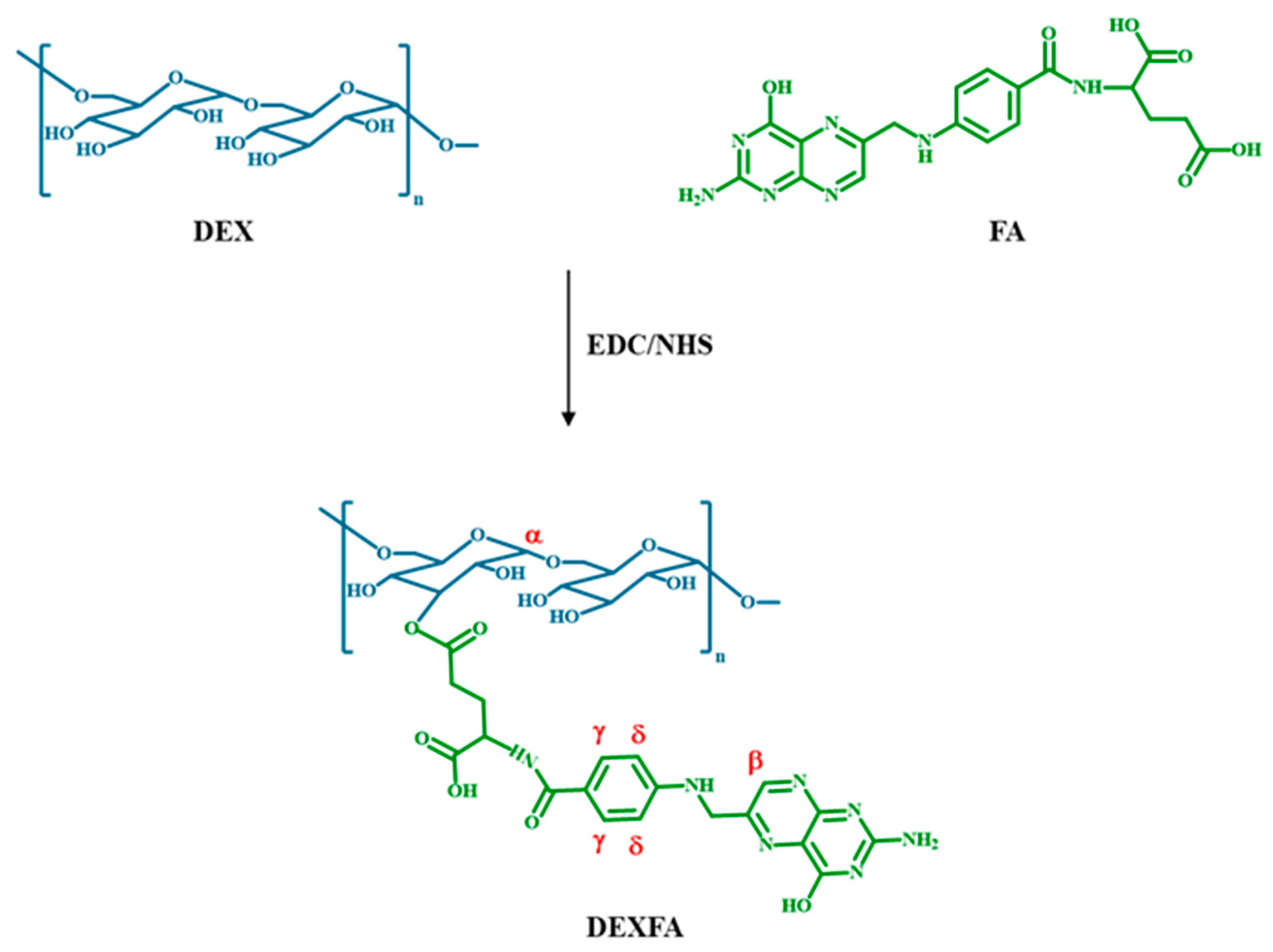
2.3. Synthesis of DEXFA
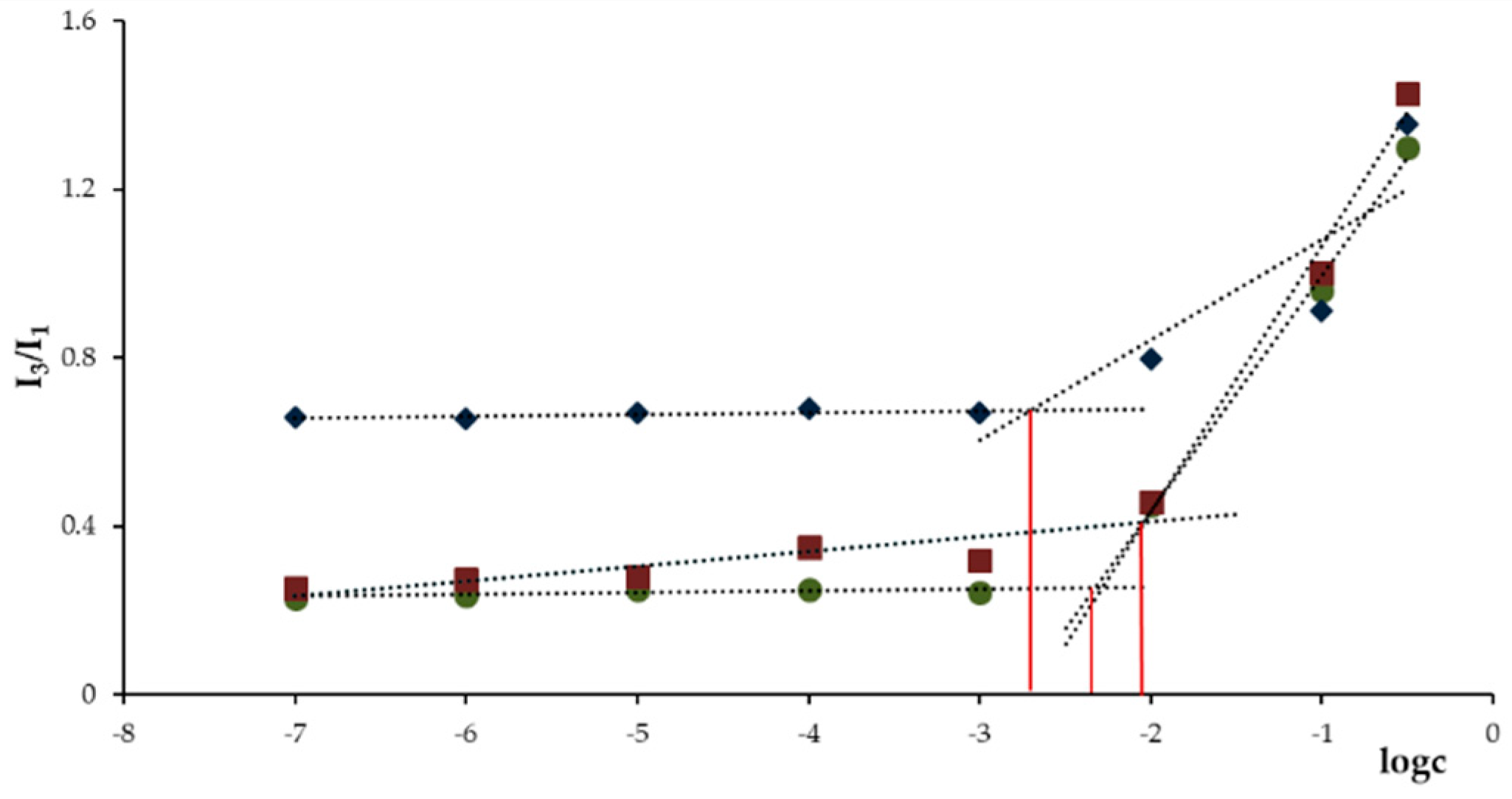
2.4. Determination of the Critical Aggregation Concentration (CAC)
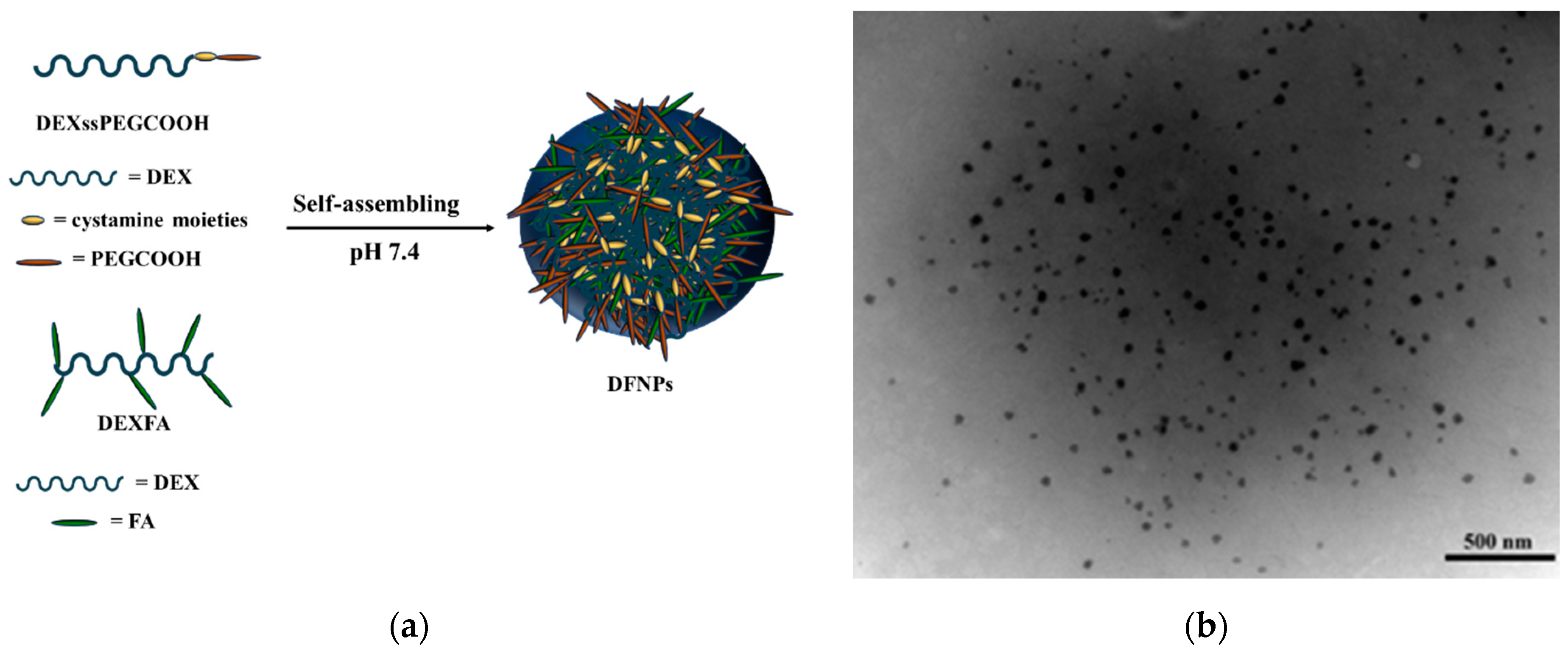
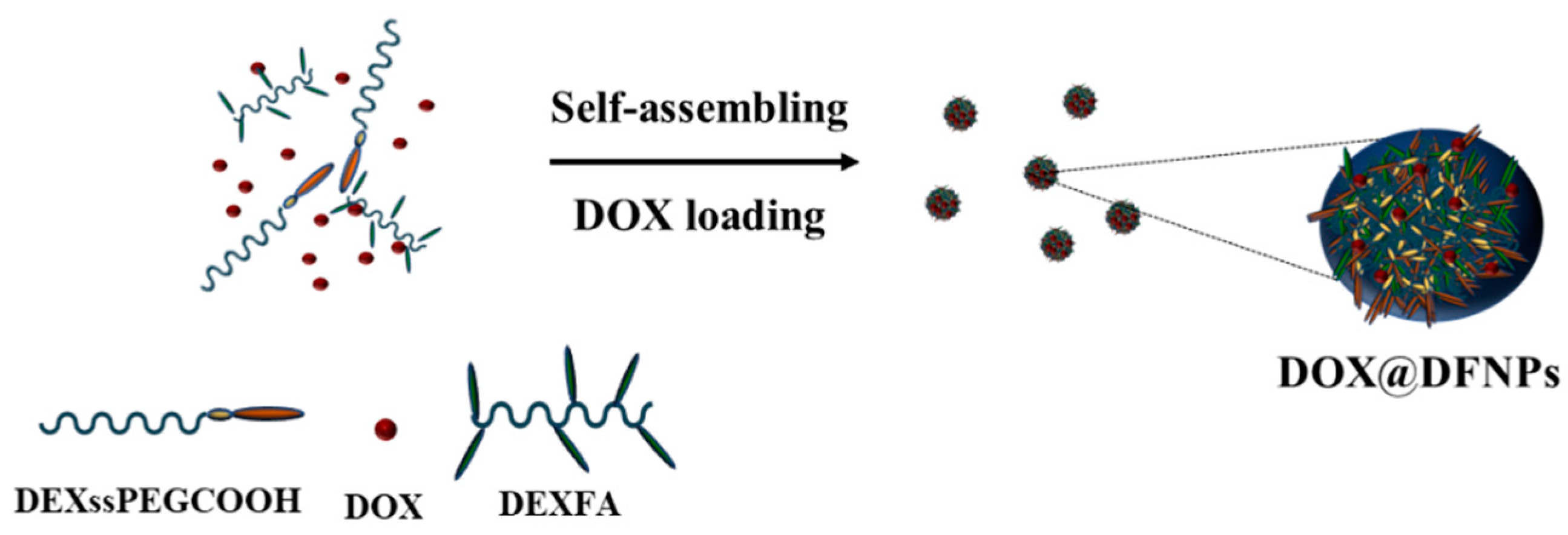
2.5. Preparation of Nanoparticles and DOX Loading
2.6. Destabilization Experiments
2.7. Release Experiments
2.8. Stability in Plasma Simulating Medium
2.9. Cell Culture
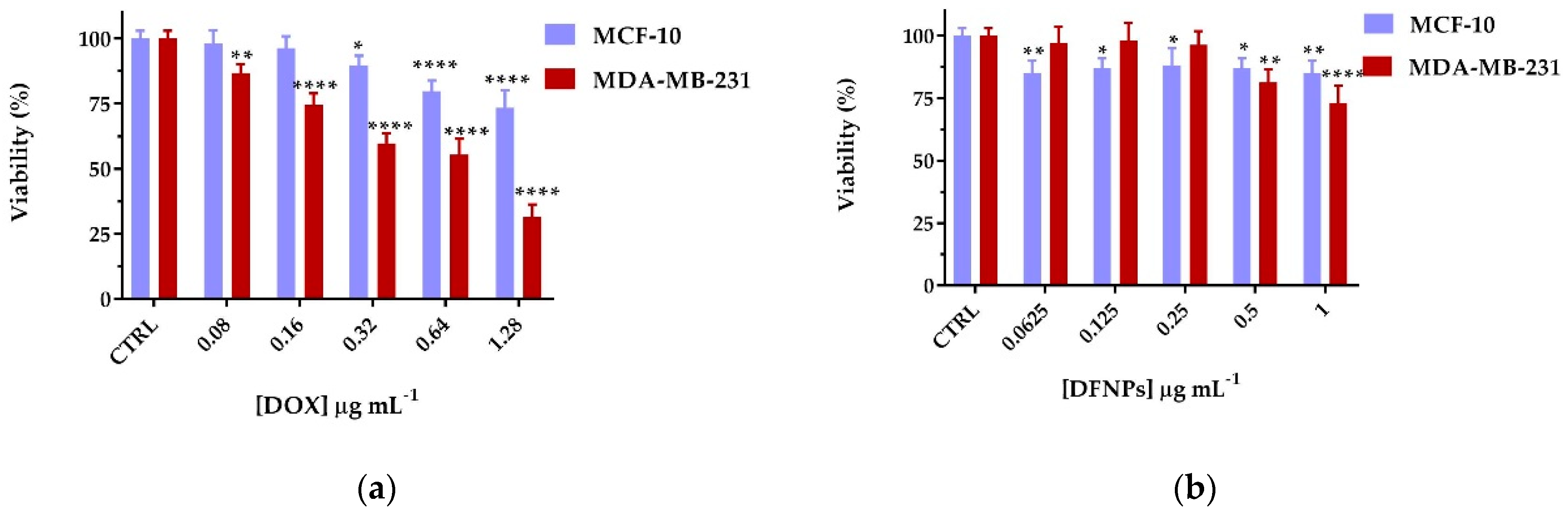
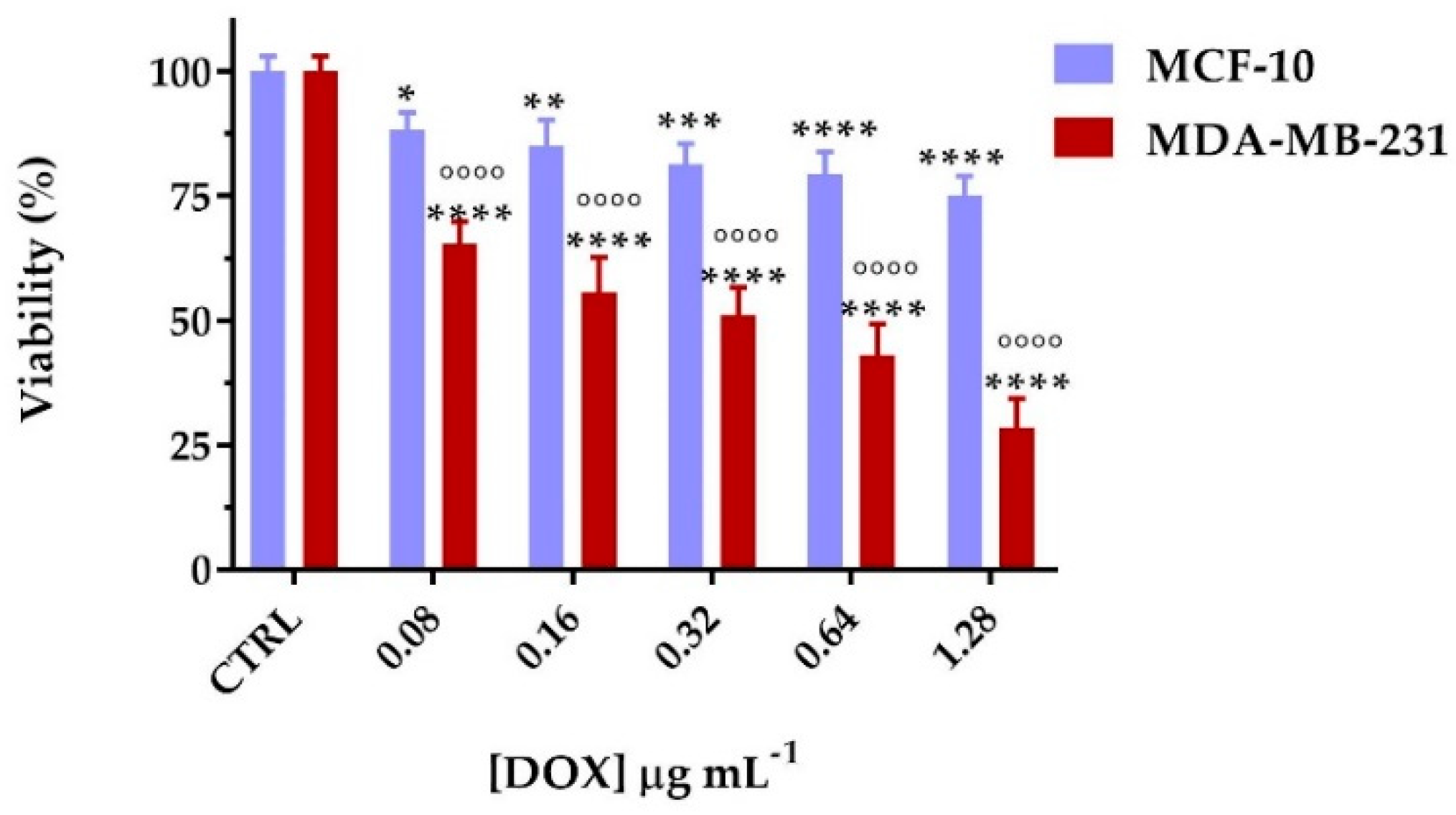
2.10. Cell Viability Assay
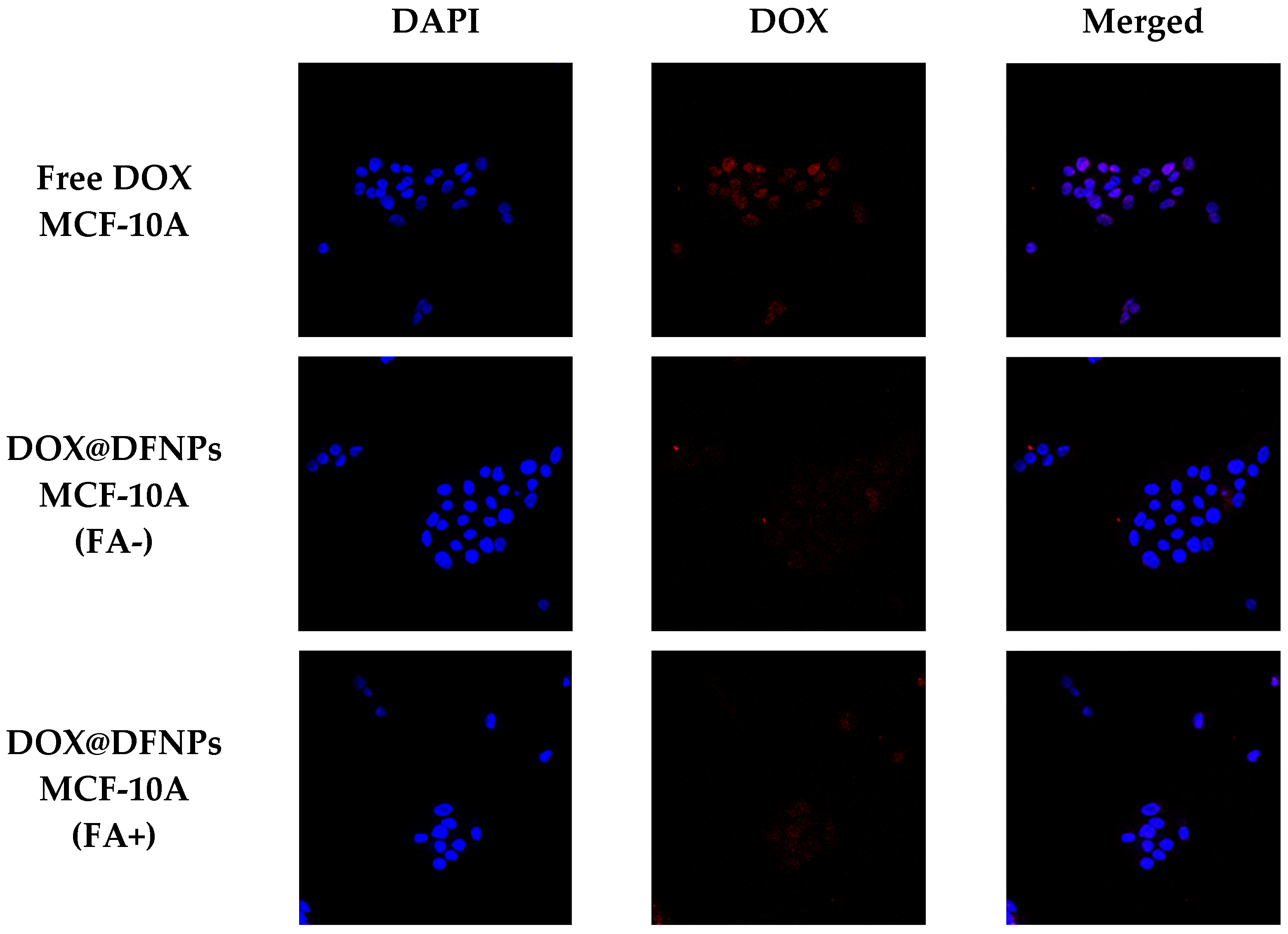
2.11. Cellular Uptake Experiments
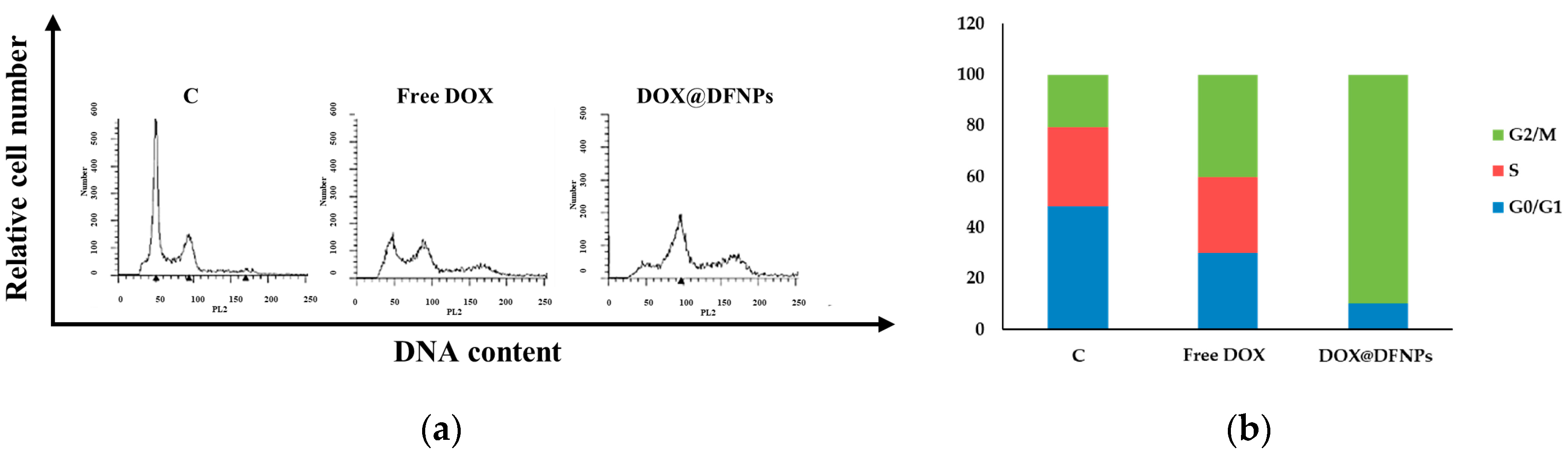
2.12. DNA Flow Cytometry
3. Results and Discussion
3.1. Synthesis and Characterization of DEXssPEGCOOH
3.2. Synthesis and Characterization of DEXFA Conjugate
3.3. Determination of CAC of DEXssPEGCOOH and DEXFA
3.4. Preparation of DFNPs
3.5. Destabilization Experiments
3.6. DOX Loading and Release Experiments
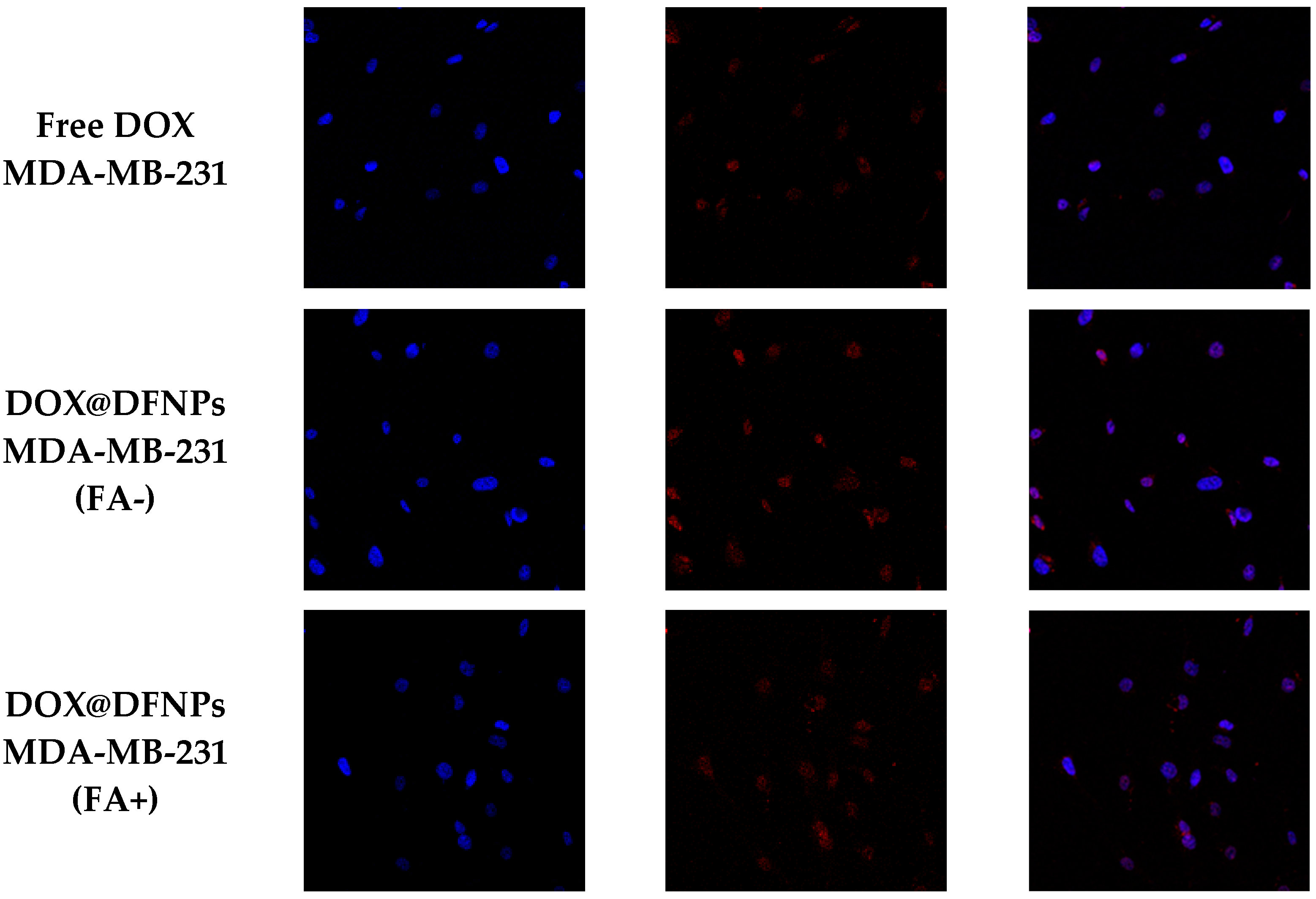
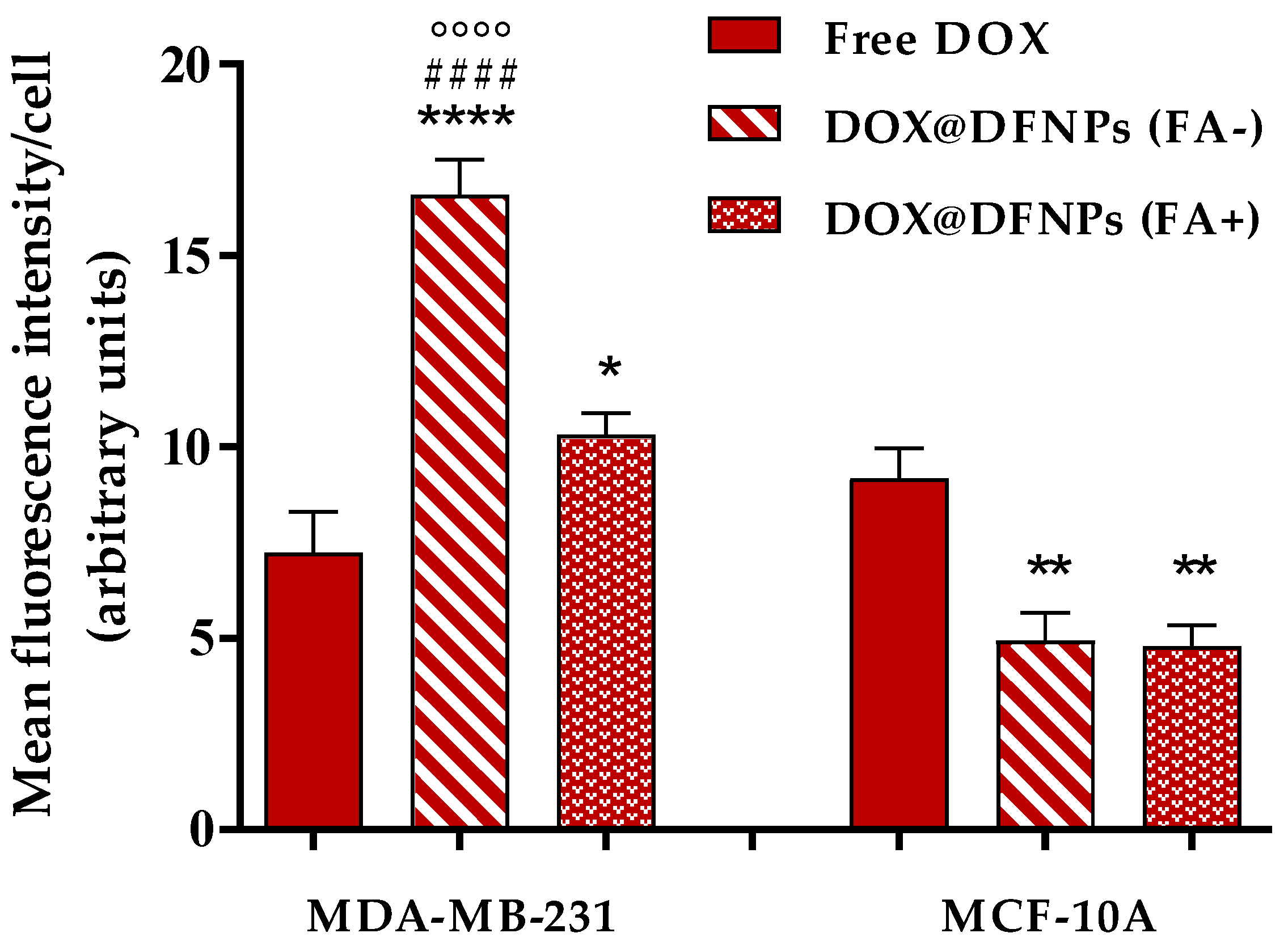
3.7. Biological Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Shi, Y.; Van der Meel, R.; Chen, X.Y.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Hao, F.; Yan, G.D.; Teng, L.S.; Lee, R.J.; Xie, J. Actively Targeted Nanoparticles for Drug Delivery to Tumor. Curr. Drug Metab. 2016, 17, 763–782. [Google Scholar] [CrossRef] [PubMed]
- Biffi, S.; Voltan, R.; Bortot, B.; Zauli, G.; Secchiero, P. Actively targeted nanocarriers for drug delivery to cancer cells. Expert Opin. Drug Del. 2019, 16, 481–496. [Google Scholar] [CrossRef]
- Du, J.Z.; Lane, L.A.; Nie, S.M. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef]
- Fernandez, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [PubMed]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the folate receptor alpha in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef]
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004, 14, 198–206. [Google Scholar] [CrossRef]
- Kim, J.W.; Dang, C.V. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006, 66, 8927–8930. [Google Scholar] [CrossRef] [PubMed]
- Kanamala, M.; Wilson, W.R.; Yang, M.M.; Palmer, B.D.; Wu, Z.M. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Xu, H.T.; Paxton, J.W.; Wu, Z.M. Enhanced pH-Responsiveness, Cellular Trafficking, Cytotoxicity and Long-circulation of PEGylated Liposomes with Post-insertion Technique Using Gemcitabine as a Model Drug. Pharm. Res. 2015, 32, 2428–2438. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Curcio, M.; Diaz-Gomez, L.; Cirillo, G.; Concheiro, A.; Iemma, F.; Alvarez-Lorenzo, C. pH/redox dual-sensitive dextran nanogels for enhanced intracellular drug delivery. Eur. J. Pharm. Biopharm. 2017, 117, 324–332. [Google Scholar] [CrossRef]
- Fu, C.P.; Li, H.L.; Li, N.N.; Miao, X.W.; Xie, M.Q.; Du, W.J.; Zhang, L.M. Conjugating an anticancer drug onto thiolated hyaluronic acid by acid liable hydrazone linkage for its gelation and dual stimuli-response release. Carbohyd. Polym. 2015, 128, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Jiao, Y.P.; Wang, Y.F.; Zhou, C.R.; Zhang, Z.Y. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Zhu, L.; Liu, Z.Z.; Cheng, R.; Meng, F.H.; Cui, J.H.; Ji, S.J.; Zhong, Z.Y. Reversibly Stabilized Multifunctional Dextran Nanoparticles Efficiently Deliver Doxorubicin into the Nuclei of Cancer Cells. Angew Chem. Int. Ed. 2009, 48, 9914–9918. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Shi, B.H.; Yue, C.X.; Gao, G.H.; Li, P.; Yi, H.Q.; Li, M.X.; Wang, B.; Ma, Y.F.; Cai, L.T. Dextran-based redox-responsive doxorubicin prodrug micelles for overcoming multidrug resistance. Polym. Chem. 2013, 4, 5793–5799. [Google Scholar] [CrossRef]
- Banerjee, A.; Bandopadhyay, R. Use of dextran nanoparticle: A paradigm shift in bacterial exopolysaccharide based biomedical applications. Int. J. Biol. Macromol. 2016, 87, 295–301. [Google Scholar] [CrossRef]
- Curcio, M.; Cirillo, G.; Paoli, A.; Naimo, G.D.; Mauro, L.; Amantea, D.; Leggio, A.; Nicoletta, F.P.; Lemma, F. Self-assembling Dextran prodrug for redox- and pH-responsive co-delivery of therapeutics in cancer cells. Colloid Surf. B 2020, 185, 110537. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.X.; Li, Y.H.; Xu, R.; Li, S.; Hu, H.; Xiao, C.; Wu, H.L.; Zhu, L.; Ming, J.X.; Chu, Z.; et al. Self-assembly of folic acid dextran conjugates for cancer chemotherapy. Nanoscale 2018, 10, 17265–17274. [Google Scholar] [CrossRef] [PubMed]
- Butzbach, K.; Konhauser, M.; Fach, M.; Bamberger, D.N.; Breitenbach, B.; Epe, B.; Wich, P.R. Receptor-mediated Uptake of Folic Acid-functionalized Dextran Nanoparticles for Applications in Photodynamic Therapy. Polymers 2019, 11, 896. [Google Scholar] [CrossRef]
- Wang, H.; Dai, T.T.; Zhou, S.Y.; Huang, X.X.; Li, S.Y.; Sun, K.; Zhou, G.D.; Dou, H.J. Self-Assembly Assisted Fabrication of Dextran-Based Nanohydrogels with Reduction-Cleavable Junctions for Applications as Efficient Drug Delivery Systems. Sci. Rep. 2017, 7, 40011. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Wang, H.L.; Mukerabigwi, J.F.; Liu, M.; Luo, S.Y.; Lei, S.J.; Cao, Y.; Huang, X.Y.; He, H.X. Self-organized nanoparticle drug delivery systems from a folate-targeted dextran-doxorubicin conjugate loaded with doxorubicin against multidrug resistance. RSC Adv. 2015, 5, 71164–71173. [Google Scholar] [CrossRef]
- Bar-On, O.; Shapira, M.; Hershko, D.D. Differential effects of doxorubicin treatment on cell cycle arrest and Skp2 expression in breast cancer cells. Anti-Cancer Drug 2007, 18, 1113–1121. [Google Scholar] [CrossRef]
- Volokhova, A.S.; Edgar, K.J.; Matson, J.B. Polysaccharide-containing block copolymers: Synthesis and applications. Mater. Chem. Front. 2020, 4, 99–112. [Google Scholar] [CrossRef]
- Bosker, W.T.E.; Agoston, K.; Stuart, M.A.C.; Norde, W.; Timmermans, J.W.; Slaghek, T.M. Synthesis and interfacial behavior of polystyrene-polysaccharide diblock copolymers. Macromolecules 2003, 36, 1982–1987. [Google Scholar] [CrossRef]
- Besheer, A.; Hause, G.; Kressler, J.; Mader, K. Hydrophobically modified hydroxyethyl starch: Synthesis, characterization and aqueous self-assembly into nano-sized polymeric micelles and vesicles. Biomacromolecules 2007, 8, 359–367. [Google Scholar] [CrossRef]
- Curcio, M.; Mauro, L.; Naimo, G.D.; Amantea, D.; Cirillo, G.; Tavano, L.; Casaburi, I.; Nicoletta, F.P.; Alvarez-Lorenzo, C.; Iemma, F. Facile synthesis of pH-responsive polymersomes based on lipidized PEG for intracellular co-delivery of curcumin and methotrexate. Colloid Surf. B 2018, 167, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Jiang, T.T.; Yuan, Z.X.; Lu, Y. Self-Assembled Casein Nanoparticles Loading Triptolide for the Enhancement of Oral Bioavailability. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Tavano, L.; Muzzalupo, R.; Mauro, L.; Pellegrino, M.; Ando, S.; Picci, N. Transferrin-Conjugated Pluronic Niosomes as a New Drug Delivery System for Anticancer Therapy. Langmuir 2013, 29, 12638–12646. [Google Scholar] [CrossRef]
- Provencher, S.W. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput. Phys. Commun. 1982, 27, 213–227. [Google Scholar] [CrossRef]
- Zhuo, X.Z.; Lei, T.; Miao, L.L.; Chu, W.; Li, X.W.; Luo, L.F.; Gou, J.X.; Zhang, Y.; Yin, T.; He, H.B.; et al. Disulfiram-loaded mixed nanoparticles with high drug-loading and plasma stability by reducing the core crystallinity for intravenous delivery. J. Colloid Interf. Sci. 2018, 529, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Soe, Z.C.; Poudel, B.K.; Nguyen, H.T.; Thapa, R.K.; Ou, W.Q.; Gautam, M.; Poudel, K.; Jin, S.G.; Jeong, J.H.; Ku, S.K.; et al. Folate-targeted nanostructured chitosan/chondroitin sulfate complex carriers for enhanced delivery of bortezomib to colorectal cancer cells. Asian J. Pharm. Sci. 2019, 14, 40–51. [Google Scholar] [CrossRef]
- Kuz’mina, N.E.; Moiseev, S.V.; Krylov, V.I.; Yashkir, V.A.; Merkulov, V.A. Quantitative determination of the average molecular weights of dextrans by diffusion ordered NMR spectroscopy. J. Anal. Chem. 2014, 69, 953–959. [Google Scholar] [CrossRef]
- Spijker, H.J.; Dirks, A.J.; Van Hest, J.C.M. Synthesis and assembly behavior of nucleobase-functionalized block copolymers. J. Polym. Sci. Pol. Chem. 2006, 44, 4242–4250. [Google Scholar] [CrossRef]
- Wang, H.B.; Li, Y.; Bai, H.S.; Shen, J.; Chen, X.; Ping, Y.; Tang, G.P. A Cooperative Dimensional Strategy for Enhanced Nucleus-Targeted Delivery of Anticancer Drugs. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.H.; Deng, C.; Klok, H.A.; Zhong, Z.Y. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Curcio, M.; Altimari, I.; Spizzirri, U.G.; Cirillo, G.; Vittorio, O.; Puoci, F.; Picci, N.; Iemma, F. Biodegradable gelatin-based nanospheres as pH-responsive drug delivery systems. J. Nanopart. Res. 2013, 15, 1581. [Google Scholar] [CrossRef]
- Lin, C.; Zhong, Z.Y.; Lok, M.C.; Jiang, X.L.; Hennink, W.E.; Feijen, J.; Engbersen, J.F.J. Novel bioreducible poly(amido amine)s for highly efficient gene delivery. Bioconjugate Chem. 2007, 18, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.Y.; Zhou, Y.F.; Yan, D.Y.; Li, H.Q.; Liu, Y. PH-responsive self-assembly of carboxyl-terminated hyperbranched polymers. Phys. Chem. Chem. Phys. 2007, 9, 1255–1262. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curcio, M.; Paolì, A.; Cirillo, G.; Di Pietro, S.; Forestiero, M.; Giordano, F.; Mauro, L.; Amantea, D.; Di Bussolo, V.; Nicoletta, F.P.; et al. Combining Dextran Conjugates with Stimuli-Responsive and Folate-Targeting Activity: A New Class of Multifunctional Nanoparticles for Cancer Therapy. Nanomaterials 2021, 11, 1108. https://doi.org/10.3390/nano11051108
Curcio M, Paolì A, Cirillo G, Di Pietro S, Forestiero M, Giordano F, Mauro L, Amantea D, Di Bussolo V, Nicoletta FP, et al. Combining Dextran Conjugates with Stimuli-Responsive and Folate-Targeting Activity: A New Class of Multifunctional Nanoparticles for Cancer Therapy. Nanomaterials. 2021; 11(5):1108. https://doi.org/10.3390/nano11051108
Chicago/Turabian StyleCurcio, Manuela, Alessandro Paolì, Giuseppe Cirillo, Sebastiano Di Pietro, Martina Forestiero, Francesca Giordano, Loredana Mauro, Diana Amantea, Valeria Di Bussolo, Fiore Pasquale Nicoletta, and et al. 2021. "Combining Dextran Conjugates with Stimuli-Responsive and Folate-Targeting Activity: A New Class of Multifunctional Nanoparticles for Cancer Therapy" Nanomaterials 11, no. 5: 1108. https://doi.org/10.3390/nano11051108
APA StyleCurcio, M., Paolì, A., Cirillo, G., Di Pietro, S., Forestiero, M., Giordano, F., Mauro, L., Amantea, D., Di Bussolo, V., Nicoletta, F. P., & Iemma, F. (2021). Combining Dextran Conjugates with Stimuli-Responsive and Folate-Targeting Activity: A New Class of Multifunctional Nanoparticles for Cancer Therapy. Nanomaterials, 11(5), 1108. https://doi.org/10.3390/nano11051108







