Hierarchical Microstructure of Tooth Enameloid in Two Lamniform Shark Species, Carcharias taurus and Isurus oxyrinchus
Abstract
1. Introduction
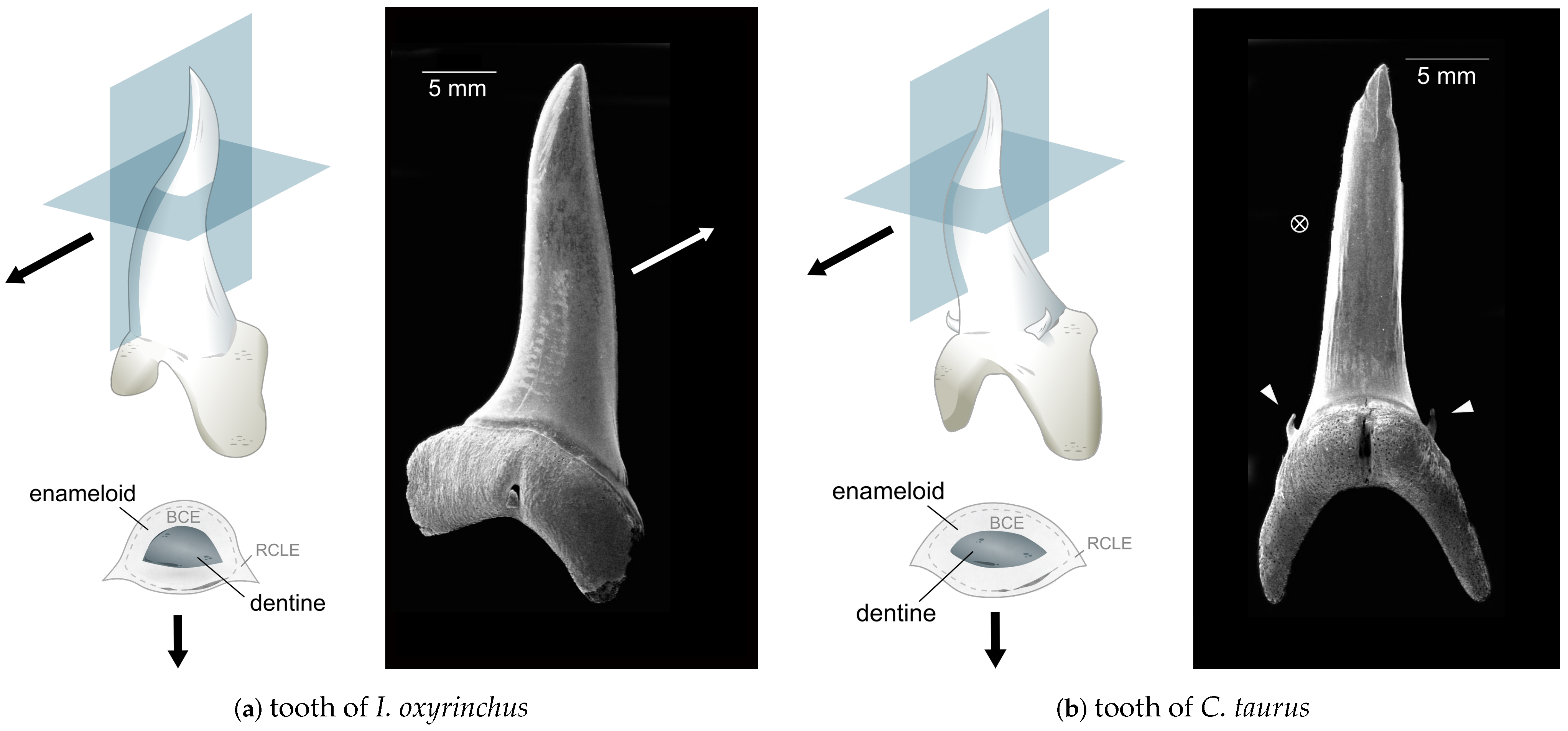
2. Materials and Methods
3. Results: Microstructure Description of Enameloid in I. oxyrinchus and C. taurus
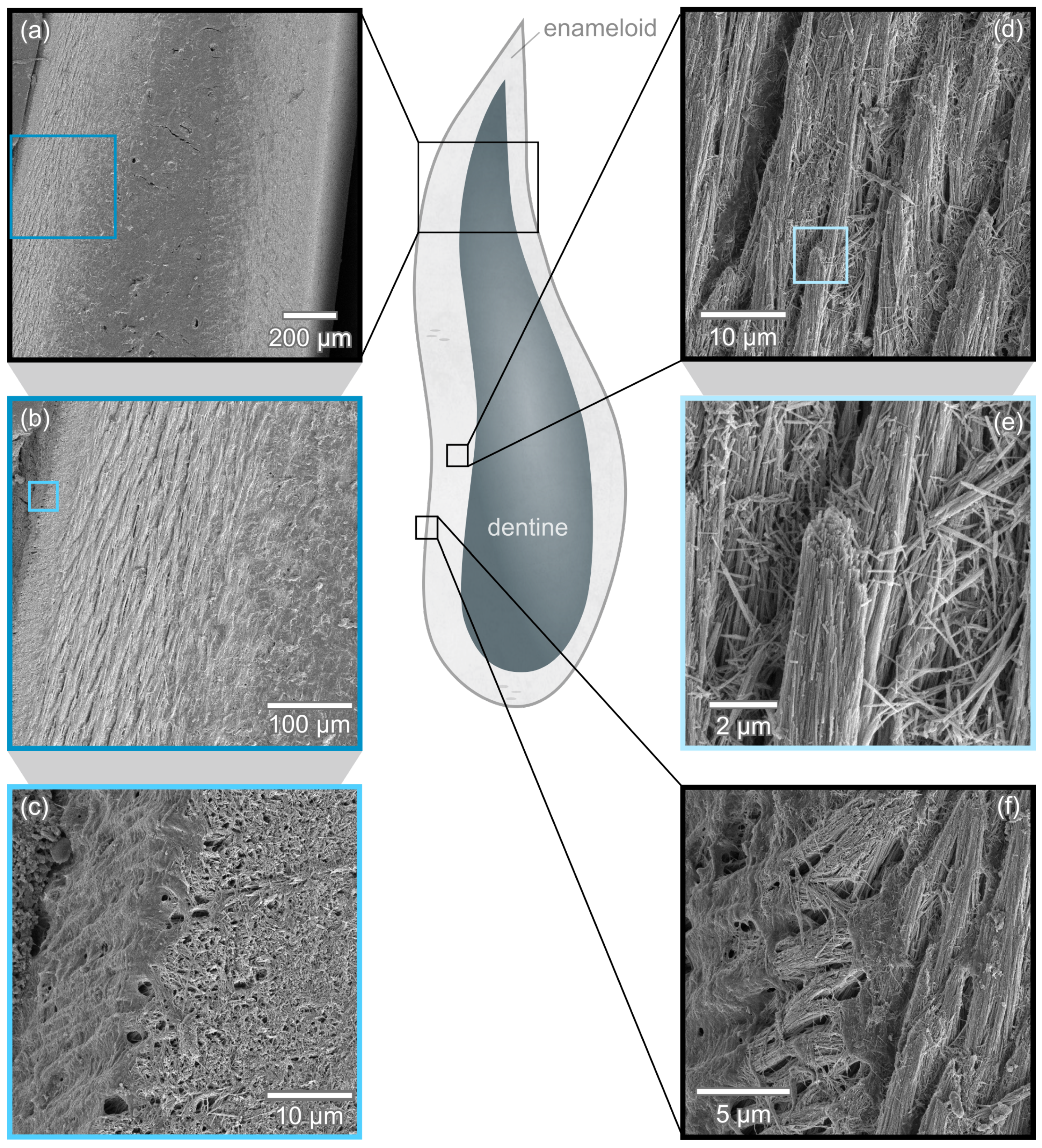
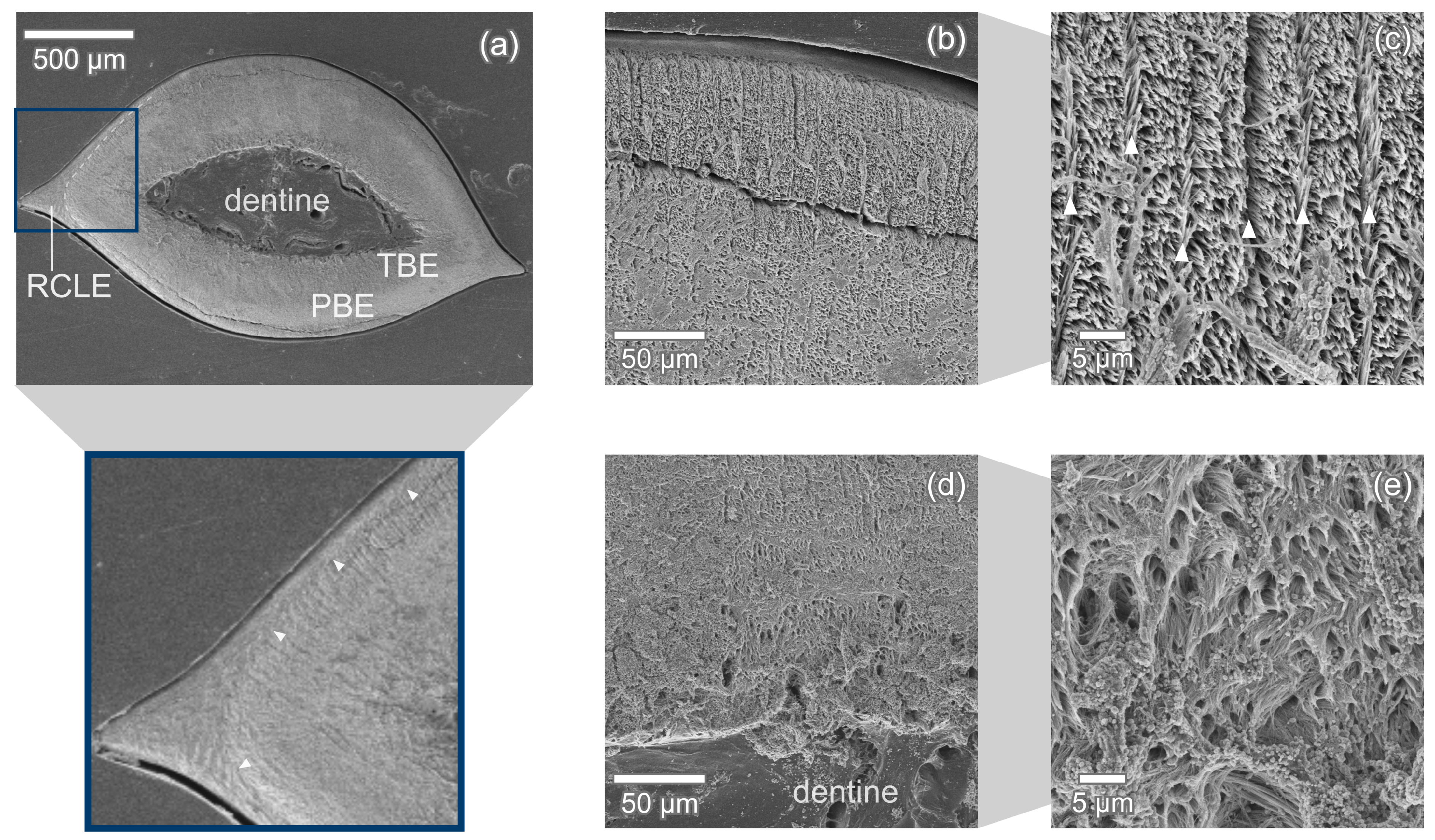
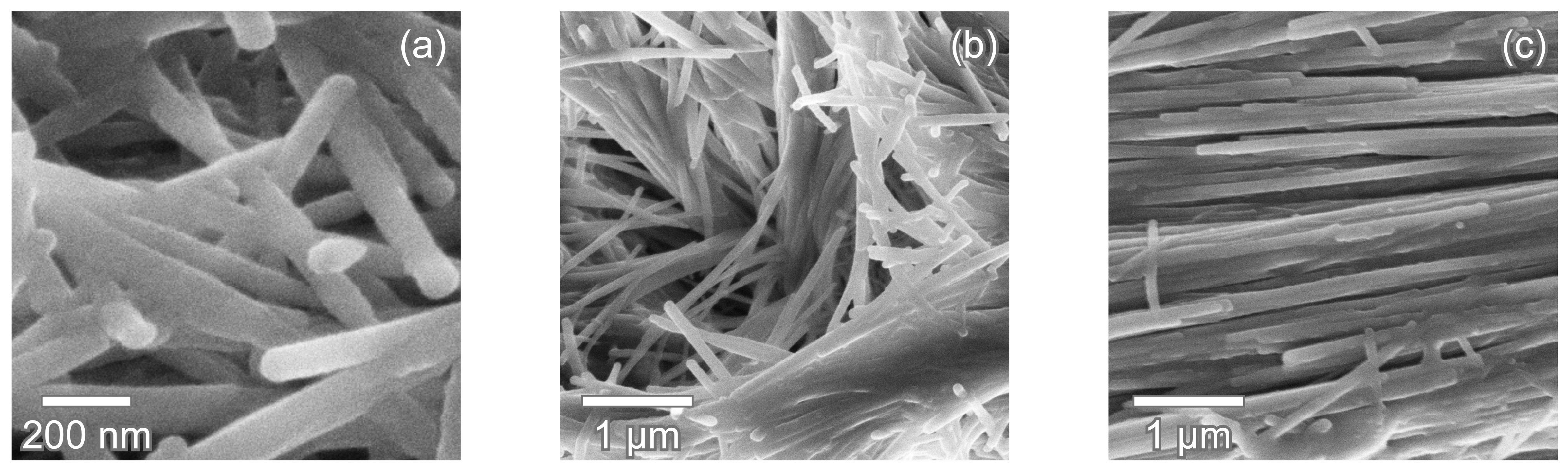
3.1. Enameloid Microstructure of Isurus oxyrinchus
3.2. Enameloid Microstructure of Carcharias taurus
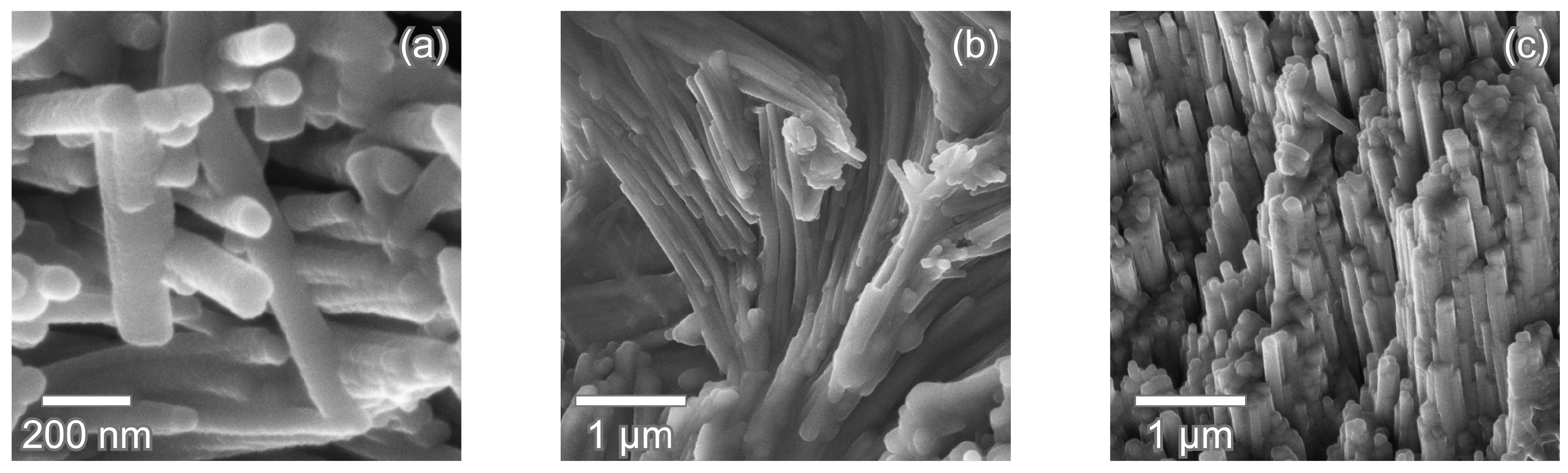
4. Microstructural Features of Modern Shark Enameloid
4.1. Lamniform Shark Enameloid Structure
4.2. Radial Elements in Enameloid
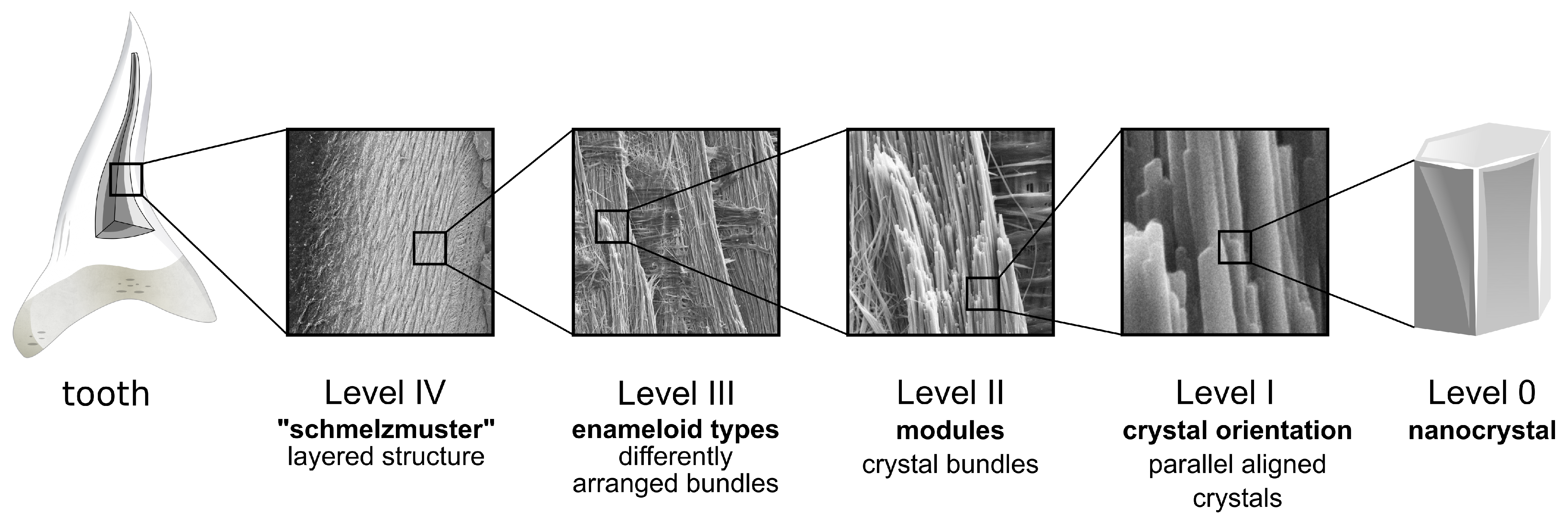
5. Structural Hierarchy in Dental Materials: Similarities between Enameloid and Enamel
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shimada, K. Dental homologies in lamniform sharks (Chondrichthyes: Elasmobranchii). J. Morphol. 2002, 251, 38–72. [Google Scholar] [CrossRef]
- Whitenack, L.B.; Gottfried, M.D. A morphometric approach for addressing tooth-based species delimitation in fossil mako sharks, Isurus (Elasmobranchii: Lamniformes). J. Vertebr. Paleontol. 2010, 30, 17–25. [Google Scholar] [CrossRef]
- Corn, K.A.; Farina, S.C.; Brash, J.; Summers, A.P. Modelling tooth–prey interactions in sharks: The importance of dynamic testing. R. Soc. Open Sci. 2016, 3, 160141. [Google Scholar] [CrossRef]
- Enax, J.; Prymak, O.; Raabe, D.; Epple, M. Structure, composition, and mechanical properties of shark teeth. J. Struct. Biol. 2012, 178, 290–299. [Google Scholar] [CrossRef]
- Frazzetta, T. The mechanics of cutting and the form of shark teeth (Chondrichthyes, Elasmobranchii). Zoomorphology 1988, 108, 93–107. [Google Scholar] [CrossRef]
- Correia, J.P. Tooth loss rate from two captive sandtiger sharks (Carcharias Taurus). Zoo Biol. 1999, 18, 313–317. [Google Scholar] [CrossRef]
- Luer, C.A.; Blum, P.C.; Gilbert, P.W. Rate of Tooth Replacement in the Nurse Shark, Ginglymostoma Cirratum. Copeia 1990, 1, 182–191. [Google Scholar] [CrossRef]
- Botella, H.; Valenzuela-Ríos, J.I.; Martínez-Pérez, C. Tooth replacement rates in early chondrichthyans: A qualitative approach. Lethaia 2009, 42, 365–376. [Google Scholar] [CrossRef]
- Whitenack, L.B.; Simkins, D.C.; Motta, P.J. Biology meets engineering: The structural mechanics of fossil and extant shark teeth. J. Morphol. 2010, 272, 169–179. [Google Scholar] [CrossRef]
- Weber, K.; Winkler, D.E.; Kaiser, T.M.; Žigaitė, Ž.; Tütken, T. Dental microwear texture analysis on extant and extinct sharks: Anteor post-mortem tooth wear? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 562, 110147. [Google Scholar] [CrossRef]
- Barthelat, F. Architectured materials in engineering and biology: Fabrication, structure, mechanics and performance. Int. Mater. Rev. 2015, 60, 413–430. [Google Scholar] [CrossRef]
- Gao, H.; Ji, B.; Jäger, I.L.; Arzt, E.; Fratzl, P. Materials become insensitive to flaws at nanoscale: Lessons from nature. Proc. Natl. Acad. Sci. USA 2003, 100, 5597–5600. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Ritchie, R.O. On the Materials Science of Nature’s Arms Race. Adv. Mater. 2018, 30, 1705220. [Google Scholar] [CrossRef] [PubMed]
- Wilmers, J.; Bargmann, S. Nature’s design solutions in dental enamel: Uniting high strength and extreme damage resistance. Acta Biomater. 2020, 107, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Naleway, S.E.; Wang, B. Biological and bioinspired materials: Structure leading to functional and mechanical performance. Bioact. Mater. 2020, 5, 745–757. [Google Scholar] [CrossRef]
- Daculsi, G.; Kerebel, L. Ultrastructural study and comparative analysis of fluoride content of enameloid in sea-water and fresh-water sharks. Arch. Oral Biol. 1980, 25, 145–151. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Saito, M.; Tohei, T.; Takano, Y.; Ikuhara, Y. Fluorine in Shark Teeth: Its Direct Atomic-Resolution Imaging and Strengthening Function. Angew. Chem. Int. Ed. 2014, 53, 1543–1547. [Google Scholar] [CrossRef]
- Enax, J.; Janus, A.M.; Raabe, D.; Epple, M.; Fabritius, H.O. Ultrastructural organization and micromechanical properties of shark tooth enameloid. Acta Biomater. 2014, 10, 3959–3968. [Google Scholar] [CrossRef]
- Cuny, C.; Risnes, S. The enameloid microstructure of the teeth of synechodontiform sharks (Chondrichthyes: Neoselachii). Vertebr. Paleontol. 2005, 3, 8–19. [Google Scholar]
- Guinot, G.; Cappetta, H. Enameloid microstructure of some Cretaceous Hexanchiformes and Synechodontiformes (Chondrichthyes, Neoselachii): New structures and systematic implications. Microsc. Res. Tech. 2011, 74, 196–205. [Google Scholar] [CrossRef]
- Enault, S.; Guinot, G.; Koot, M.B.; Cuny, G. Chondrichthyan tooth enameloid: Past, present, and future. Zool. J. Linn. Soc. 2015, 174, 549–570. [Google Scholar] [CrossRef]
- Lübke, A.; Enax, J.; Loza, K.; Prymak, O.; Gaengler, P.; Fabritius, H.O.; Raabe, D.; Epple, M. Dental lessons from past to present: Ultrastructure and composition of teeth from plesiosaurs, dinosaurs, extinct and recent sharks. RSC Adv. 2015, 5, 61612–61622. [Google Scholar] [CrossRef]
- Enault, S.; Cappetta, H.; Adnet, S. Simplification of the enameloid microstructure of large stingrays (Chondrichthyes: Myliobatiformes): A functional approach. Zool. J. Linn. Soc. 2013, 169, 144–155. [Google Scholar] [CrossRef][Green Version]
- Manzanares, E.; Botella, H.; Delsate, D. On the enameloid microstructure of Archaeobatidae (Neoselachii, Chondrichthyes). J. Iber. Geol. 2018, 44, 67–74. [Google Scholar] [CrossRef]
- Von Koenigswald, W. Levels of complexity in the microstructure of mammalian enamel and their application in studies of systematics. Scanning Microsc. 1992, 6, 195–217. [Google Scholar]
- Von Koenigswald, W.; Sander, P.M. Glossary of terms used for enamel microstructures. In Tooth Enamel Microstructure; von Koenigswald, W., Sander, P., Eds.; Balkema: Rotterdam, The Netherlands, 1997; pp. 267–280. [Google Scholar]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef]
- Gillis, J.A.; Donoghue, P.C. The homology and phylogeny of chondrichthyan tooth enameloid. J. Morphol. 2007, 268, 33–49. [Google Scholar] [CrossRef] [PubMed]
- van Vuuren, L.J.; Loch, C.; Kieser, J.A.; Gordon, K.C.; Fraser, S.J. Structure and mechanical properties of normal and anomalous teeth in the sand tiger shark Carcharias Taurus. J. Zoo Aquar. Res. 2015, 3, 29–36. [Google Scholar]
- Beniash, E.; Stifler, C.A.; Sun, C.Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P.U.P.A. The hidden structure of human enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, H.; Shi, M.; Zheng, L.; Qian, L.; Zhou, Z. In vitro study on the wear behaviour of human tooth enamel in citric acid solution. Wear 2011, 271, 2313–2321. [Google Scholar] [CrossRef]
- Boushell, L.W.; Sturdevant, J.R. Clinical Significance of Dental Anatomy, Histology, Physiology, and Occlusion. In Sturdevant’s Art & Science of Operative Dentistry, 7th ed.; Ritter, A., Ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Fellah, C.; Douillard, T.; Maire, E.; Meille, S.; Reynard, B.; Cuny, G. 3D microstructural study of selachimorph enameloid evolution. J. Struct. Biol. 2021, 213, 107664. [Google Scholar] [CrossRef] [PubMed]
- Pfretzschner, H. Enamel microstructure and hypsodonty in large mammals. In Structure, Function and Evolution of Teeth; Smith, P., Tchernov, E., Eds.; Freund Publishing House Ltd.: Tel Aviv, Israel, 1992. [Google Scholar]
- Von Koenigswald, W. Two different strategies in enamel differentiation: Marsupialia versus Eutheria. In Development, Function and Evolution of Teeth; Teaford, M.F., Smith, M.M., Ferguson, M.W.J., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 107–118. [Google Scholar]
- Kemp, N.E. Sharks, Skates, and Rays: The Biology of Elasmobranch Fishes; Chapter Integumentary System and Teeth; The Johns Hopkins University Press: Baltimore, MD, USA, 1999; pp. 43–68. [Google Scholar]
- Ferretti, M.P. Enamel Structure of Cuvieronius Hyodon (Proboscidea, Gomphotheriidae) discussion on enamel evolution in elephantoids. J. Mamm. Evol. 2008, 15, 37–58. [Google Scholar] [CrossRef]
- Meyers, M.A.; Lin, A.Y.M.; Lin, Y.S.; Olevsky, E.A.; Georgalis, S. The cutting edge: Sharp biological materials. JOM 2008, 60, 19–24. [Google Scholar] [CrossRef]
- Yin, Z.; Hannard, F.; Barthelat, F. Impact-resistant nacre-like transparent materials. Science 2019, 364, 1260–1263. [Google Scholar] [CrossRef] [PubMed]




| I. oxyrinchus Enameloid | C. taurus Enameloid | Wallaby Enamel | Human Enamel | |
|---|---|---|---|---|
| thickness TBE [μm] | 270–290 | 215 | - | - |
| thickness PBE [μm] | 250 | 115 | - | - |
| thickness outer enameloid [μm] | 30–35 | 10–30 | - | - |
| crystal width [nm] | 50–75 | 55–70 | 45–50 | 50–70 [30,31] |
| bundle diameter [μm] | 4–9 | 5 | 3–4 | 4–8 [32] |
| thickness of radial elements [μm] | 1.5 | 1.5 | 1–1.5 | - |
| distance radial elements [μm] | 6–10 | 5 | 4 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilmers, J.; Waldron, M.; Bargmann, S. Hierarchical Microstructure of Tooth Enameloid in Two Lamniform Shark Species, Carcharias taurus and Isurus oxyrinchus. Nanomaterials 2021, 11, 969. https://doi.org/10.3390/nano11040969
Wilmers J, Waldron M, Bargmann S. Hierarchical Microstructure of Tooth Enameloid in Two Lamniform Shark Species, Carcharias taurus and Isurus oxyrinchus. Nanomaterials. 2021; 11(4):969. https://doi.org/10.3390/nano11040969
Chicago/Turabian StyleWilmers, Jana, Miranda Waldron, and Swantje Bargmann. 2021. "Hierarchical Microstructure of Tooth Enameloid in Two Lamniform Shark Species, Carcharias taurus and Isurus oxyrinchus" Nanomaterials 11, no. 4: 969. https://doi.org/10.3390/nano11040969
APA StyleWilmers, J., Waldron, M., & Bargmann, S. (2021). Hierarchical Microstructure of Tooth Enameloid in Two Lamniform Shark Species, Carcharias taurus and Isurus oxyrinchus. Nanomaterials, 11(4), 969. https://doi.org/10.3390/nano11040969






