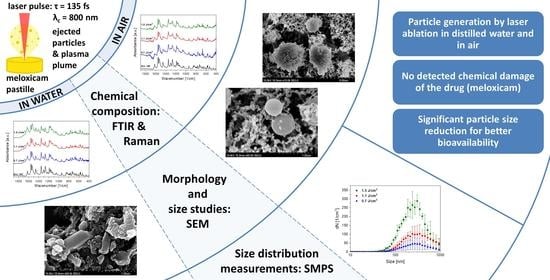
Fabrication of Submicrometer-Sized Meloxicam Particles Using Femtosecond Laser Ablation in Gas and Liquid Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Meloxicam
2.2. Laser Source
2.3. Target
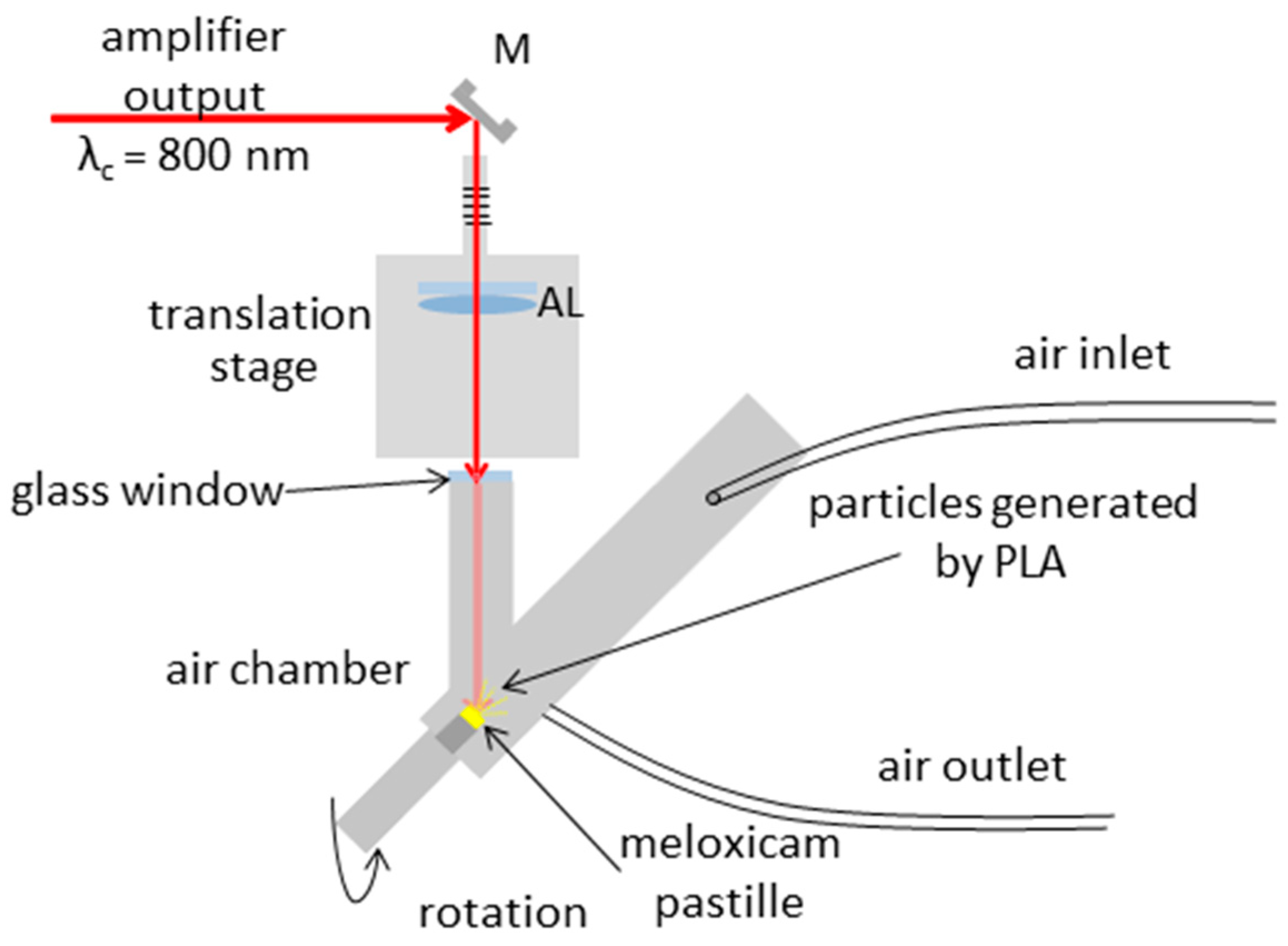
2.4. Pulsed Laser Ablation in Air (PLA)
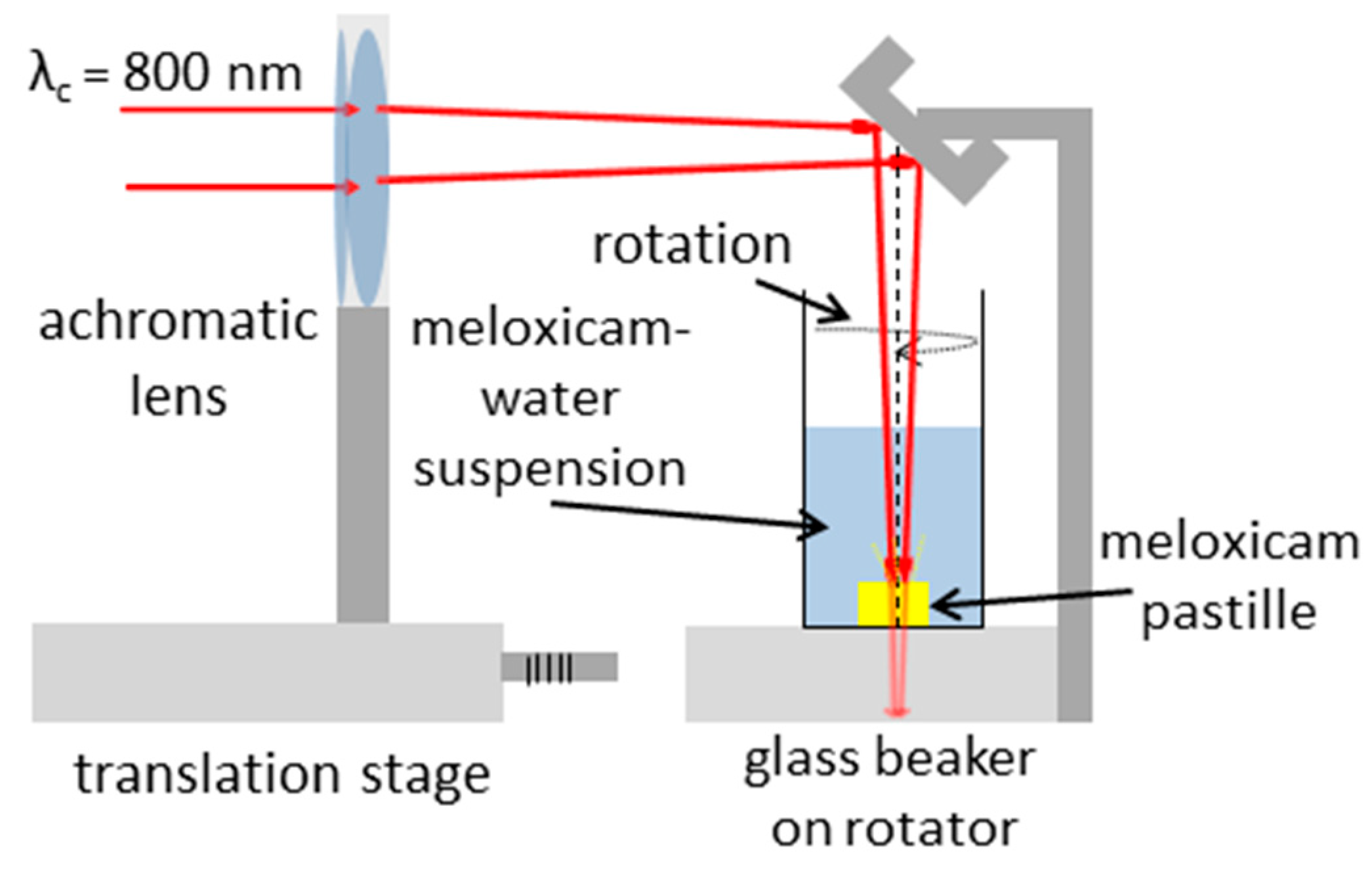
2.5. Pulsed Laser Ablation in Liquid (PLAL)
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Raman Spectroscopy
2.8. Scanning Electron Microscopy (SEM)
2.9. Scanning Mobility Particle Sizer (SMPS)
3. Results
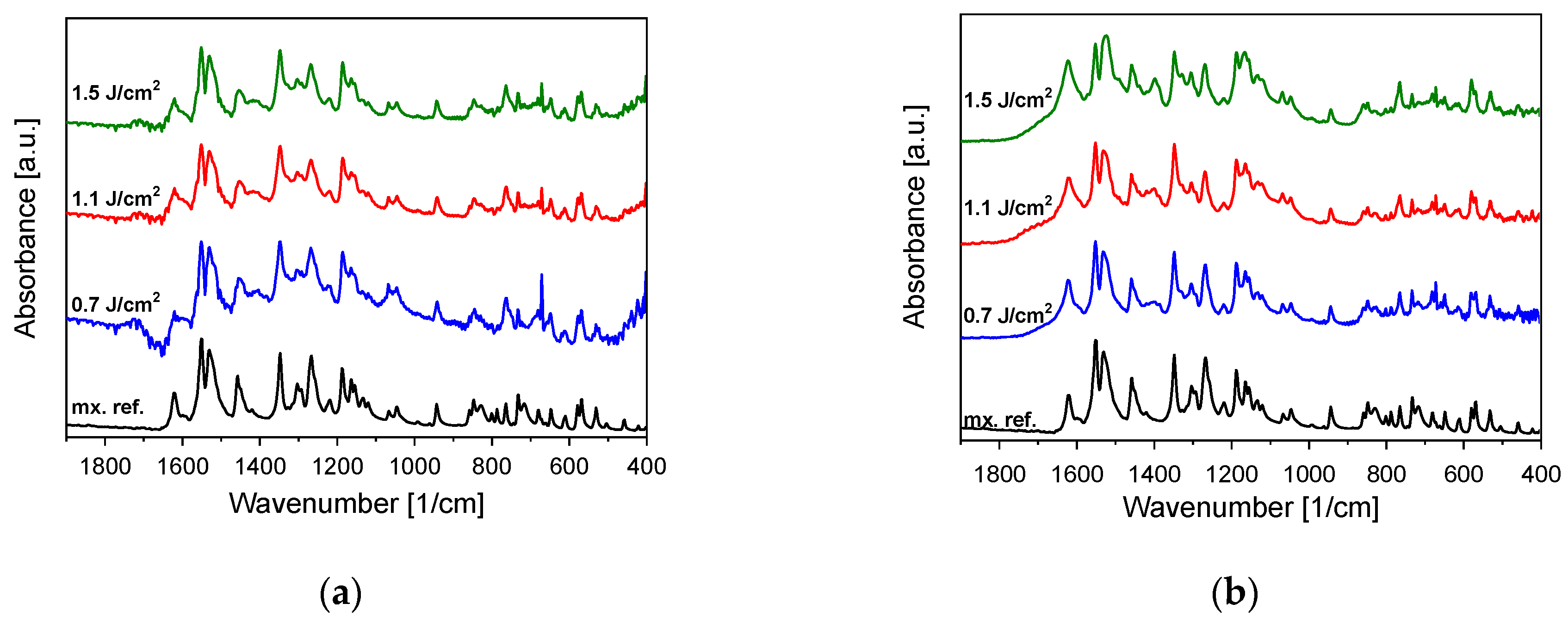
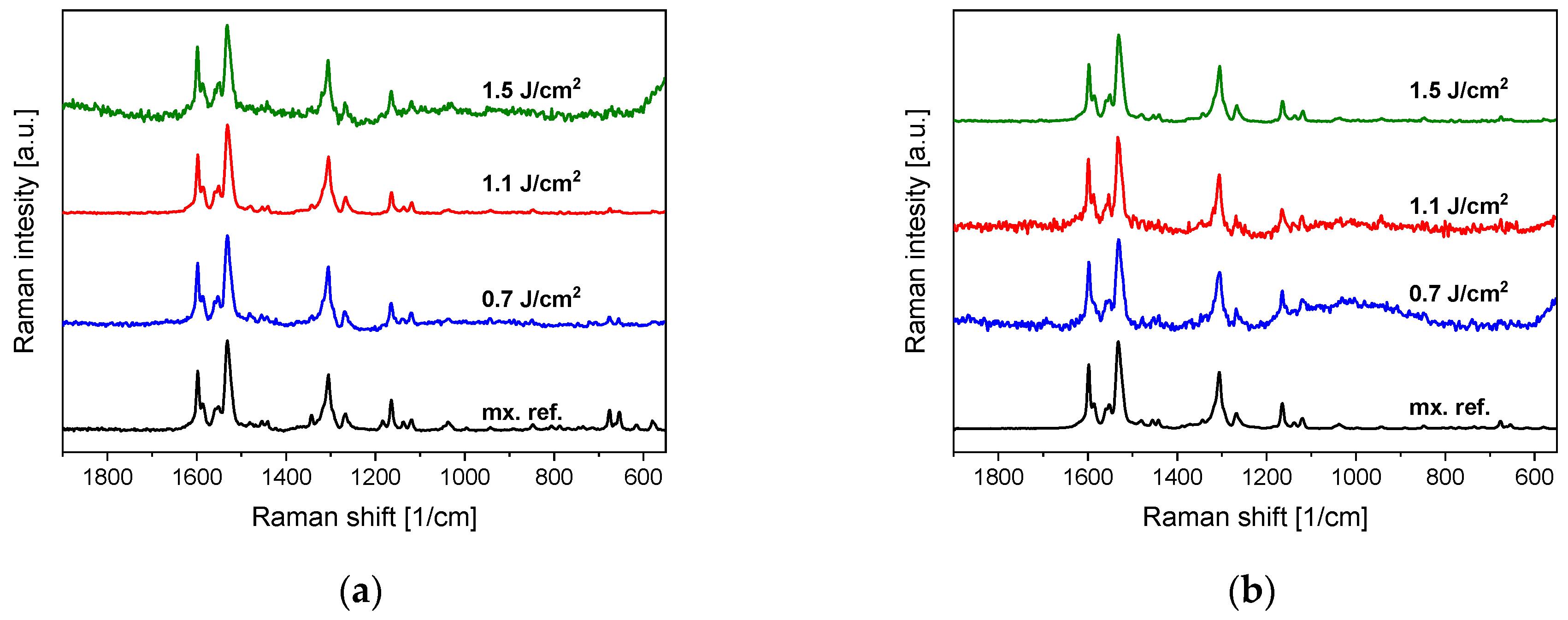
3.1. Chemical Composition (FTIR, Raman)
3.2. Particle Size Comparison (SEM, SMPS)
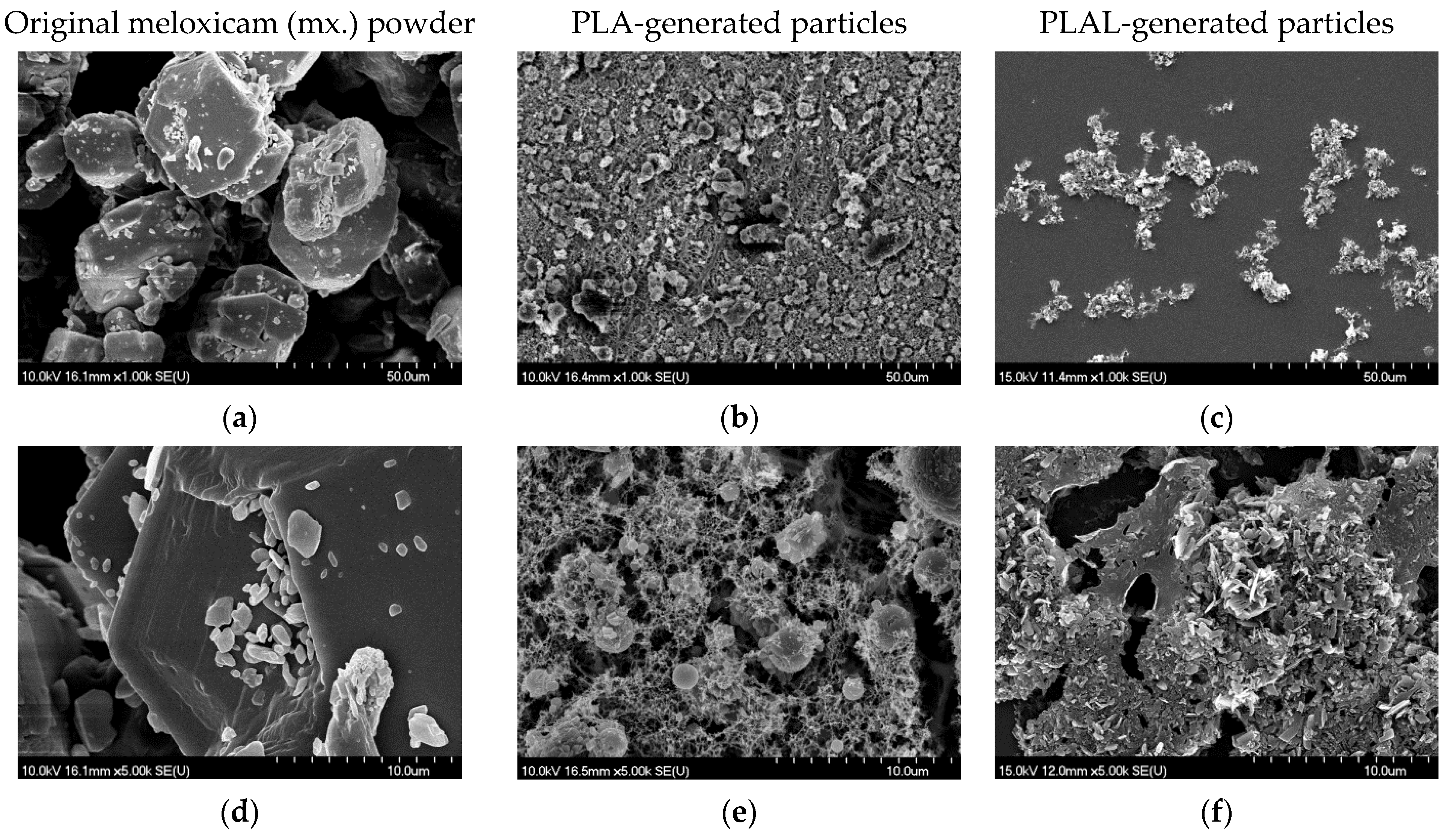
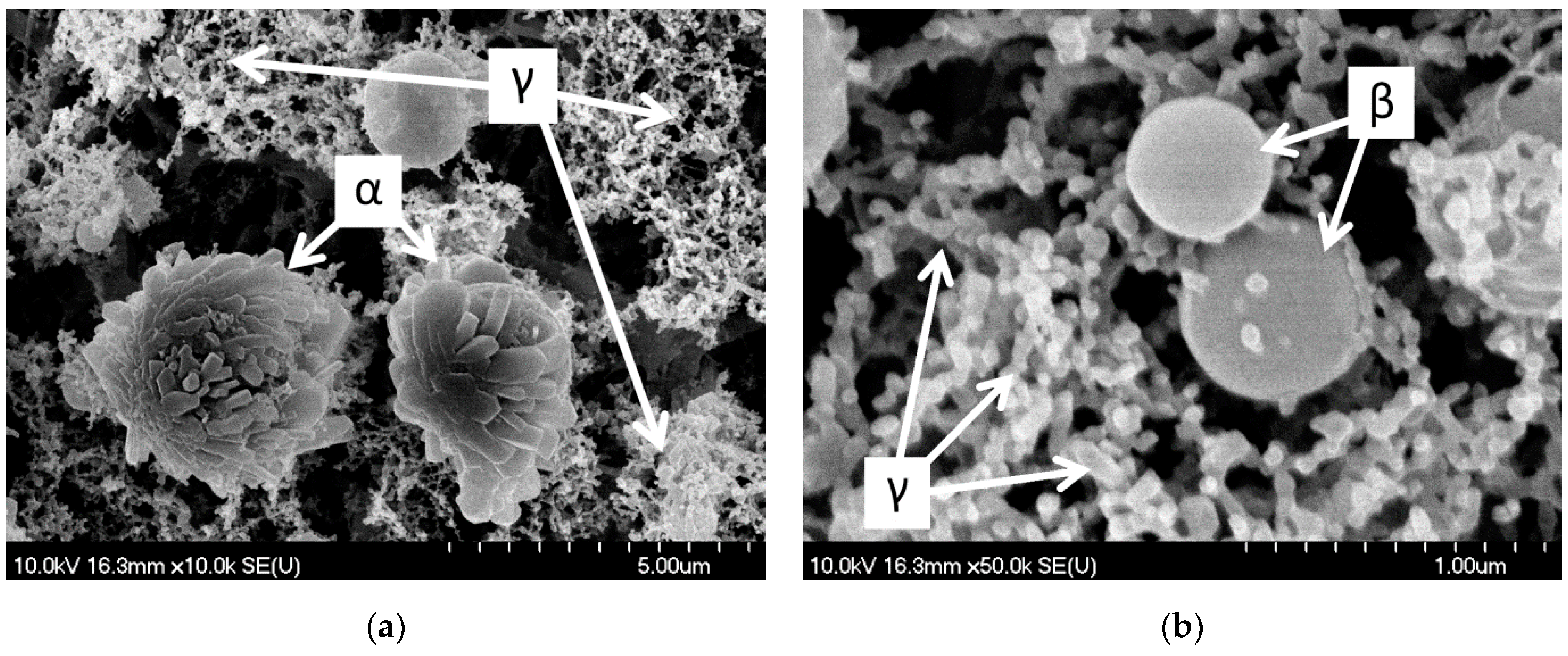
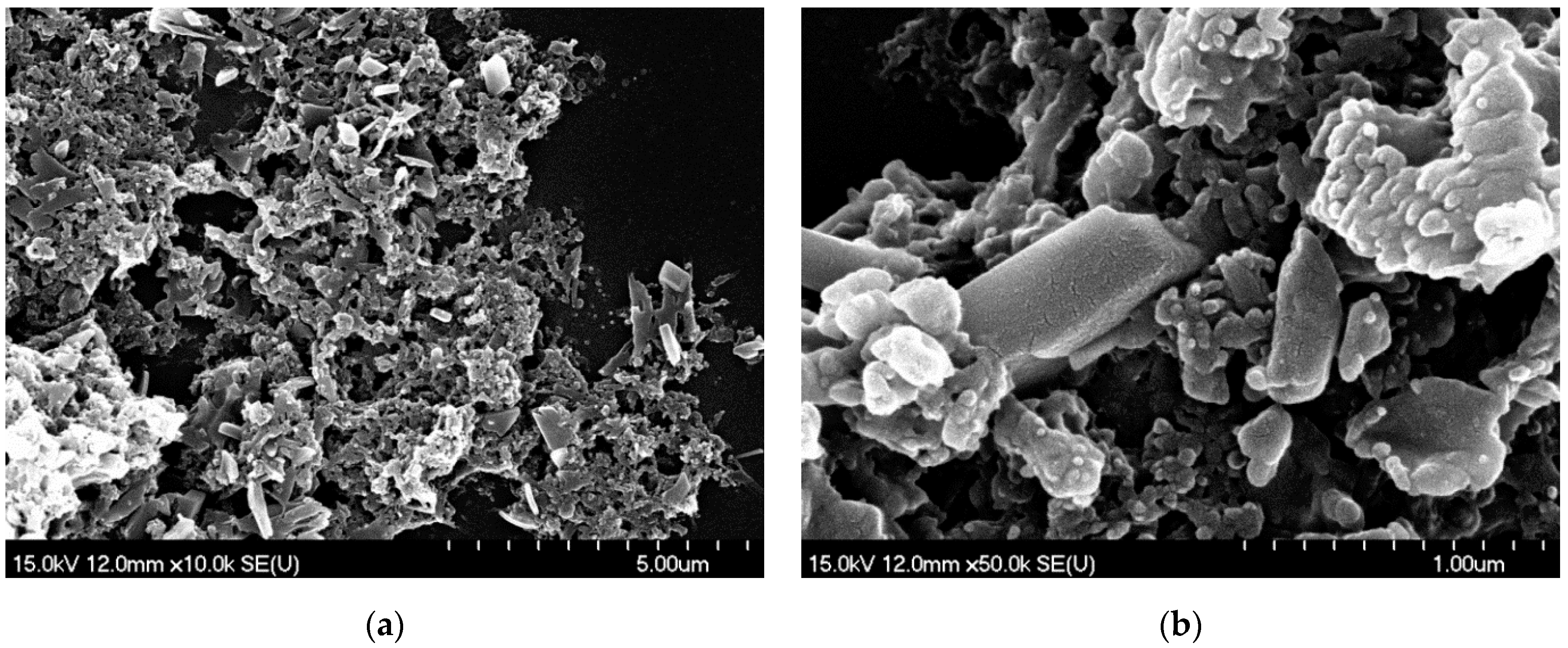
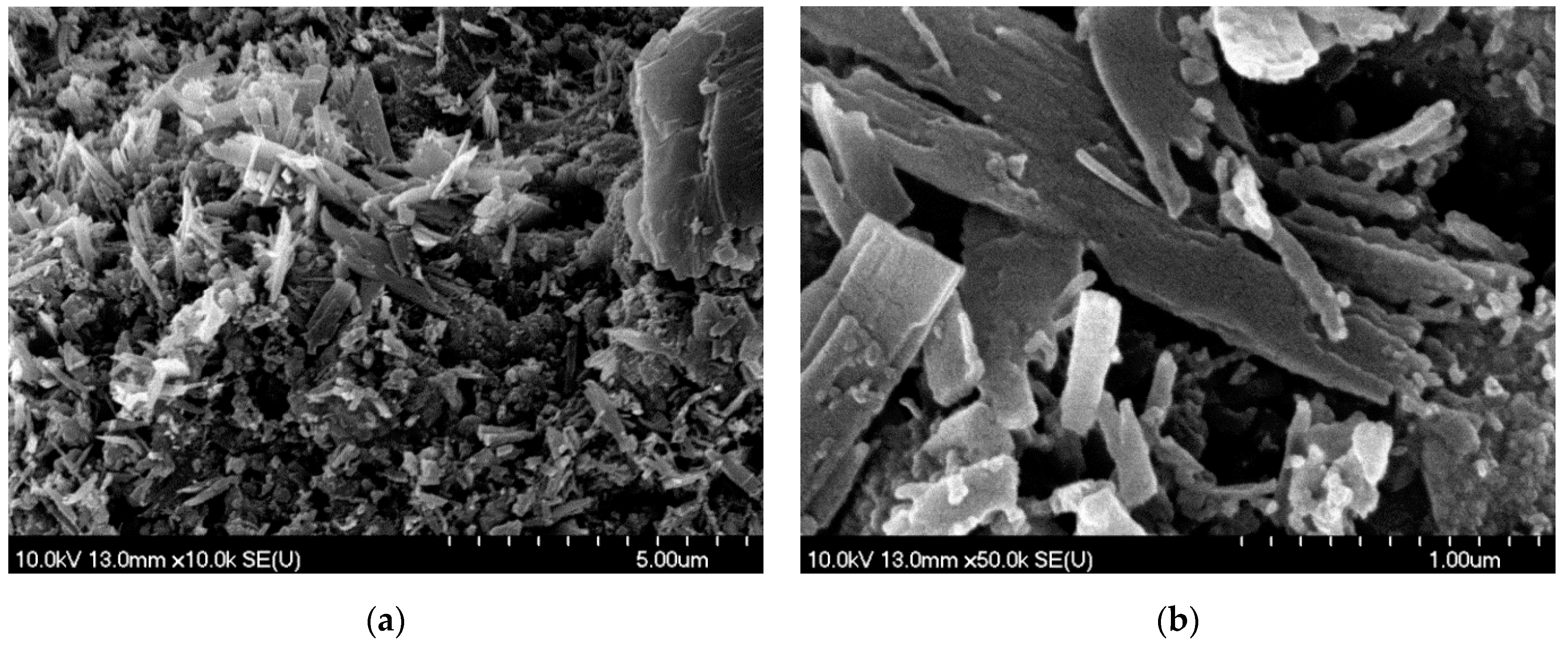
3.2.1. SEM
3.2.2. SMPS PLA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazakevich, V.S.; Kazakevich, P.V.; Yaresko, P.S.; Kazakevich, D.A. Laser ablation of gold and titanium targets in heavy water. J. Phys. Conf. Ser. 2018, 1096. [Google Scholar] [CrossRef]
- Pyatenko, A.; Shimokawa, K.; Yamaguchi, M.; Nishimura, O.; Suzuki, M. Synthesis of silver nanoparticles by laser ablation in pure water. Appl. Phys. A Mater. Sci. Process. 2004, 79, 803–806. [Google Scholar] [CrossRef]
- Karimzadeh, R.; Anvari, J.Z.; Mansour, N. Nanosecond pulsed laser ablation of silicon in liquids. Appl. Phys. A Mater. Sci. Process. 2009, 94, 949–955. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Comesaña, R.; Lusquiños, F.; Riveiro, A.; del Val, J.; Pou, J. Production of silver nanoparticles by laser ablation in open air. Appl. Surface Sci. 2015, 336, 108–111. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, N.; Zhu, X.; Wu, Z. Femtosecond laser ablation of silicon in air and vacuum. Chin. Opt. Lett. 2011, 9, 093201. [Google Scholar]
- Pershin, S.M.; Lednev, V.N.; Bunkin, A.F. Laser ablation of alloys: Selective evaporation model. Phys. Wave Phenom. 2011, 19, 261–274. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Chen, M.; Zhao, M. Laser ablation of Ti-Al alloy in vacuum and air environments. Appl. Mech. Mater. 2012, 217, 2257–2264. [Google Scholar] [CrossRef]
- Zheng, B.; Jiang, G.; Wang, W.; Wang, K.; Mei, X. Ablation experiment and threshold calculation of titanium alloy irradiated by ultra-fast pulse lase. AIP Adv. 2014, 4, 031310. [Google Scholar] [CrossRef]
- Asahi, T.; Sugiyama, T.; Masuhara, H. Laser fabrication and spectroscopy of organic nanoparticles. Acc. Chem. Res. 2008, 41, 1790–1798. [Google Scholar] [CrossRef]
- Wagener, P.; Barcikowski, S. Laser fragmentation of organic microparticles into colloidal nanoparticles in a free liquid jet. Appl. Phys. A Mater. Sci. Process. 2010, 101, 435–439. [Google Scholar] [CrossRef]
- Sylvestre, J.P.; Tang, M.C.; Furtos, A.; Leclair, G.; Meunier, M.; Leroux, J.C. Nanonization of megestrol acetate by laser fragmentation in aqueous milieu. J. Control. Release 2011, 149, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Sylvestre, J.-P.; Leclair, G.; Meunier, M. Laser Fragmentation as an Efficient Size-Reduction Method for Pulmonary Drug Discovery: Proof-of-Concept Study of Beclomethasone Dipropionate. Int. J. Theor. Appl. Nanotechnol. 2012, 1, 99–104. [Google Scholar] [CrossRef]
- Kenth, S.; Sylvestre, J.P.; Fuhrmann, K.; Meunier, M.; Leroux, J.C. Fabrication of paclitaxel nanocrystals by femtosecond laser ablation and fragmentation. J. Pharm. Sci. 2011, 100, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Mosharraf, M.; Nyström, C. The effect of particle size and shape on the surface specific dissolution rate of microsized practically insoluble drugs. Int. J. Pharm. 1995, 122, 35–47. [Google Scholar] [CrossRef]
- Rasenack, N.; Hartenhauer, H.; Müller, B.W. Microcrystals for dissolution rate enhancement of poorly water-soluble drugs. Int. J. Pharm. 2003, 254, 137–145. [Google Scholar] [CrossRef]
- Sud, S.; Kamath, A. Methods of size reduction and factors affecting size reduction in pharmaceutics. Int. Res. J. Pharm. 2013, 4, 57–64. [Google Scholar] [CrossRef]
- Loh, Z.H.; Samanta, A.K.; Heng, P.W.S. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J. Pharm. Sci. 2014, 10, 255–274. [Google Scholar] [CrossRef]
- Pawar, S.R.; Barhate, S.D. Solubility enhancement (Solid Dispersions) novel boon to increase bioavailability. J. Drug Deliv. Ther. 2019, 9, 583–590. [Google Scholar] [CrossRef]
- Andrews, G.; Jones, D.; Zhai, H.; Diak, O.A.; Walker, G. Effects of grinding in pharmaceutical tablet production. In Pharmaceutical Sciences Encyclopedia; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Bartos, C.; Szabó-Révész, P.; Bartos, C.; Katona, G.; Jójárt-Laczkovich, O.; Ambrus, R. The Effect of an Optimized Wet Milling Technology on the Crystallinity, Morphology and Dissolution Properties of Micro- and Nanonized Meloxicam. Molecules 2016, 21, 507. [Google Scholar] [CrossRef] [PubMed]
- Bartos, C.; Ambrus, R.; Szabóné, R.P. Particle size reduction using acoustic cavitation. Acta Pharm. Hung. 2014, 84, 131–135. [Google Scholar]
- Gera, T.; Smausz, T.; Kopniczky, J.; Galbács, G.; Ambrus, R.; Szabó-Révész, P.; Hopp, B. Production of ibuprofen in crystalline and amorphous forms by Pulsed Laser Deposition (PLD). Appl. Surf. Sci. 2019, 493, 359–367. [Google Scholar] [CrossRef]
- Hopp, B.; Nagy, E.; Peták, F.; Smausz, T.; Kopniczky, J.; Tápai, C.; Budai, J.; Papp, I.Z.; Kukovecz, Á.; Ambrus, R.; et al. Production of meloxicam suspension using pulsed laser ablation in liquid (PLAL) technique. J. Phys. D Appl. Phys. 2018, 51, 16. [Google Scholar] [CrossRef]
- Ding, W.; Sylvestre, J.P.; Bouvier, E.; Leclair, G.; Meunier, M. Ultrafast laser processing of drug particles in water for pharmaceutical discovery. Appl. Phys. A Mater. Sci. Process. 2014, 114, 267–276. [Google Scholar] [CrossRef]
- Ambrus, R.; Szabó-Révész, P.; Kiss, T.; Nagy, E.; Szűcs, T.; Smausz, T.; Hopp, B. Application of a suitable particle engineering technique by pulsed laser ablation in liquid (PLAL) to modify the physicochemical properties of poorly soluble drugs. J. Drug Deliv. Sci. Technol. 2020, 57, 101727. [Google Scholar] [CrossRef]
- Gera, T.; Nagy, E.; Smausz, T.; Budai, J.; Ajtai, T.; Kun-Szabó, F.; Homik, Z.; Kopniczky, J.; Bozóki, Z.; Szabó-Révész, P.; et al. Application of pulsed laser ablation (PLA) for the size reduction of non-steroidal anti-inflammatory drugs (NSAIDs). Sci. Rep. 2020, 10, 15806. [Google Scholar] [CrossRef]
- Singh, A.; Kutscher, H.L.; Bulmahn, J.C.; Mahajan, S.D.; He, G.S.; Prasad, P.N. Laser ablation for pharmaceutical nanoformulations: Multi-drug nanoencapsulation and theranostics for HIV. Nanomed. Nanotechnol. Biol. Med. 2020, 25, 102172. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.A.; Bakhtiar, H.; Ghoshal, S.K.; Huyop, F. Customised structural, optical and antibacterial characteristics of cinnamon nanoclusters produced inside organic solvent using 532 nm Q-switched Nd:YAG-pulse laser ablation. Opt. Laser Technol. 2020, 130, 106331. [Google Scholar] [CrossRef]
- M.|C.- PubChem. Meloxicam|C14H13N3O4S2—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Meloxicam#section=Solubility&fullscreen=true (accessed on 5 February 2020).
- Hanft, G.; Türck, D.; Scheuerer, S.; Sigmund, R. Meloxicam oral suspension: A treatment alternative to solid meloxicam formulations. Inflamm. Res. 2001, 50, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Venkatakrishnan, K. A femtosecond laser-induced periodical surface structure on crystalline silicon. J. Micromech. Microeng. 2006, 16, 1080–1085. [Google Scholar] [CrossRef]
- Arpagaus, C. Pharmaceutical Particle Engineering via Nano Spray Drying—Process Parameters and Application Examples on the Laboratory-Scale. Int. J. Med. Nano Res. 2018, 5, 26. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Mohyeldin, S.M.; Elgindy, N.A. Rifampicin-carbohydrate spray-dried nanocomposite: A futuristic multiparticulate platform for pulmonary delivery. Int. J. Nanomed. 2019, 14, 9089–9112. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, A.; Ambrus, R.; Party, P.; Katona, G.; Jójárt-Laczkovich, O.; Szabó-Révész, P.; Fattal, E.; Tsapis, N. Formulation and comparison of spray dried non-porous and large porous particles containing meloxicam for pulmonary drug delivery. Int. J. Pharm. 2019, 559, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, S.; Tan, B.; Venkatakrishnan, K. Critical Time to Nucleation: Graphite and Silicon Nanoparticle Generation by Laser Ablation. J. Nanotechnol. 2009, 2009, 590763. [Google Scholar] [CrossRef]
- Ajtai, T.; Utry, N.; Pintér, M.; Kiss-Albert, G.; Puskás, R.; Tápai, C.; Kecskeméti, G.; Smausz, T.; Hopp, B.; Bozóki, Z.; et al. Microphysical properties of carbonaceous aerosol particles generated by laser ablation of a graphite target. Atmos. Meas. Tech. 2015, 8, 1207–1215. [Google Scholar] [CrossRef]
- Gelencsér, A. Carbonaceous Aerosol; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, E.; Andrásik, A.; Smausz, T.; Ajtai, T.; Kun-Szabó, F.; Kopniczky, J.; Bozóki, Z.; Szabó-Révész, P.; Ambrus, R.; Hopp, B. Fabrication of Submicrometer-Sized Meloxicam Particles Using Femtosecond Laser Ablation in Gas and Liquid Environments. Nanomaterials 2021, 11, 996. https://doi.org/10.3390/nano11040996
Nagy E, Andrásik A, Smausz T, Ajtai T, Kun-Szabó F, Kopniczky J, Bozóki Z, Szabó-Révész P, Ambrus R, Hopp B. Fabrication of Submicrometer-Sized Meloxicam Particles Using Femtosecond Laser Ablation in Gas and Liquid Environments. Nanomaterials. 2021; 11(4):996. https://doi.org/10.3390/nano11040996
Chicago/Turabian StyleNagy, Eszter, Attila Andrásik, Tamás Smausz, Tibor Ajtai, Fruzsina Kun-Szabó, Judit Kopniczky, Zoltán Bozóki, Piroska Szabó-Révész, Rita Ambrus, and Béla Hopp. 2021. "Fabrication of Submicrometer-Sized Meloxicam Particles Using Femtosecond Laser Ablation in Gas and Liquid Environments" Nanomaterials 11, no. 4: 996. https://doi.org/10.3390/nano11040996
APA StyleNagy, E., Andrásik, A., Smausz, T., Ajtai, T., Kun-Szabó, F., Kopniczky, J., Bozóki, Z., Szabó-Révész, P., Ambrus, R., & Hopp, B. (2021). Fabrication of Submicrometer-Sized Meloxicam Particles Using Femtosecond Laser Ablation in Gas and Liquid Environments. Nanomaterials, 11(4), 996. https://doi.org/10.3390/nano11040996