Synthesis and Performance of Large-Scale Cost-Effective Environment-Friendly Nanostructured Thermoelectric Materials
Abstract
1. Introduction
2. Recent Advancements in Large-Scale Thermoelectric Synthesis and Fabrication Methods
| Technology | Materials | Processing Cost | Time | Scalability (Max Weight Per Batch) | Ztmax (Material-Ref) | Strengths | Drawbacks |
|---|---|---|---|---|---|---|---|
| Microwave-assisted hydrothermal/solvothermal | Inorganic (Bi2S3, SnTe, α- MgAgSb,etc) nanostructures | Medium | Moderate | ≈10 g | 2.2 (SnSe [15]) | Lower operating temperatures, minimum material loss, good dispersion, and eco-friendliness, highly crystalline nanostructures | Inability to observe and monitor the reaction process |
| Chemical synthesis | Inorganic (Bi2Te3, Copper-based) | Low | Moderate | ≈20 g | 1.2 (Bi2Te2.5Se0.5 [17]) | Simple, inexpensive, better mechanical properties, better characterization | Controlling the parameters of deposition is difficult to achieve |
| Gas Atomization | Inorganic | Low | Ultra-fast | 3–5 Kg/min | ≈1 (Bi2Te3+ 75% Sb2Te3 [19]) | High-quality pure powders, high powder flow rates, economical, very high scalability | Powder properties vary with the equipment from different suppliers |
| Cryogenic grinding | Inorganic, hybrid | High | Fast | High | 1.5 (Yb single-filled CoSb3 [18]) | Fine sintered powders, improved mechanical abilities, power saver, no oxidation | Formation of ice around the delivery nozzle and piping system blocks delivery of liquid nitrogen |
3. Various Synthesis and Fabrication Techniques for Large-Scale Production
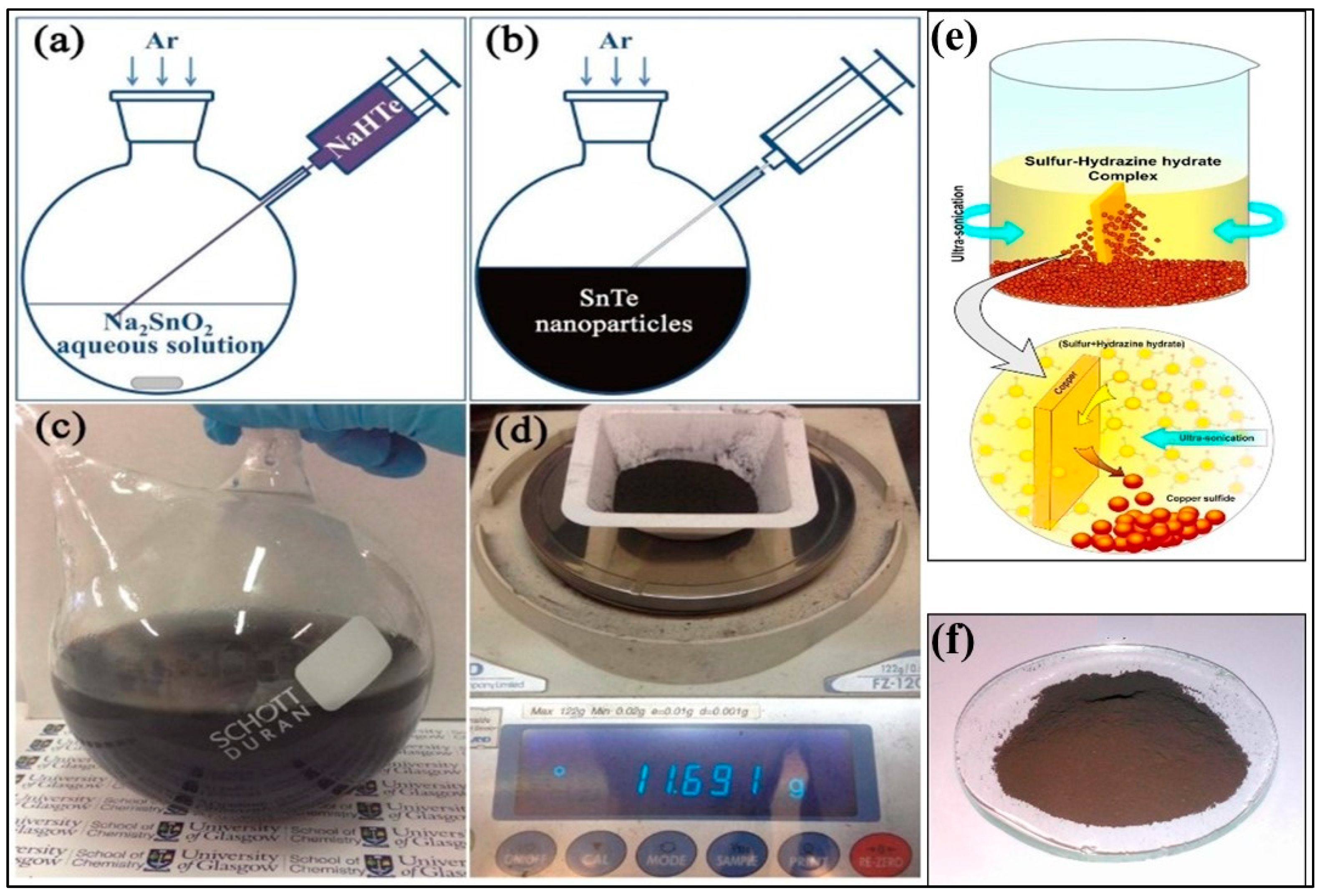
3.1. Microwave-Assisted Solvothermal/Hydrothermal Synthesis
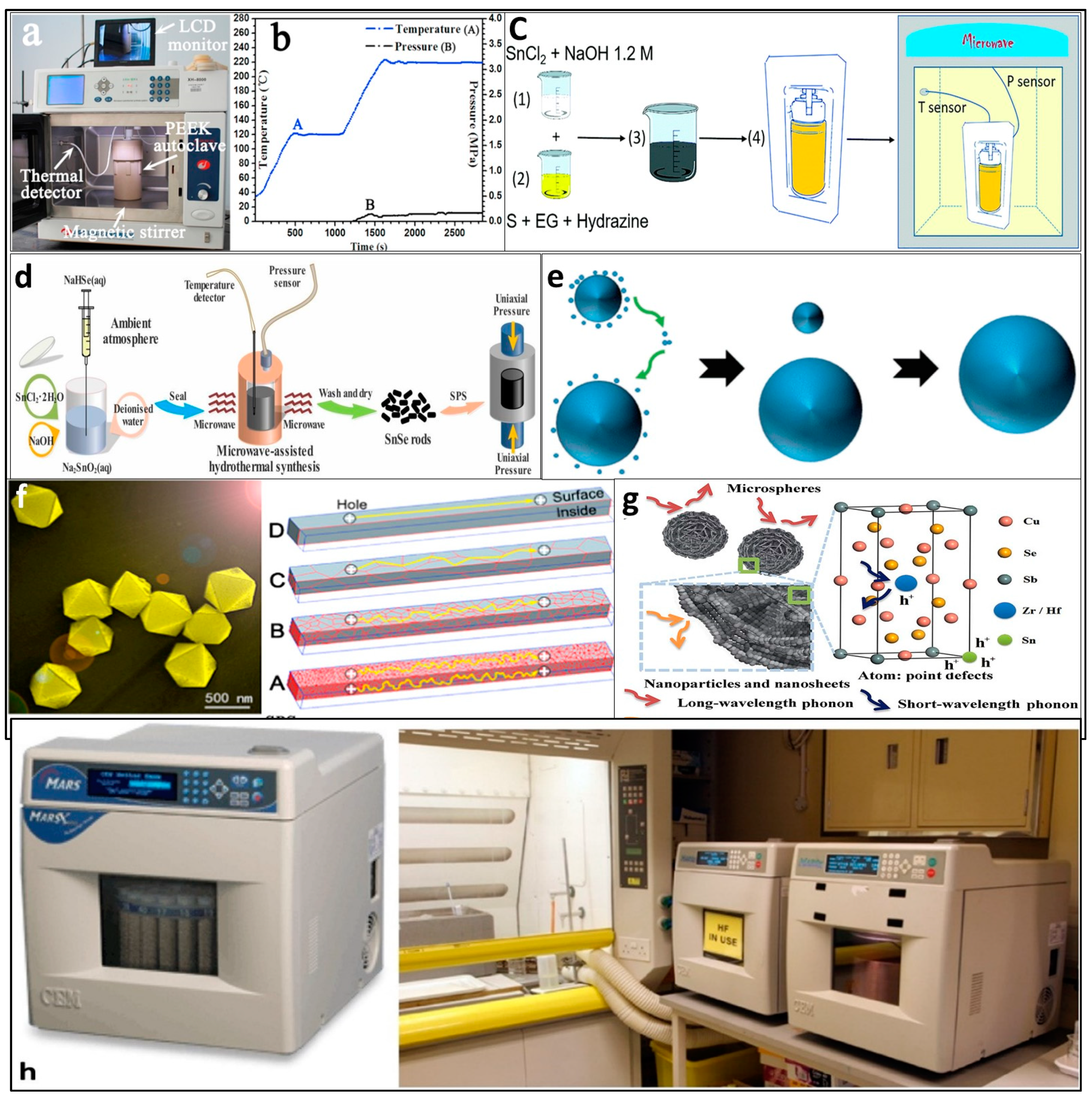
3.2. Melt-Spinning
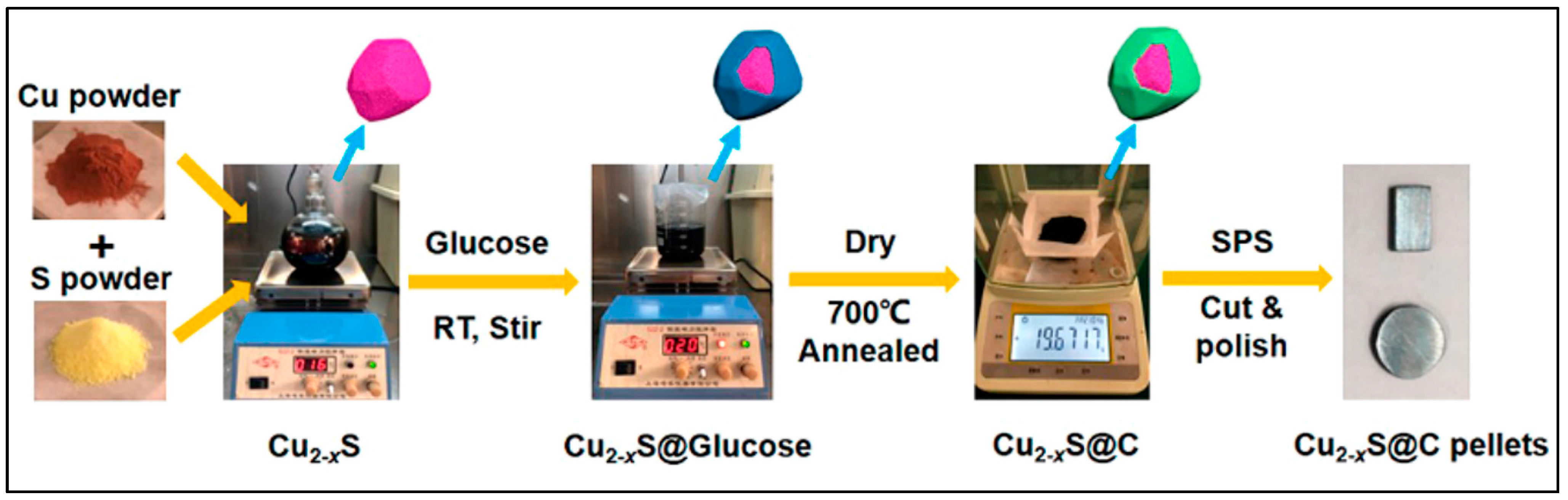
3.3. Chemical Synthesis
3.3.1. Colloidal Synthesis
3.3.2. Wet Chemical Method
3.3.3. Solution Synthesis
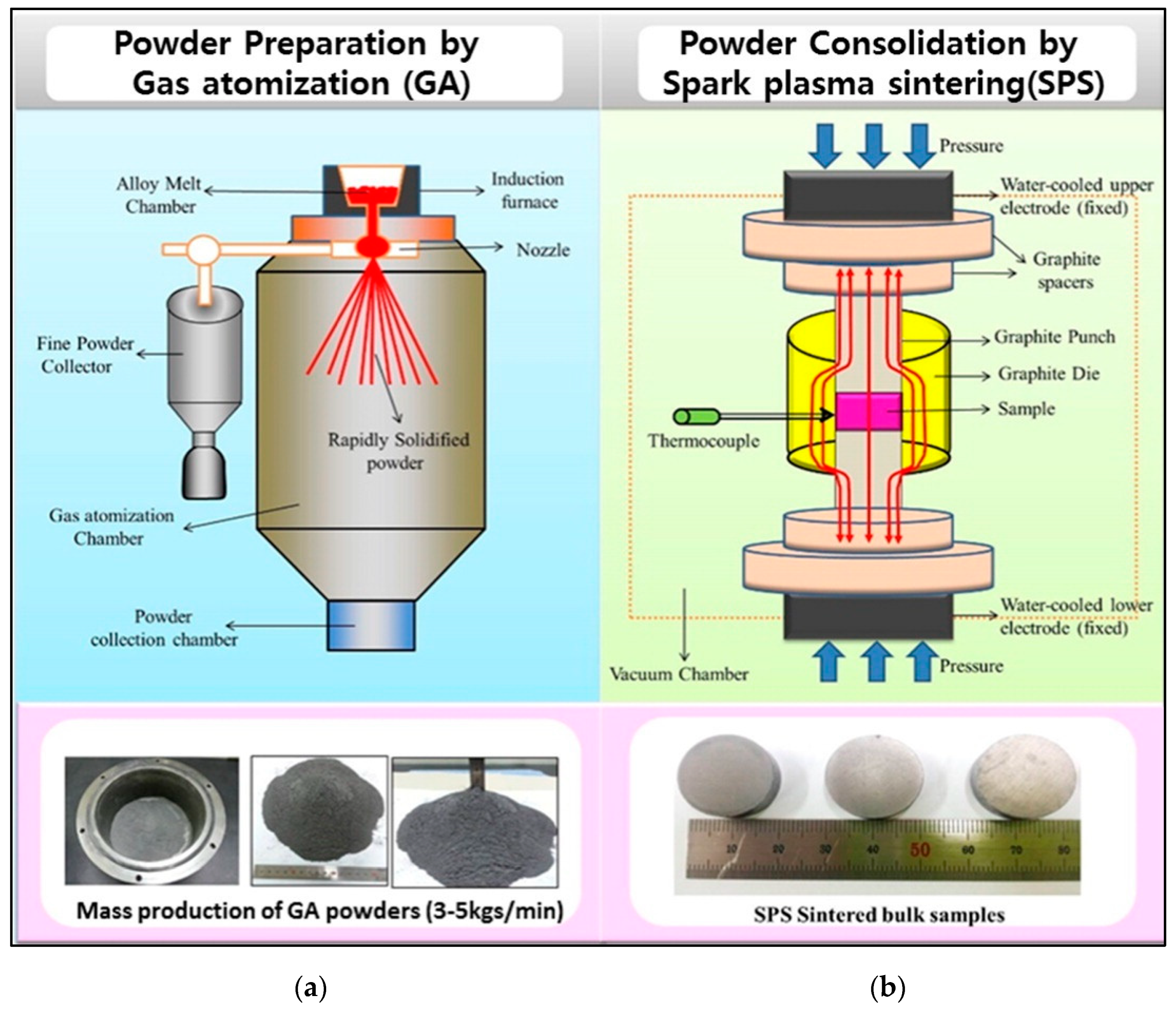
3.4. Powder Metallurgy
3.4.1. Gas Atomization
3.4.2. Cryogenic Grinding
3.5. Printable Technologies
3.5.1. Inkjet Printing
3.5.2. Screen Printing
3.5.3. Dispenser Printing
3.5.4. Aerosol Printing
3.5.5. Photonic Sintering
3.6. Other Methods
4. Limitations, Future Scope, and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Snyder, G.J.; Snyder, A.H. Figure of merit ZT of a thermoelectric device defined from materials properties. Energy Environ. Sci. 2017, 10, 2280–2283. [Google Scholar] [CrossRef]
- Gayner, C.; Kar, K.K.; Kim, W. Recent progress and futuristic development of PbSe thermoelectric materials and devices. Mater. Today Energy 2018, 9, 359–376. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Agarwal, A.; Coutant, Z.A.; Hall, M.J.; Liu, J.; Liu, R.; Malhotra, A.; Norouzzadeh, P.; Öztürk, M.C.; Ramesh, V.P.; et al. Thermoelectric silicides: A review. Jpn. J. Appl. Phys. 2017, 56, 05DA04. [Google Scholar] [CrossRef]
- Madar, N.; Givon, T.; Mogilyansky, D.; Gelbstein, Y. High thermoelectric potential of Bi2Te3 alloyed GeTe-rich phases. J. Appl. Phys. 2016, 120, 035102. [Google Scholar] [CrossRef]
- Ben-Ayoun, D.; Sadia, Y.; Gelbstein, Y. High temperature thermoelectric properties evolution of Pb1-xSnxTe based alloys. J. Alloy. Compod. 2017, 722, 33–38. [Google Scholar] [CrossRef]
- Sadia, Y.; Madar, N.; Kaler, I.; Gelbstein, Y. Thermoelectric properties of the quasi-binary MnSi1.73–FeSi2 system. J. Electron. Mater. 2014, 44, 1637–1643. [Google Scholar] [CrossRef]
- Jaldurgam, F.; Ahmad, Z.; Touati, F. Low-toxic, earth-abundant nanostructured materials for thermoelectric applications. Nanomaterials 2021, 11, 895. [Google Scholar] [CrossRef] [PubMed]
- Slack, G. New materials and performance limits for thermoelectric cooling. CRC Handb. Thermoelectr. 1995, 407–440. [Google Scholar] [CrossRef]
- Fan, F.-J.; Yu, B.; Wang, Y.-X.; Zhu, Y.-L.; Liu, X.-J.; Yu, S.-H.; Ren, Z. Colloidal synthesis of Cu2CdSnSe4 nanocrystals and hot-pressing to enhance the thermoelectric figure-of-merit. J. Am. Chem. Soc. 2011, 133, 15910–15913. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Aizawa, T.; Yamamoto, A.; Ohta, T. Thermoelectric properties of n-type (Bi2Se3)x(Bi2Te3)1−x prepared by bulk mechanical alloying and hot pressing. J. Alloy. Compd. 2000, 312, 326–330. [Google Scholar] [CrossRef]
- Kim-Hak, O.; Soulier, M.; Szkutnik, P.-D.; Saunier, S.; Simon, J.; Goeuriot, D. Microwave sintering and thermoelectric properties of p-type (Bi0.2Sb0.8)2Te3 powder. Powder Technol. 2012, 226, 231–234. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, T.; Zhao, X. Nanostructured PbTe compound synthesized by a simple chemical route. J. Alloy. Compd. 2010, 493, 423–426. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Hwang, C.-S.; Jeng, M.-S.; Su, W.-S.; Chou, Y.-W.; Ku, J.-R. Thermoelectric transport properties of bismuth telluride bulk materials fabricated by ball milling and spark plasma sintering. J. Alloy. Compd. 2010, 496, 687–690. [Google Scholar] [CrossRef]
- Ioannou, M.; Polymeris, G.; Hatzikraniotis, E.; Khan, A.U.; Paraskevopoulos, K.M.; Kyratsi, T.; Kyratsi, T. Solid-state synthesis and thermoelectric properties of Sb-doped Mg2Si materials. J. Electron. Mater. 2013, 42, 1827–1834. [Google Scholar] [CrossRef]
- Chandra, S.; Biswas, K. Realization of high thermoelectric figure of merit in solution synthesized 2D SnSe nanoplates via Ge alloying. J. Am. Chem. Soc. 2019, 141, 6141–6145. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-Y.; Wang, B.; Ma, M.-G.; Lin, K.-L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1–2, 63–83. [Google Scholar] [CrossRef]
- Xu, B.; Feng, T.; Agne, M.T.; Zhou, L.; Ruan, X.; Snyder, G.J.; Wu, Y. Highly porous thermoelectric nanocomposites with low thermal conductivity and high figure of merit from large-scale solution-synthesized Bi2Te2.5Se0.5 hollow nanostructures. Angew. Chem. Int. Ed. 2017, 56, 3546–3551. [Google Scholar] [CrossRef]
- Zhou, Z.; Agne, M.T.; Zhang, Q.; Wan, S.; Song, Q.; Xu, Q.; Lu, X.; Gu, S.; Fan, Y.; Jiang, W.; et al. Microstructure and composition engineering Yb single-filled CoSb3 for high thermoelectric and mechanical performances. J. Mater. 2019, 5, 702–710. [Google Scholar] [CrossRef]
- Madavali, B.; Kim, H.-S.; Lee, K.-H.; Isoda, Y.; Gascoin, F.; Hong, S.-J. Large scale production of high efficient and robust p-type Bi-Sb-Te based thermoelectric materials by powder metallurgy. Mater. Des. 2016, 112, 485–494. [Google Scholar] [CrossRef]
- Chen, B.; Das, S.R.; Zheng, W.; Zhu, B.; Xu, B.; Hong, S.; Sun, C.; Wang, X.; Wu, Y.; Claussen, J.C. Inkjet printing of single-crystalline Bi2Te3 thermoelectric nanowire networks. Adv. Electron. Mater. 2017, 3, 1600524. [Google Scholar] [CrossRef]
- Choi, H.; Kim, Y.J.; Kim, C.S.; Yang, H.M.; Oh, M.-W.; Cho, B.J. Enhancement of reproducibility and reliability in a high-performance flexible thermoelectric generator using screen-printed materials. Nano Energy 2018, 46, 39–44. [Google Scholar] [CrossRef]
- Madan, D.; Chen, A.; Wright, P.K.; Evans, J.W. Dispenser printed composite thermoelectric thick films for thermoelectric generator applications. J. Appl. Phys. 2011, 109, 034904. [Google Scholar] [CrossRef]
- Yu, S.; Shu, L.; Qian, Y.; Xie, Y.; Yang, J.; Yang, L. Hydrothermal preparation and characterization of nanocrystalline powder of β-Indium sulfide. Mater. Res. Bull. 1998, 33, 717–721. [Google Scholar] [CrossRef]
- Yu, S.; Qian, Y.; Shu, L.; Xie, Y.; Yang, L.; Wang, C. Solvent thermal synthesis and characterization of ultrafine powder of bismuth sulfide. Mater. Lett. 1998, 35, 116–119. [Google Scholar] [CrossRef]
- Yu, S.-H.; Yang, J.; Wu, Y.-S.; Han, Z.-H.; Xie, Y.; Qian, Y.-T. Hydrothermal preparation and characterization of rod-like ultrafine powders of bismuth sulfide. Mater. Res. Bull. 1998, 33, 1661–1666. [Google Scholar] [CrossRef]
- Fu, J.; Song, S.; Zhang, X.; Cao, F.; Zhou, L.; Li, X.; Zhang, H. Bi2Te3 nanoplates and nanoflowers: Synthesized by hydrothermal process and their enhanced thermoelectric properties. Cryst. Eng. Commun. 2012, 14, 2159–2165. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, J.-H.; Yu, S.-H.; Yang, L.; Zhang, Y.-H.; Qian, Y.-T. Pressure-controlled fabrication of stibnite nanorods by the solvothermal decomposition of a simple single-source precursor. Chem. Mater. 2000, 12, 2924–2929. [Google Scholar] [CrossRef]
- Cui, H.; Liu, H.; Li, X.; Wang, J.; Han, F.; Zhang, X.; Boughton, R. Synthesis of Bi2Se3 thermoelectric nanosheets and nanotubes through hydrothermal co-reduction method. J. Solid State Chem. 2004, 177, 4001–4006. [Google Scholar] [CrossRef]
- Wang, D.; Yu, D.; Mo, M.; Liu, X.; Qian, Y. Preparation and characterization of wire-like Sb2Se3 and flake-like Bi2Se3 nanocrystals. J. Cryst. Growth 2003, 253, 445–451. [Google Scholar] [CrossRef]
- Shi, W.; Zhou, L.; Song, S.; Yang, J.; Zhang, H. Hydrothermal synthesis and thermoelectric transport properties of impurity-free antimony telluride hexagonal nanoplates. Adv. Mater. 2008, 20, 1892–1897. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, P.; Miao, H.; Zhu, J.; Bian, X.; Liu, Y.; Chen, B.; Wang, X. Large-scale synthesis of ultralong Sb2S3 sub-microwires via a hydrothermal process. Mater. Res. Bull. 2008, 43, 2636–2642. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Du, Y.; Sun, Y.; Li, J.; Zhang, R.; Li, Q.; Chen, P.; Zhao, G.; Fang, Y.; et al. P-type quaternary chalcogenides of Cu2ZnSn(S,Se)4 nanocrystals: Large-scale synthesis, bandgap engineering and their thermoelectric performances. J. Alloy. Compd. 2018, 738, 484–490. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, S.; Xie, Q.; Hu, Z.; Yang, Y.; Zhang, S.; Qian, Y. Large-scale synthesis of ultralong Bi2S3 nanoribbons via a solvothermal process. Adv. Mater. 2003, 15, 936–940. [Google Scholar] [CrossRef]
- Wu, J.; Qin, F.; Cheng, G.; Li, H.; Zhang, J.; Xie, Y.; Yang, H.-J.; Lu, Z.; Yu, X.; Chen, R. Large-scale synthesis of bismuth sulfide nanorods by microwave irradiation. J. Alloy. Compd. 2011, 509, 2116–2126. [Google Scholar] [CrossRef]
- Xin, J.; Yang, J.; Li, S.; Basit, A.; Sun, B.; Li, S.; Long, Q.; Li, X.; Chen, Y.; Jiang, Q. Thermoelectric performance of rapidly microwave-synthesized α-MgAgSb with SnTe nanoinclusions. Chem. Mater. 2019, 31, 2421–2430. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Li, J.-F.; Chen, H.; Wang, L.; Zheng, S.; Lu, G. Systhesizing SnTe nanocrystals leading to thermoelectric performance enhancement via an ultra-fast microwave hydrothermal method. Nano Energy 2016, 28, 78–86. [Google Scholar] [CrossRef]
- Wang, L.; Chang, S.; Zheng, S.; Fang, T.; Cui, W.; Bai, P.-P.; Yue, L.; Chen, Z.-G. Thermoelectric performance of Se/Cd codoped SnTe via microwave solvothermal method. ACS Appl. Mater. Interface 2017, 9, 22612–22619. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, S.; Wang, Q.; Li, Z.; Li, J.; Zhang, Z.; Wu, Y.; Zhu, B.; Wang, S.; Chen, Y.; et al. Synergistic modulation of power factor and thermal conductivity in Cu3SbSe4 towards high thermoelectric performance. Nano Energy 2020, 71, 104658. [Google Scholar] [CrossRef]
- Zhai, J.; Wang, H.; Su, W.; Wang, T.; Wang, X.; Chen, T.; Wang, C. The synthesis and microstructure of CuFeO2 powders via microwave hydrothermal reaction. J. Ceram. Soc. Jpn. 2019, 127, 22–27. [Google Scholar] [CrossRef]
- Jantrasee, S.; Moontragoon, P.; Pinitsoontorn, S.; Ruttanapun, C.; Moontragoon, P. Enhancing thermoelectric properties of nanostructure Ga-doped ZnO prepared by microwave-hydrothermal synthesizing with comparing to calculation results. Mater. Res. Express 2019, 6, 045047. [Google Scholar] [CrossRef]
- Prado-Gonjal, J.; Gainza, J.; Aguayo, I.; Dura, O.J.; Perez, S.R.; Serrano-Sánchez, F.; Nemes, N.; Fernandez-Diaz, M.T.; Alonso, J.A.; Morán, E. High thermoelectric performance of rapidly microwave-synthesized Sn1-δS. Mater. Adv. 2020, 1, 845–853. [Google Scholar] [CrossRef]
- Sheng, J.; Liu, X.; Niu, C.; Sun, Y.; Chen, Y.; Wang, H.; Zhang, B.; Wang, G.; Zhou, X.; Han, G. Facile microwave-assisted hydrothermal synthesis of SnSe: Impurity removal and enhanced thermoelectric properties. J. Mater. Chem. C 2020, 8, 10333–10341. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Kharissova, O.V.; Méndez, U.O. Microwave hydrothermal and solvothermal processing of materials and compounds. Dev. Appl. Microw. Heat. 2012, 5, 107–140. [Google Scholar]
- Wei, Z. Research process of polymer nanofibers prepared by melt spinning. IOP Conf. Ser. Mater. Sci. Eng. 2018, 452, 022002. [Google Scholar] [CrossRef]
- Luo, W.; Li, H.; Yan, Y.; Lin, Z.; Tang, X.; Zhang, Q.; Uher, C. Rapid synthesis of high thermoelectric performance higher manganese silicide with in-situ formed nano-phase of MnSi. Intermetallics 2011, 19, 404–408. [Google Scholar] [CrossRef]
- Xie, W.; He, J.; Kang, H.J.; Tang, X.; Zhu, S.; Laver, M.; Wang, S.; Copley, J.R.D.; Brown, C.M.; Zhang, Q.; et al. Identifying the specific nanostructures responsible for the high thermoelectric performance of (Bi,Sb)2Te3 nanocomposites. Nano Lett. 2010, 10, 3283–3289. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Qi, D.; Xie, W.; Tang, X. Enhancement of the thermoelectric performance of β-Zn4Sb3 by in situ nanostructures and minute Cd-doping. Acta Mater. 2011, 59, 4805–4817. [Google Scholar] [CrossRef]
- Li, H.; Tang, X.; Zhang, Q.; Uher, C. Rapid preparation method of bulk nanostructured Yb0.3Co4Sb12+y compounds and their improved thermoelectric performance. Appl. Phys. Lett. 2008, 93, 252109. [Google Scholar] [CrossRef]
- Muthiah, S.; Singh, R.C.; Pathak, B.P.; Avasthi, P.K.; Kumar, R.; Kumar, A.; Srivastava, A.K.; Dhar, A. Significant enhancement in thermoelectric performance of nanostructured higher manganese silicides synthesized employing a melt spinning technique. Nanoscale 2018, 10, 1970–1977. [Google Scholar] [CrossRef]
- Tan, H.; Guo, L.; Wang, G.; Wu, H.; Shen, X.; Zhang, B.; Lu, X.; Wang, G.; Zhang, X.; Zhou, X. Synergistic effect of bismuth and indium codoping for high thermoelectric performance of melt spinning SnTe alloys. ACS Appl. Mater. Interface 2019, 11, 23337–23345. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, S.; Li, X.; Liu, Z.; Zhong, H.; Feng, S. Ultralow thermal conductivity and enhanced thermoelectric properties of SnTe based alloys prepared by melt spinning technique. J. Alloy. Compd. 2020, 837, 155568. [Google Scholar] [CrossRef]
- Fan, F.-J.; Wang, Y.-X.; Liu, X.-J.; Wu, L.; Yu, S.-H. Large-scale colloidal synthesis of non-stoichiometric Cu2ZnSnSe4 Nanocrystals for thermoelectric applications. Adv. Mater. 2012, 24, 6158–6163. [Google Scholar] [CrossRef] [PubMed]
- Son, J.S.; Park, K.; Han, M.-K.; Kang, C.; Park, S.-G.; Kim, J.-H.; Kim, W.; Kim, S.-J.; Hyeon, T. Large-scale synthesis and characterization of the size-dependent thermoelectric properties of uniformly sized bismuth nanocrystals. Angew. Chem. Int. Ed. 2010, 50, 1363–1366. [Google Scholar] [CrossRef]
- Son, J.S.; Choi, M.K.; Han, M.-K.; Park, K.; Kim, J.-Y.; Lim, S.J.; Oh, M.; Kuk, Y.; Park, C.; Kim, S.-J.; et al. n-Type nanostructured thermoelectric materials prepared from chemically synthesized ultrathin Bi2Te3 Nanoplates. Nano Lett. 2012, 12, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Chen, Q.; Yao, W.; Yang, D.; Wang, G.; Zhou, X. Large-scale colloidal synthesis of Co-doped Cu2SnSe3 nanocrystals for thermoelectric applications. J. Electron. Mater. 2016, 45, 1935–1941. [Google Scholar] [CrossRef]
- Ibáñez, M.; Cadavid, D.; Anselmi-Tamburini, U.; Zamani, R.; Gorsse, S.; Li, W.; López, A.M.; Morante, J.R.; Arbiol, J.; Cabot, A. Colloidal synthesis and thermoelectric properties of Cu2SnSe3 nanocrystals. J. Mater. Chem. A 2013, 1, 1421–1426. [Google Scholar] [CrossRef]
- Song, J.-M.; Liu, Y.; Niu, H.-L.; Mao, C.-J.; Cheng, L.-J.; Zhang, S.-Y.; Shen, Y.-H. Hot-injection synthesis and characterization of monodispersed ternary Cu2SnSe3 nanocrystals for thermoelectric applications. J. Alloy. Compd. 2013, 581, 646–652. [Google Scholar] [CrossRef]
- Wang, W.; Feng, W.; Ding, T.; Yang, Q. Phosphine-free synthesis and characterization of cubic-phase Cu2SnTe3 nanocrystals with optical and optoelectronic properties. Chem. Mater. 2015, 27, 6181–6184. [Google Scholar] [CrossRef]
- Yin, D.; Dun, C.; Zhang, H.; Fu, Z.; Gao, X.; Wang, X.; Singh, D.J.; Carroll, D.L.; Liu, Y.; Swihart, M.T. Binary and ternary colloidal Cu-Sn-Te nanocrystals for thermoelectric thin films. Small 2021, 17, 2006729. [Google Scholar] [CrossRef]
- Zhang, A.; Shen, X.; Xie, D.; Lu, X.; Yao, W.; Dai, J.; Guo, L.; Wang, G.; Zhou, X. Large-scale colloidal synthesis of Cu5FeS4 compounds and their application in thermoelectrics. J. Mater. Chem. C 2016, 5, 301–308. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Zhao, Y.; Liu, W.-D.; Dai, W.; Wu, T.; Lu, X.; Wu, C.; Luo, W.; Fan, Y.; et al. Carbon-encapsulated copper sulfide leading to enhanced thermoelectric properties. ACS Appl. Mater. Interfaces 2019, 11, 22457–22463. [Google Scholar] [CrossRef]
- Han, G.; Zhang, R.; Popuri, S.R.; Greer, H.F.; Reece, M.J.; Bos, J.-W.G.; Zhou, W.; Knox, A.R.; Gregory, D.H. Large-scale surfactant-free synthesis of p-type SnTe nanoparticles for thermoelectric applications. Materials 2017, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Mulla, R.; Rabinal, M. Large-scale synthesis of copper sulfide by using elemental sources via simple chemical route. Ultrason. Sonochem. 2017, 39, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Popuri, S.R.; Greer, H.F.; Llin, L.F.; Bos, J.-W.G.; Zhou, W.; Paul, D.J.; Ménard, H.; Knox, A.R.; Montecucco, A.; et al. Chlorine-enabled electron doping in solution-synthesized SnSe thermoelectric nanomaterials. Adv. Energy Mater. 2017, 7, 1602328. [Google Scholar] [CrossRef]
- Li, X.; Chen, C.; Xue, W.; Li, S.; Cao, F.; Chen, Y.; He, J.; Sui, J.; Liu, X.; Wang, Y.; et al. N-type Bi-doped SnSe thermoelectric nanomaterials synthesized by a facile solution method. Inorg. Chem. 2018, 57, 13800–13808. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lu, J.; Ma, D.; Ma, C.; Zhang, B.; Wang, H.; Wang, G.; Gregory, D.H.; Zhou, X.; Han, G. Facile in situ solution synthesis of SnSe/rGO nanocomposites with enhanced thermoelectric performance. J. Mater. Chem. A 2019, 8, 1394–1402. [Google Scholar] [CrossRef]
- Hong, S.-J.; Chun, B.-S. Microstructure and thermoelectric properties of extruded n-type 95%Bi2Te2–5%Bi2Se3 alloy along bar length. Mater. Sci. Eng. A 2003, 356, 345–351. [Google Scholar] [CrossRef]
- Hong, S.-J.; Lee, Y.-S.; Byeon, J.-W.; Chun, B.-S. Optimum dopant content of n-type 95%Bi2Te3+5%Bi2Se3 compounds fabricated by gas atomization and extrusion process. J. Alloy. Compd. 2006, 414, 146–151. [Google Scholar] [CrossRef]
- Moon, C.; Shin, S.; Kim, D.; Kim, T.-S. Microstructure and thermoelectric properties of p-type Bi2Te3–Sb2Te3 alloys produced by rapid solidification and spark plasma sintering. J. Alloy. Compd. 2010, 504, S504–S507. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Dong, Y.; Wang, L.; Chen, L.; Jiang, W. Preparation of nano-sized Bi2Te3 thermoelectric material powders by cryogenic grinding. Prog. Nat. Sci. 2012, 22, 201–206. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Q.; Li, J.; Liu, X.; Wang, L.; Zhu, J.; Luo, W.; Jiang, W. An efficient thermoelectric material: Preparation of reduced graphene oxide/polyaniline hybrid composites by cryogenic grinding. RSC Adv. 2015, 5, 8988–8995. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Z.; Zhang, Q.; Zhou, Z.; Tang, Y.; Wang, L.; Zhu, J.; Luo, W.; Jiang, W. Preparation of bulk AgNWs/PEDOT: PSS composites: A new model towards high-performance bulk organic thermoelectric materials. RSC Adv. 2015, 5, 45106–45112. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, J.; Fan, Y.; Zhang, Q.; Lu, X.; Fan, S.; Kikuchi, K.; Nomura, N.; Kawasaki, A.; Wang, L.; et al. Uniform dispersion of SiC in Yb-filled skutterudite nanocomposites with high thermoelectric and mechanical performance. Scr. Mater. 2019, 162, 166–171. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Z.; Dylla, M.; Agne, M.T.; Pei, Y.; Wang, L.; Tang, Y.; Liao, J.; Li, J.; Bai, S.; et al. Realizing high-performance thermoelectric power generation through grain boundary engineering of skutterudite-based nanocomposites. Nano Energy 2017, 41, 501–510. [Google Scholar] [CrossRef]
- Orrill, M.; Leblanc, S. Printed thermoelectric materials and devices: Fabrication techniques, advantages, and challenges. J. Appl. Polym. Sci. 2016, 134, 134. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet printing for materials and devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Drahi, E.; Gupta, A.; Blayac, S.; Saunier, S.; Benaben, P. Characterization of sintered inkjet-printed silicon nanoparticle thin films for thermoelectric devices. Phys. Status Solidi (A) 2014, 211, 1301–1307. [Google Scholar] [CrossRef]
- Juntunen, T.; Jussila, H.; Ruoho, M.; Liu, S.; Hu, G.; Albrow-Owen, T.; Ng, L.W.; Howe, R.C.; Hasan, T.; Sun, Z. Inkjet printed large-area flexible few-layer graphene thermoelectrics. Adv. Funct. Mater. 2018, 28, 1800480. [Google Scholar] [CrossRef]
- Besganz, A.; Zöllmer, V.; Kun, R.; Pál, E.; Walder, L.; Busse, M. Inkjet printing as a flexible technology for the deposition of thermoelectric composite structures. Procedia Technol. 2014, 15, 99–106. [Google Scholar] [CrossRef]
- Ou, C.; Sangle, A.L.; Datta, A.; Jing, Q.; Busolo, T.; Chalklen, T.; Narayan, V.; Kar-Narayan, S. Fully printed organic-inorganic nanocomposites for flexible thermoelectric Applications. ACS Appl. Mater. Interfaces 2018, 10, 19580–19587. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Layani, M.; Zhao, X.; Tan, L.P.; Sun, T.; Fan, S.; Yan, Q.; Magdassi, S.; Hng, H.H. Fabrication of flexible thermoelectric thin film devices by inkjet printing. Small 2014, 10, 3551–3554. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Kruse, M.; Xu, B.; Tutika, R.; Zheng, W.; Bartlett, M.D.; Wu, Y.; Claussen, J.C. Flexible thermoelectric generators with inkjet-printed bismuth telluride nanowires and liquid metal contacts. Nanoscale 2018, 11, 5222–5230. [Google Scholar] [CrossRef]
- Lee, H.-B.; Yang, H.J.; We, J.H.; Kim, K.; Choi, K.C.; Cho, B.J. Thin-film thermoelectric module for power generator applications using a screen-printing method. J. Electron. Mater. 2011, 40, 615–619. [Google Scholar] [CrossRef]
- Kim, S.J.; We, J.H.; Kim, J.S.; Kim, G.S.; Cho, B.J. Thermoelectric properties of P-type Sb2Te3 thick film processed by a screen-printing technique and a subsequent annealing process. J. Alloy. Compd. 2014, 582, 177–180. [Google Scholar] [CrossRef]
- We, J.H.; Kim, S.J.; Kim, G.S.; Cho, B.J. Improvement of thermoelectric properties of screen-printed Bi2Te3 thick film by optimization of the annealing process. J. Alloy. Compd. 2013, 552, 107–110. [Google Scholar] [CrossRef]
- Varghese, T.; Hollar, C.; Richardson, J.; Kempf, N.; Han, C.; Gamarachchi, P.; Estrada, D.; Mehta, R.J.; Zhang, Y. High-performance and flexible thermoelectric films by screen printing solution-processed nanoplate crystals. Sci. Rep. 2016, 6, 33135. [Google Scholar] [CrossRef]
- Cao, Z.; Koukharenko, E.; Tudor, M.; Torah, R.; Beeby, S. Flexible screen printed thermoelectric generator with enhanced processes and materials. Sens. Actuators A Phys. 2016, 238, 196–206. [Google Scholar] [CrossRef]
- Soonshin, K.; Kumar, R.; Roh, J.W.; Ko, D.-S.; Kim, H.-S.; Kim, S.I.; Yin, L.; Schlossberg, S.M.; Cui, S.; You, J.-M.; et al. High-performance screen-printed thermoelectric films on fabrics. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, H.; Kim, Y.; We, J.H.; Shin, J.S.; Lee, H.E.; Oh, M.-W.; Lee, K.J.; Cho, B.J. Post ionized defect engineering of the screen-printed Bi2Te2.7Se0.3 thick film for high performance flexible thermoelectric generator. Nano Energy 2017, 31, 258–263. [Google Scholar] [CrossRef]
- Varghese, T.; Dun, C.; Kempf, N.; Saeidi-Javash, M.; Karthik, C.; Richardson, J.; Hollar, C.; Estrada, D.; Zhang, Y. Flexible thermoelectric devices of ultrahigh power factor by scalable printing and interface engineering. Adv. Funct. Mater. 2020, 30, 1905796. [Google Scholar] [CrossRef]
- Chen, A.; Madan, D.; Wright, P.K.; Evans, J.W. Dispenser-printed planar thick-film thermoelectric energy generators. J. Micromech. Microeng. 2011, 21, 104006. [Google Scholar] [CrossRef]
- Madan, D.; Wang, Z.; Chen, A.; Juang, R.-C.; Keist, J.; Wright, P.K.; Evans, J.W. Enhanced performance of dispenser printed MA n-type Bi2Te3Composite thermoelectric generators. ACS Appl. Mater. Interfaces 2012, 4, 6117–6124. [Google Scholar] [CrossRef]
- Madan, D.; Wang, Z.; Chen, A.; Wright, P.K.; Evans, J.W. High-performance dispenser printed MA p-Type Bi0.5Sb1.5Te3 flexible thermoelectric generators for powering wireless sensor networks. ACS Appl. Mater. Interfaces 2013, 5, 11872–11876. [Google Scholar] [CrossRef] [PubMed]
- Madan, D.; Wang, Z.; Chen, A.; Winslow, R.; Wright, P.K.; Evans, J.W. Dispenser printed circular thermoelectric devices using Bi and Bi0.5Sb1.5Te3. Appl. Phys. Lett. 2014, 104, 013902. [Google Scholar] [CrossRef]
- Wu, K.; Yan, Y.; Zhang, J.; Mao, Y.; Xie, H.; Yang, J.; Zhang, Q.; Uher, C.; Tang, X. Preparation of n-type Bi2Te3 thermoelectric materials by non-contact dispenser printing combined with selective laser melting. Phys. Status Solidi (RRL) Rapid Res. Lett. 2017, 11, 1700067. [Google Scholar] [CrossRef]
- Ou, C.; Sangle, A.L.; Chalklen, T.; Jing, Q.; Narayan, V.; Kar-Narayan, S. Enhanced thermoelectric properties of flexible aerosol-jet printed carbon nanotube-based nanocomposites. APL Mater. 2018, 6, 096101. [Google Scholar] [CrossRef]
- Dun, C.; Kuang, W.; Kempf, N.; Saeidi-Javash, M.; Singh, D.J.; Zhang, Y. 3D printing of solution-processable 2D nanoplates and 1D nanorods for flexible thermoelectrics with ultrahigh power factor at low-medium temperatures. Adv. Sci. 2019, 6, 1901788. [Google Scholar] [CrossRef]
- Saeidi-Javash, M.; Kuang, W.; Dun, C.; Zhang, Y. 3D conformal printing and photonic sintering of high-performance flexible thermoelectric films using 2D nanoplates. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Ou, C.; Zhang, L.; Jing, Q.; Narayan, V.; Kar-Narayan, S. Compositionally graded organic-inorganic nanocomposites for enhanced thermoelectric performance. Adv. Electron. Mater. 2020, 6, 1900720. [Google Scholar] [CrossRef]
- Ou, C. Aerosol-Jet Printed Nanocomposites for Flexible and Stretchable Thermoelectric Generators; University of Cambridge: Cambridge, UK, 2020. [Google Scholar]
- Hak-SungKim, H.-S.; Dhage, S.R.; Shim, D.-E.; Hahn, H.T. Intense pulsed light sintering of copper nanoink for printed electronics. Appl. Phys. A 2009, 97, 791–798. [Google Scholar] [CrossRef]
- Abbel, R.; van Lammeren, T.; Hendriks, R.; Ploegmakers, J.; Rubingh, E.J.; Meinders, E.R.; Groen, W.A. Photonic flash sintering of silver nanoparticle inks: A fast and convenient method for the preparation of highly conductive structures on foil. MRS Commun. 2012, 2, 145–150. [Google Scholar] [CrossRef]
- Niittynen, J.; Abbel, R.; Mäntysalo, M.; Perelaer, J.; Schubert, U.S.; Lupo, D. Alternative sintering methods compared to conventional thermal sintering for inkjet printed silver nanoparticle ink. Thin Solid Films 2014, 556, 452–459. [Google Scholar] [CrossRef]
- Danaei, R.; Varghese, T.; Ahmadzadeh, M.; McCloy, J.; Hollar, C.; Saleh, M.S.; Park, J.; Zhang, Y.; Panat, R. Ultrafast fabrication of thermoelectric films by pulsed light sintering of colloidal nanoparticles on flexible and rigid substrates. Adv. Eng. Mater. 2019, 21, 1800800. [Google Scholar] [CrossRef]
- Cademartiri, L.; Malakooti, R.; O’Brien, P.G.; Migliori, A.; Petrov, S.; Kherani, N.P.; Ozin, G.A. Large-scale synthesis of ultrathin Bi2S3 necklace nanowires. Angew. Chem. Int. Ed. 2008, 47, 3814–3817. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Hu, C.; Wang, S.; Xi, Y.; Zhang, K. Tunable synthesis and thermoelectric property of Bi2S3 nanowires. J. Phys. Chem. C 2013, 117, 5515–5520. [Google Scholar] [CrossRef]
- Fang, W.-C.; Liou, K.-M.; Leu, M.-S. Large-scale preparation of ternary BiSbTe films with enhanced thermoelectric properties using DC magnetron sputtering. In Proceedings of the 2009 4th International Microsystems, Packaging, Assembly and Circuits Technology Conference, Taipei, Taiwan, 21–23 October 2009; pp. 457–460. [Google Scholar]
- Mesa, F.; Gordillo, G.; Dittrich, T.; Ellmer, K.; Baier, R.; Sadewasser, S. Transient surface photovoltage of p-type Cu3BiS3. Appl. Phys. Lett. 2010, 96, 082113. [Google Scholar] [CrossRef]


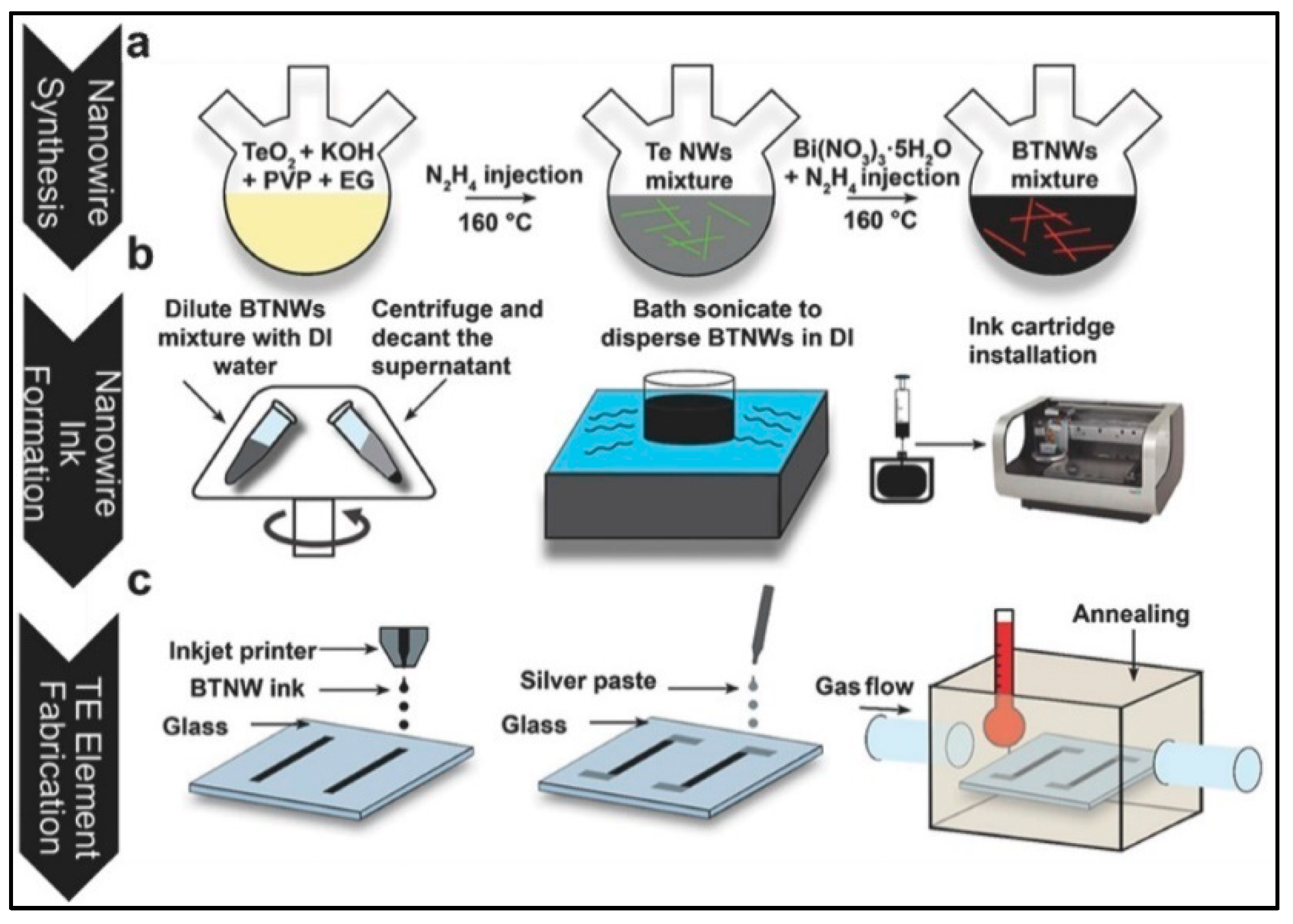
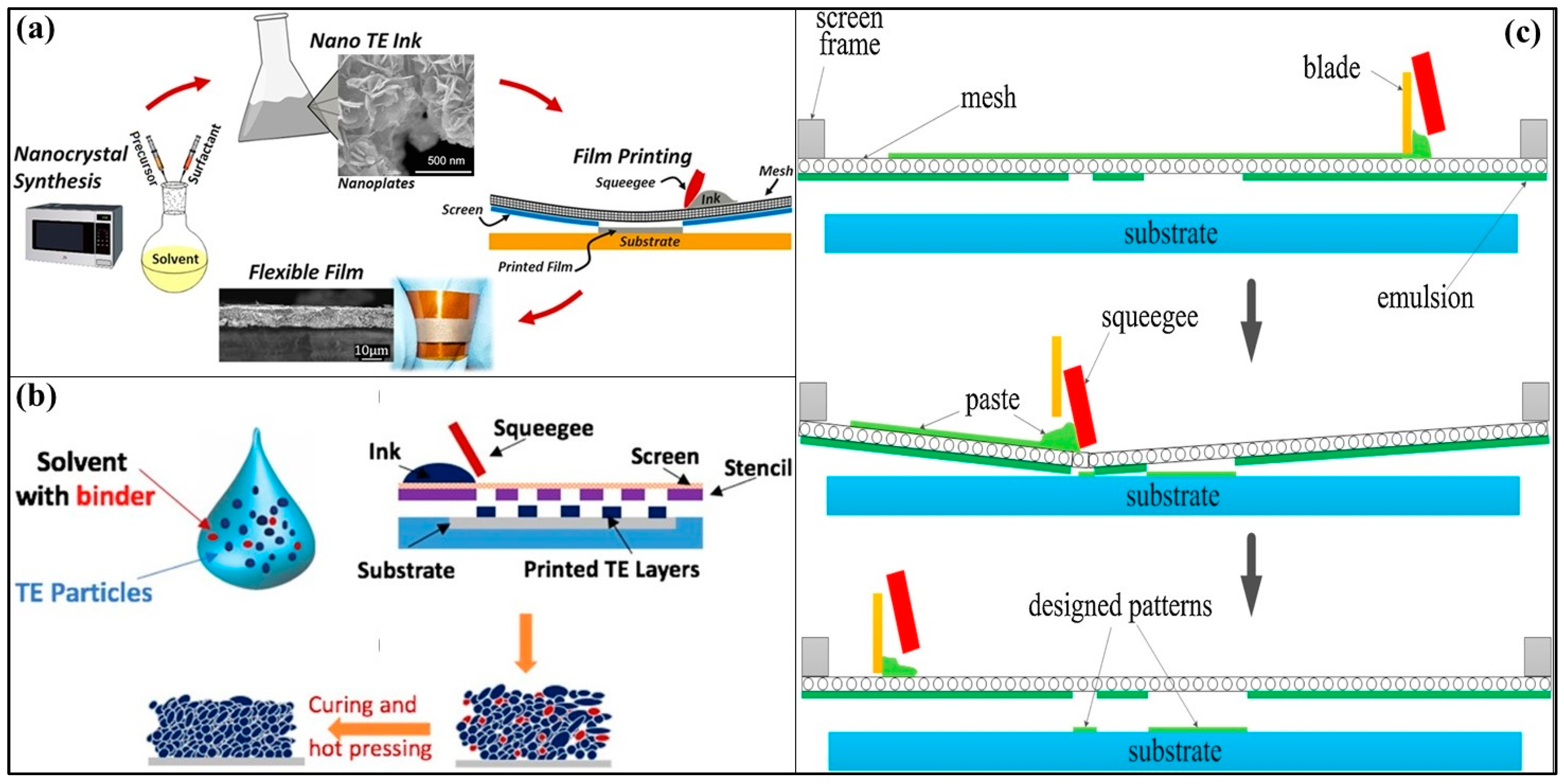



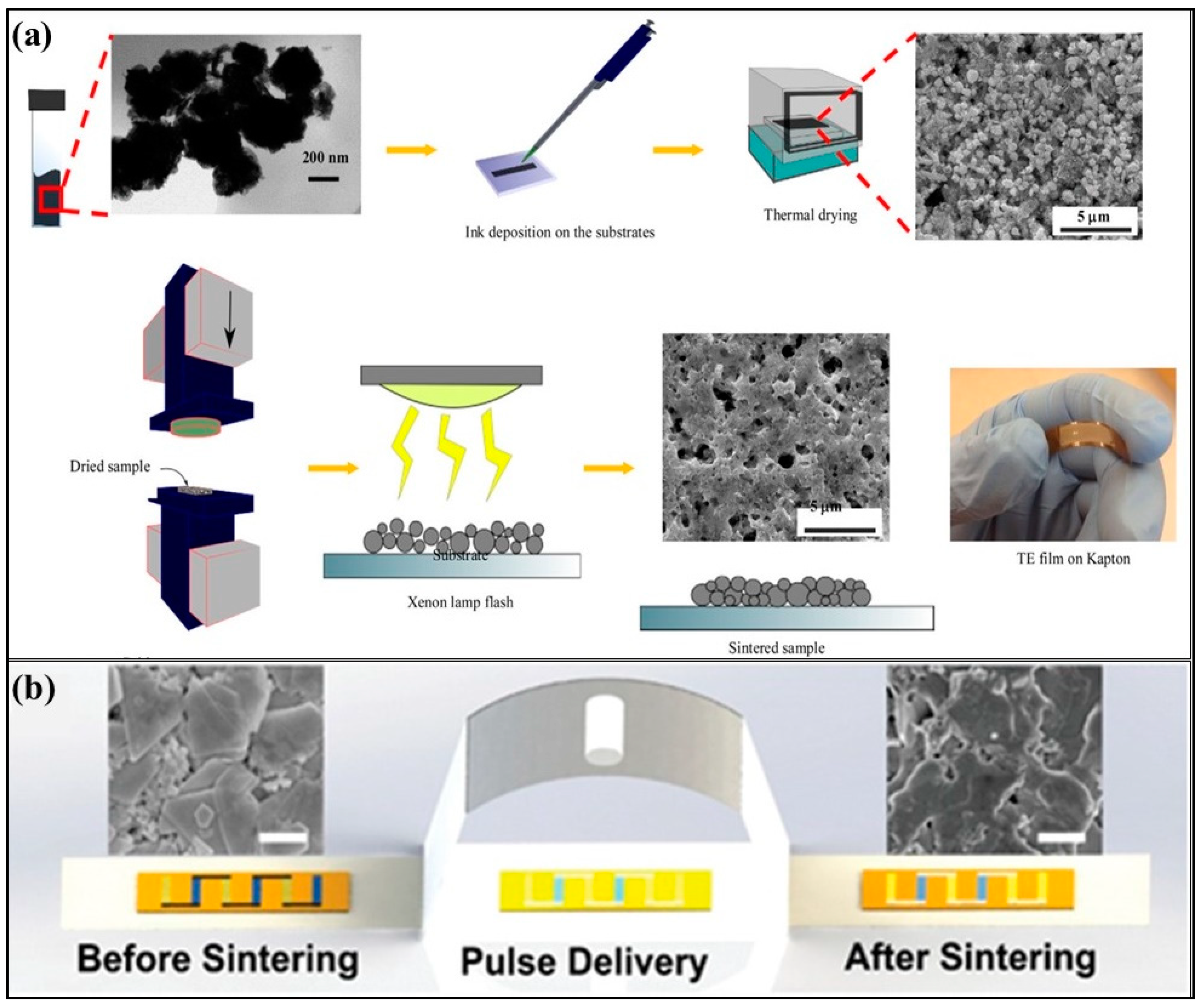
| Technology | Materials | Processing Cost | Time | Scalability (Max Weight Per Batch) | Ztmax (Material-Ref) | Strengths | Drawbacks |
|---|---|---|---|---|---|---|---|
| Inkjet printing | Organic, hybrid organic–inorganic | Low | Medium | High | 0.26 (Bi2Te3 [20]) | High quality, fine, and smooth printing. Power source in wearable and portable electronics | Nozzle clogging, nozzle plate flooding, and erratic droplet ejection |
| Screen printing | Organic, hybrid organic–inorganic | Low | Fast | High | ≈1(Bi0.5Sb1.5Te3 [21]) | Printable on diverse substrates, durable and high quality | Relatively complex and less eco-friendly |
| Dispenser Printing | Organic, hybrid organic–inorganic | Low | Medium | Medium | 0.41 (Sb2Te3-epoxy [22]) | Higher contact resistance, simple, easy, and do not have post-processing requirements | Slow dispensing and difficulty in reproducibility |
| Photonic sintering | Inorganic (Bismuth-based) | Low | Fast | High | - | Higher conductivities, shorter processing times, better adhesion, flexibility | The intense light pulses lead to increased energy consumption |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaldurgam, F.F.; Ahmad, Z.; Touati, F. Synthesis and Performance of Large-Scale Cost-Effective Environment-Friendly Nanostructured Thermoelectric Materials. Nanomaterials 2021, 11, 1091. https://doi.org/10.3390/nano11051091
Jaldurgam FF, Ahmad Z, Touati F. Synthesis and Performance of Large-Scale Cost-Effective Environment-Friendly Nanostructured Thermoelectric Materials. Nanomaterials. 2021; 11(5):1091. https://doi.org/10.3390/nano11051091
Chicago/Turabian StyleJaldurgam, Farheen F., Zubair Ahmad, and Farid Touati. 2021. "Synthesis and Performance of Large-Scale Cost-Effective Environment-Friendly Nanostructured Thermoelectric Materials" Nanomaterials 11, no. 5: 1091. https://doi.org/10.3390/nano11051091
APA StyleJaldurgam, F. F., Ahmad, Z., & Touati, F. (2021). Synthesis and Performance of Large-Scale Cost-Effective Environment-Friendly Nanostructured Thermoelectric Materials. Nanomaterials, 11(5), 1091. https://doi.org/10.3390/nano11051091






