Anisotropic Growth and Magnetic Properties of α″-Fe16N2@C Nanocones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of α″-Fe16N2@C Nanocones
2.3. Characterization
3. Results
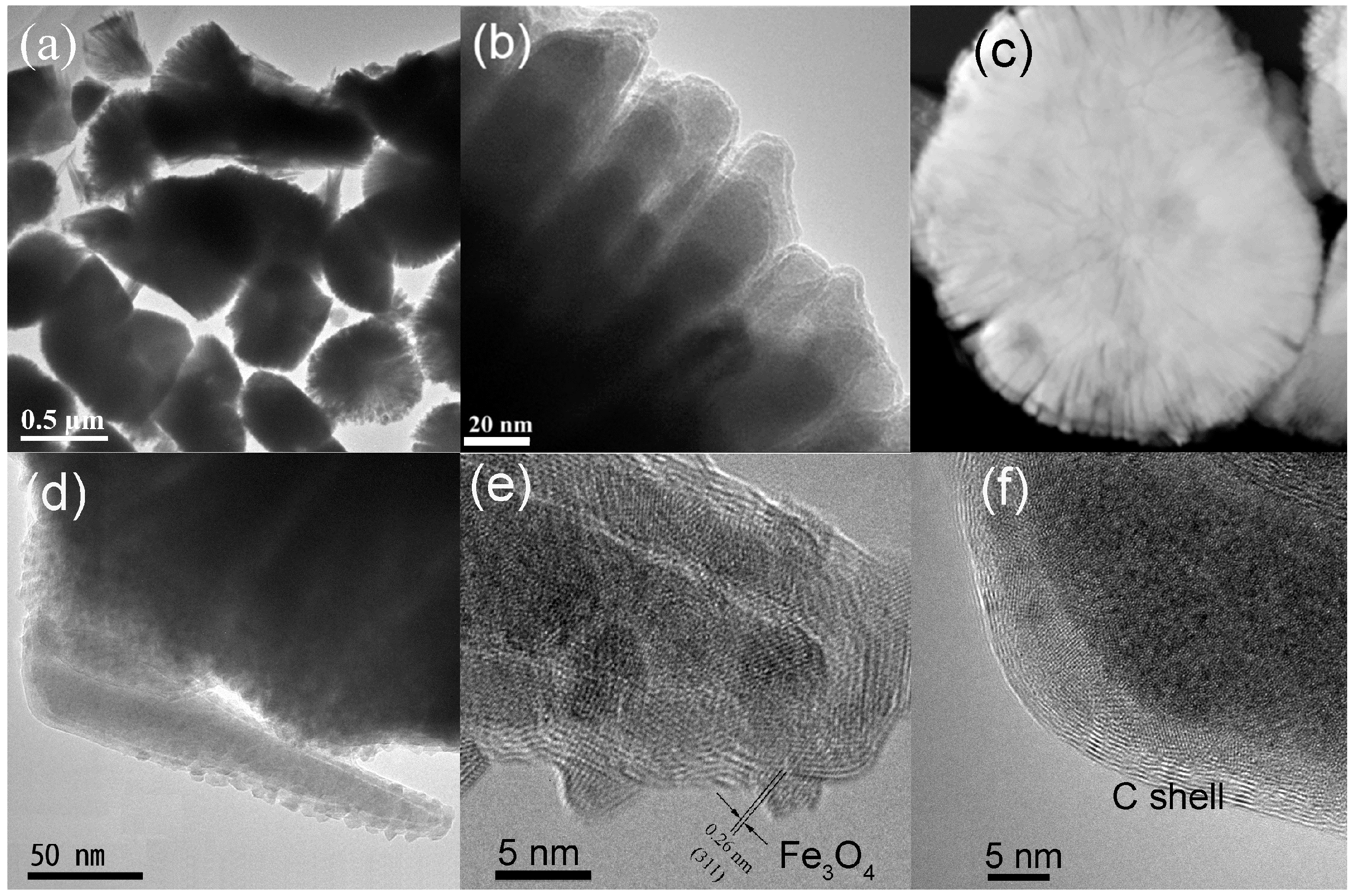
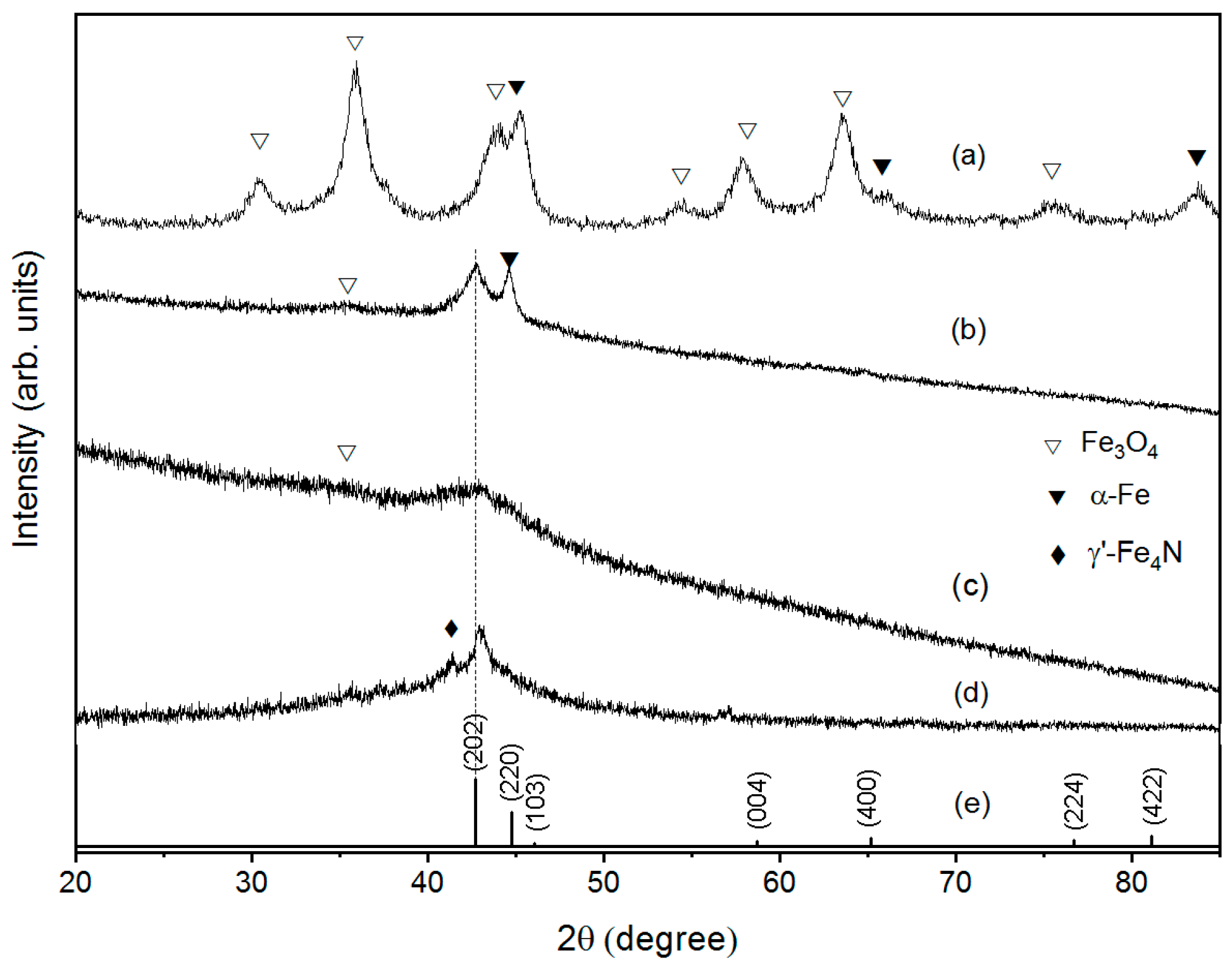
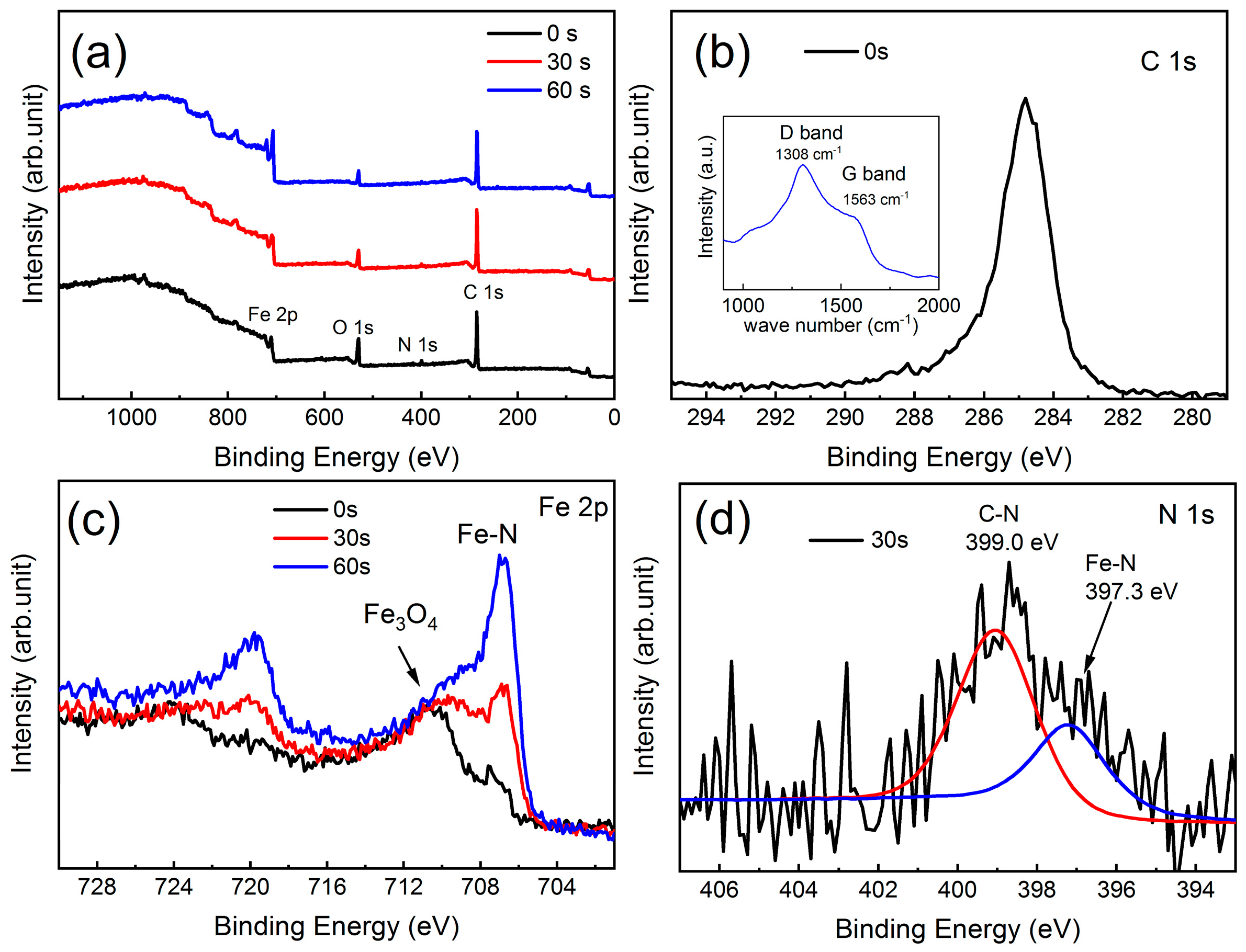
3.1. Structural Properties
3.2. Anisotropic Growth
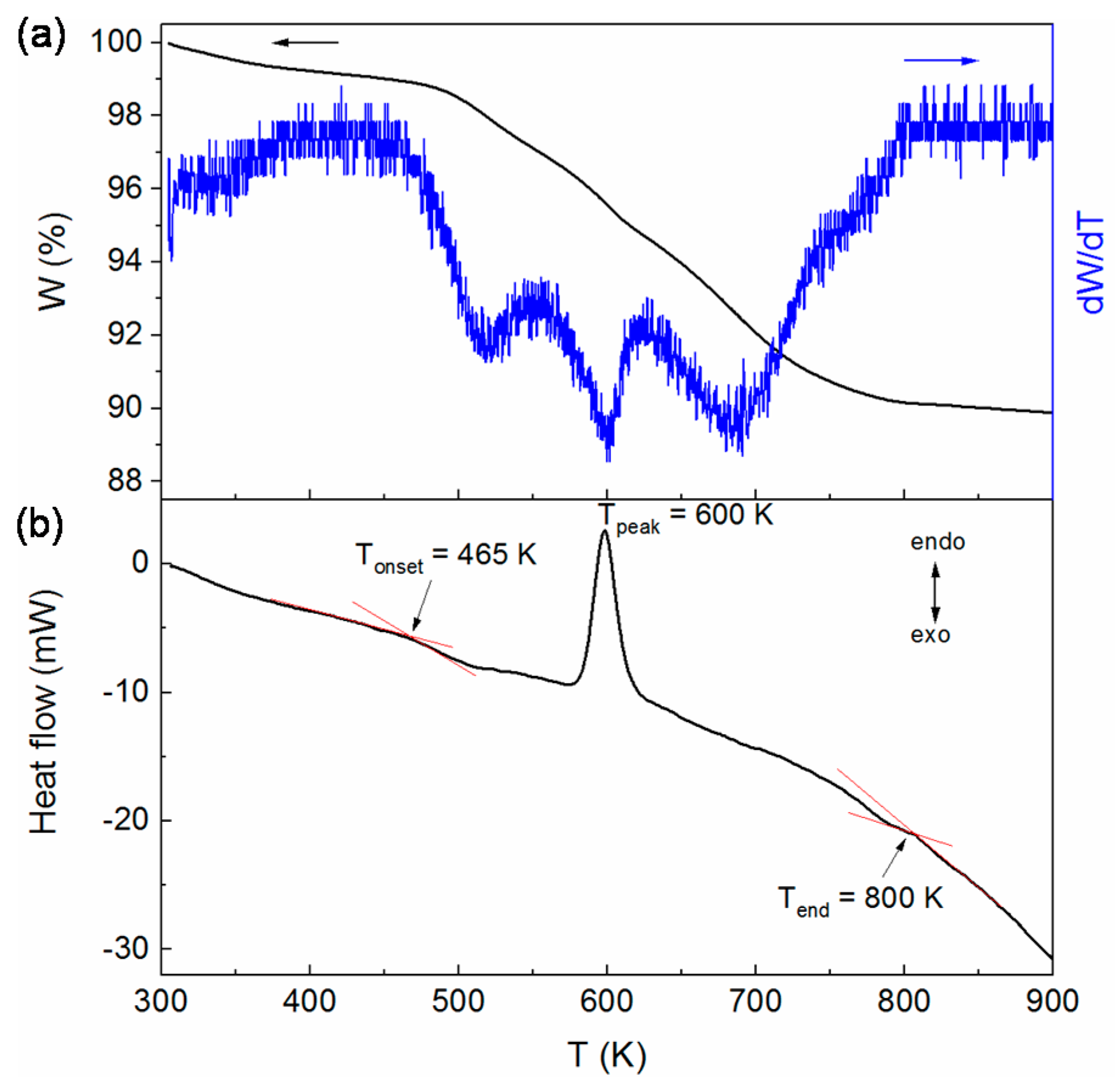
3.3. Thermal Property
3.4. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coey, J.M.D. Hard Magnetic Materials: A Perspective. IEEE Trans. Magn. 2011, 47, 4671–4681. [Google Scholar] [CrossRef]
- Li, D.; Pan, D.S.; Li, S.J.; Zhang, Z.D. Recent developments of rare-earth-free hard-magnetic materials. Sci. China Phys. Mech. Astron. 2016, 59, 617501. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Pan, D.S.; Zhang, Z.D.; Choi, C.J. Prospect and status of iron-based rare-earth-free permanent magnetic materials. J. Magn. Magn. Mater. 2019, 469, 535–544. [Google Scholar] [CrossRef]
- Gao, C.; Doyle, W.D.; Shamsuzzoha, M. Quantitative Correlation of phase-structure with the magnetic-moments in rf-sputtered Fe-N Films. J. Appl. Phys. 1993, 73, 6579–6581. [Google Scholar] [CrossRef]
- Komuro, M.; Kozono, Y.; Hanazono, M.; Sugita, Y. Epitaxial-growth and magnetic-properties of Fe16N2 films with high saturation magnetic-flux density. J. Appl. Phys. 1990, 67, 5126–5130. [Google Scholar] [CrossRef]
- Okamoto, S.; Kitakami, O.; Shimada, Y. Alpha”-Fe16N2 phase epitaxially grown by sputter beam method. J. Appl. Phys. 1996, 79, 5250–5252. [Google Scholar] [CrossRef]
- Nakajima, K.; Yamashita, T.; Takata, M.; Okamoto, S. Mössbauer study on Fe16N2 films prepared by ion-implant nitrification of iron films. J. Appl. Phys. 1991, 70, 6033–6035. [Google Scholar] [CrossRef]
- Takahashi, H.; Mitsuoka, K.; Komuro, M.; Sugita, Y. Ferromagnetic-resonance studies of Fe16N2 films with a giant magnetic-moment. J. Appl. Phys. 1993, 73, 6060–6062. [Google Scholar] [CrossRef]
- Kim, T.K.; Takahash, M. New magentic mateial having ultrahigh magnetic moment. Appl. Phys. Lett. 1972, 20, 492–494. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Smith, P.A.I. Magnetic nitrides. J. Magn. Magn. Mater. 1999, 200, 405–424. [Google Scholar] [CrossRef]
- Li, Y.; Pan, D.S.; Zhou, Y.T.; Kuang, Q.F.; Wang, C.W.; Li, B.; Zhang, B.S.; Park, J.; Li, D.; Choi, C.; et al. Enhanced magnetic properties and thermal stability of highly ordered ε-Fe3N1+x (−0.12 ≤ x ≤-0.01) nanoparticles. Nanoscale 2020, 12, 10834–10841. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Kitawatase, K. Electronic-structures and magnetic-properties of iron nitrides. J. Magn. Magn. Mater. 1992, 104, 1933–1934. [Google Scholar] [CrossRef]
- Ji, N.A.; Allard, L.F.; Lara-Curzio, E.; Wang, J.P. N site ordering effect on partially ordered Fe16N2. Appl. Phys. Lett. 2011, 98, 092506. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.F.; Dabade, V.; Allard, L.F.; Lara-Curzio, E.; James, R.; Wang, J.P. Synthesis of α″-Fe16N2 compound anisotropic magnet by the strained-wire method. Phys. Rev. Appl. 2016, 6, 10. [Google Scholar]
- Gandha, K.; Elkins, K.; Poudyal, N.; Liu, X.B.; Liu, J.P. High energy product developed from cobalt nanowires. Sci. Rep. 2014, 4, 5345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.W.; Long, G.; Li, D.; Sabirianov, R.; Zeng, H. Fe3Se4 nanostructures with giant coercivity synthesized by solution chemistry. Chem. Mater. 2011, 23, 3769–3774. [Google Scholar] [CrossRef]
- Wang, C.; Hou, Y.L.; Kim, J.M.; Sun, S.H. A general strategy for synthesizing FePt nanowires and nanorods. Angew. Chem. Int. Ed. 2007, 46, 6333–6335. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.F.; Liu, J.M.; Suri, P.K.; Kennedy, G.; Thadhani, N.N.; Flannigan, D.J.; Wang, J.P. Preparation of an α″-Fe16N2 magnet via a ball milling and shock compaction approach. Adv. Eng. Mater. 2016, 18, 1009–1016. [Google Scholar] [CrossRef]
- Kita, E.; Shibata, K.; Yanagihara, H.; Sasaki, Y.; Kishimoto, M. Magnetic properties of core-shell type Fe16N2 nanoparticles. J. Magn. Magn. Mater. 2007, 310, 2411–2413. [Google Scholar] [CrossRef]
- Zulhijah, R.; Nandiyanto, A.B.D.; Ogi, T.; Iwaki, T.; Nakamura, K.; Okuyama, K. Gas phase preparation of spherical core-shell α″-Fe16N2/SiO2 magnetic nanoparticles. Nanoscale 2014, 6, 6487–6491. [Google Scholar] [CrossRef]
- Bridges, C.A.; Rios, O.; Allard, L.F.; Meyer, H.M.; Huq, A.; Jiang, Y.; Wang, J.P.; Brady, M.P. The impact of carbon coating on the synthesis and properties of α″-Fe16N2 powders. Phys. Chem. Chem. Phys. 2016, 18, 13010–13017. [Google Scholar] [CrossRef]
- Ogi, T.; Nandiyanto, A.B.D.; Kisakibaru, Y.; Iwaki, T.; Nakamura, K.; Okuyama, K. Facile synthesis of single-phase spherical α″-Fe16N2/Al2O3 core-shell nanoparticles via a gas-phase method. J. Appl. Phys. 2013, 113, 164301. [Google Scholar] [CrossRef]
- Ogawa, T.; Ogata, Y.; Gallage, R.; Kobayashi, N.; Hayashi, N.; Kusano, Y.; Yamamoto, S.; Kohara, K.; Doi, M.; Takano, M.; et al. Challenge to the synthesis of α″-Fe16N2 compound nanoparticle with high saturation magnetization for rare earth free new permanent magnetic material. Appl. Phys. Express 2013, 6, 073007. [Google Scholar] [CrossRef]
- Kikkawa, S.; Yamada, A.; Masubuchi, Y.; Takeda, T. Fine Fe16N2 powder prepared by low-temperature nitridation. Mater. Res. Bull. 2008, 43, 3352–3357. [Google Scholar] [CrossRef]
- Li, D.; Li, S.J.; Zhou, Y.T.; Bai, Y.; Zhu, Y.L.; Ren, W.J.; Long, G.; Zeng, H.; Zhang, Z.D. Magnetization reversal and coercivity of Fe3Se4 nanowire arrays. J. Appl. Phys. 2015, 117, 17E702. [Google Scholar] [CrossRef]
- Robbins, M.; White, J.G. Magnetic properties of ε-ion nitride. J. Phys. Chem. Solids 1964, 25, 717–720. [Google Scholar] [CrossRef]
- Chen, G.M.; Jaggi, N.K.; Butt, J.B.; Yeh, E.B.; Schwartz, L.H. Mossbauer and magnetic studies of ε-FexN, 2 < x < 3. J. Phys. Chem. 1983, 87, 5326–5332. [Google Scholar]
- Jack, K.H. Binary and ternary interstitial alloys. 1. The iron-nitrogen system- the structure of Fe4N and Fe2N. Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 1948, 195, 34–40. [Google Scholar]
- Li, D.; Choi, C.J.; Kim, B.K.; Zhang, Z.D. Characterization of Fe/N nanoparticles synthesized by the chemical vapor condensation process. J. Magn. Magn. Mater. 2004, 277, 64–70. [Google Scholar] [CrossRef]
- Yu, Y.S.; Mukherjee, P.; Tian, Y.; Li, X.Z.; Shield, J.E.; Sellmyer, D.J. Direct chemical synthesis of L10-FePtAu nanoparticles with high coercivity. Nanoscale 2014, 6, 12050–12055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Pan, D.; Li, D.; Feng, Y.; Choi, C.J.; Liu, W.; Zhang, Z. Catalytic synthesis and enhanced Curie temperature of ε-Fe3N@C nanostructure synthesized in a tetraethylenepentamine solution. J. Magn. Magn. Mater. 2018, 465, 736–742. [Google Scholar] [CrossRef]
- Bhattacharyya, S. Iron Nitride Family at Reduced Dimensions: A Review of Their Synthesis Protocols and Structural and Magnetic Properties. J. Phys. Chem. C 2015, 119, 1601–1622. [Google Scholar] [CrossRef]
- Kikkawa, S.; Kubota, K.; Takeda, T. Particle size dependence in low temperature nitridation reaction for Fe16N2. J. Alloy. Compd. 2008, 449, 7–10. [Google Scholar] [CrossRef]
- Dirba, I.; Schwobel, C.A.; Diop, L.V.B.; Duerrschnabel, M.; Molina-Luna, L.; Hofmann, K.; Komissinskiy, P.; Kleebe, H.J.; Gutfleisch, O. Synthesis, morphology, thermal stability and magnetic properties of α″-Fe16N2 nanoparticles obtained by hydrogen reduction of γ-Fe2O3 and subsequent nitrogenation. Acta Mater. 2017, 123, 214–222. [Google Scholar] [CrossRef]
- Kartikowati, C.W.; Suhendi, A.; Zulhijah, R.; Ogi, T.; Iwaki, T.; Okuyama, K. Effect of magnetic field strength on the alignment of α″-Fe16N2 nanoparticle films. Nanoscale 2016, 8, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, S.J.; Zhang, Y.; Jiang, J.J.; Gong, W.J.; Wang, J.H.; Zhang, Z.D. Monodisperse water-soluble γ-Fe2O3/polyvinylpyrrolidone nanoparticles for a magnetic resonance imaging contrast agent. Mater. Res. Innov. 2015, 19, S358–S362. [Google Scholar] [CrossRef]
- Li, D.; Choi, C.J.; Yu, J.H.; Kim, B.K.; Zhang, Z.D. Nanocrystalline α-Fe and ε-Fe3N particles prepared by chemical vapor condensation process. J. Magn. Magn. Mater. 2004, 283, 8–15. [Google Scholar] [CrossRef]
- Li, D.; Han, Z.; Wu, B.; Geng, D.; Zhang, Z. Ferromagnetic and spin-glass behaviour of nanosized oriented pyrolytic graphite in Pb-C nanocomposites. J. Phys. D Appl. Phys. 2008, 41, 115005. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.B.; Hou, Y.L.; Ma, D. Fe5C2 Nanoparticles: A facile bromide-induced synthesis and as an active phase for fischer-tropsch synthesis. J. Am. Chem. Soc. 2012, 134, 15814–15821. [Google Scholar] [CrossRef] [PubMed]
- Strijkers, G.J.; Dalderop, J.H.J.; Broeksteeg, M.A.A.; Swagten, H.J.M.; de Jonge, W.J.M. Structure and magnetization of arrays of electrodeposited Co wires in anodic alumina. J. Appl. Phys. 1999, 86, 5141–5145. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Kuang, Q.; Men, X.; Wang, S.; Li, D.; Choi, C.; Zhang, Z. Anisotropic Growth and Magnetic Properties of α″-Fe16N2@C Nanocones. Nanomaterials 2021, 11, 890. https://doi.org/10.3390/nano11040890
Li Y, Kuang Q, Men X, Wang S, Li D, Choi C, Zhang Z. Anisotropic Growth and Magnetic Properties of α″-Fe16N2@C Nanocones. Nanomaterials. 2021; 11(4):890. https://doi.org/10.3390/nano11040890
Chicago/Turabian StyleLi, Yong, Qifeng Kuang, Xiaoling Men, Shenggang Wang, Da Li, Chuljin Choi, and Zhidong Zhang. 2021. "Anisotropic Growth and Magnetic Properties of α″-Fe16N2@C Nanocones" Nanomaterials 11, no. 4: 890. https://doi.org/10.3390/nano11040890





