Polysaccharide-Based Multilayer Nano-Emulsions Loaded with Oregano Oil: Production, Characterization, and In Vitro Digestion Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Multilayer Nanoemulsions Production
2.2.1. Solutions Preparation
2.2.2. Nano-Emulsions Production
2.2.3. Nano-Emulsions Loaded with OEO
2.2.4. Stability Analysis
2.3. Particle Size, Polydispersity Index, and Zeta Potential
2.4. Encapsulation Efficiency
2.5. Morphological and Chemical Characterization
2.5.1. Transmission Electron Microscopy
2.5.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5.3. X-ray Diffraction
2.6. Simulated In Vitro Gastric and Intestinal Conditions
2.7. Statistical Analysis
3. Results and Discussion
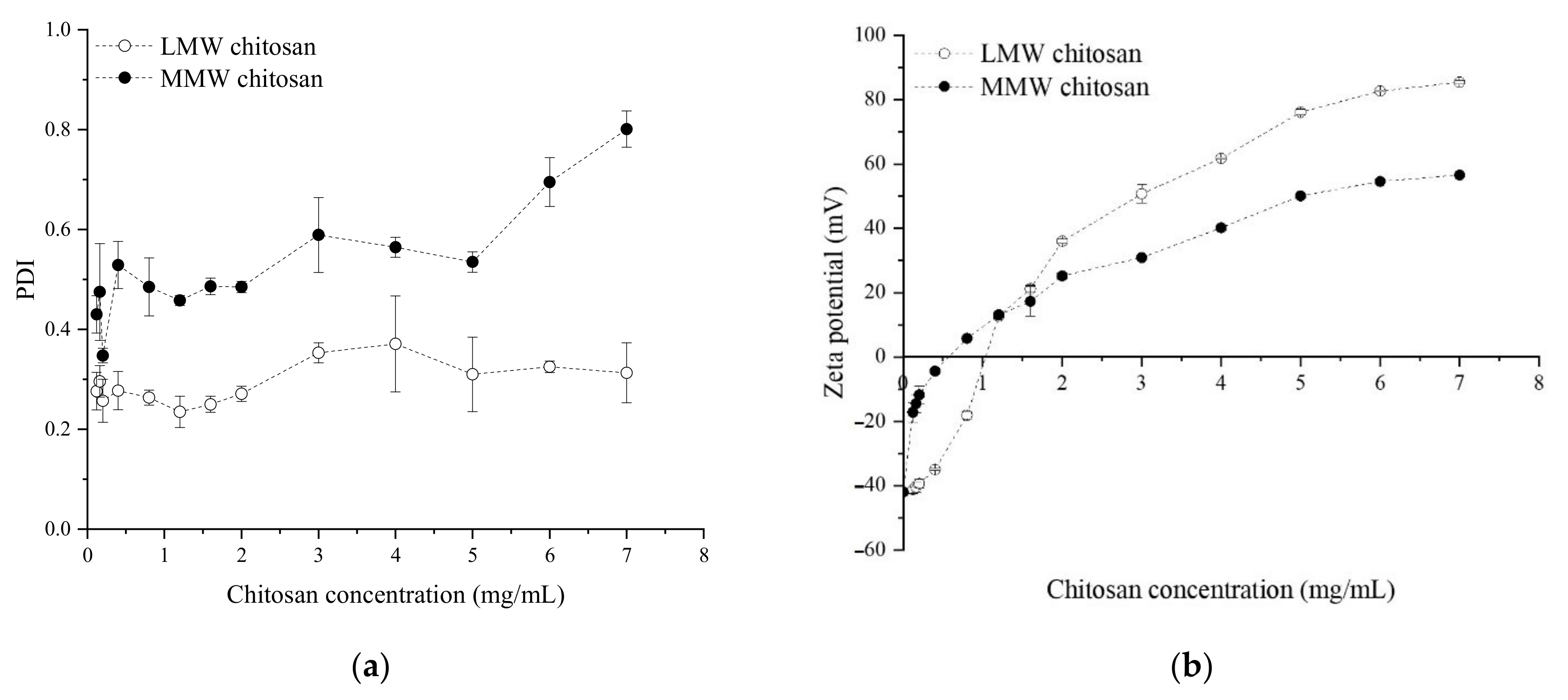
3.1. Nanoemulsions without OEO
Effect of the Polysaccharide’s Concentration and Chitosan Molecular Weight
3.2. Nanoemulsions Loaded with OEO
3.2.1. Effect of OEO on Nano-Emulsions
3.2.2. Encapsulation Efficiency
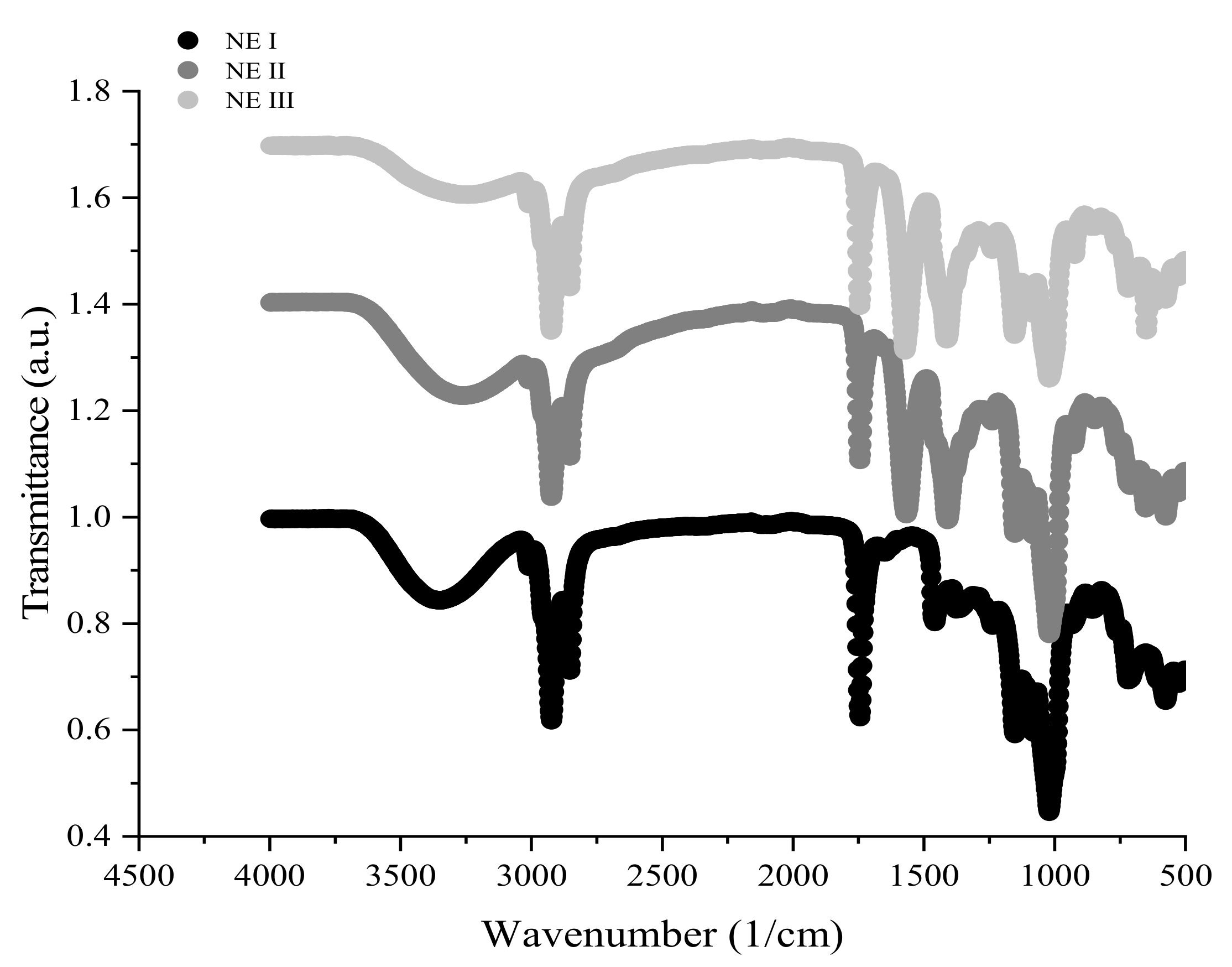
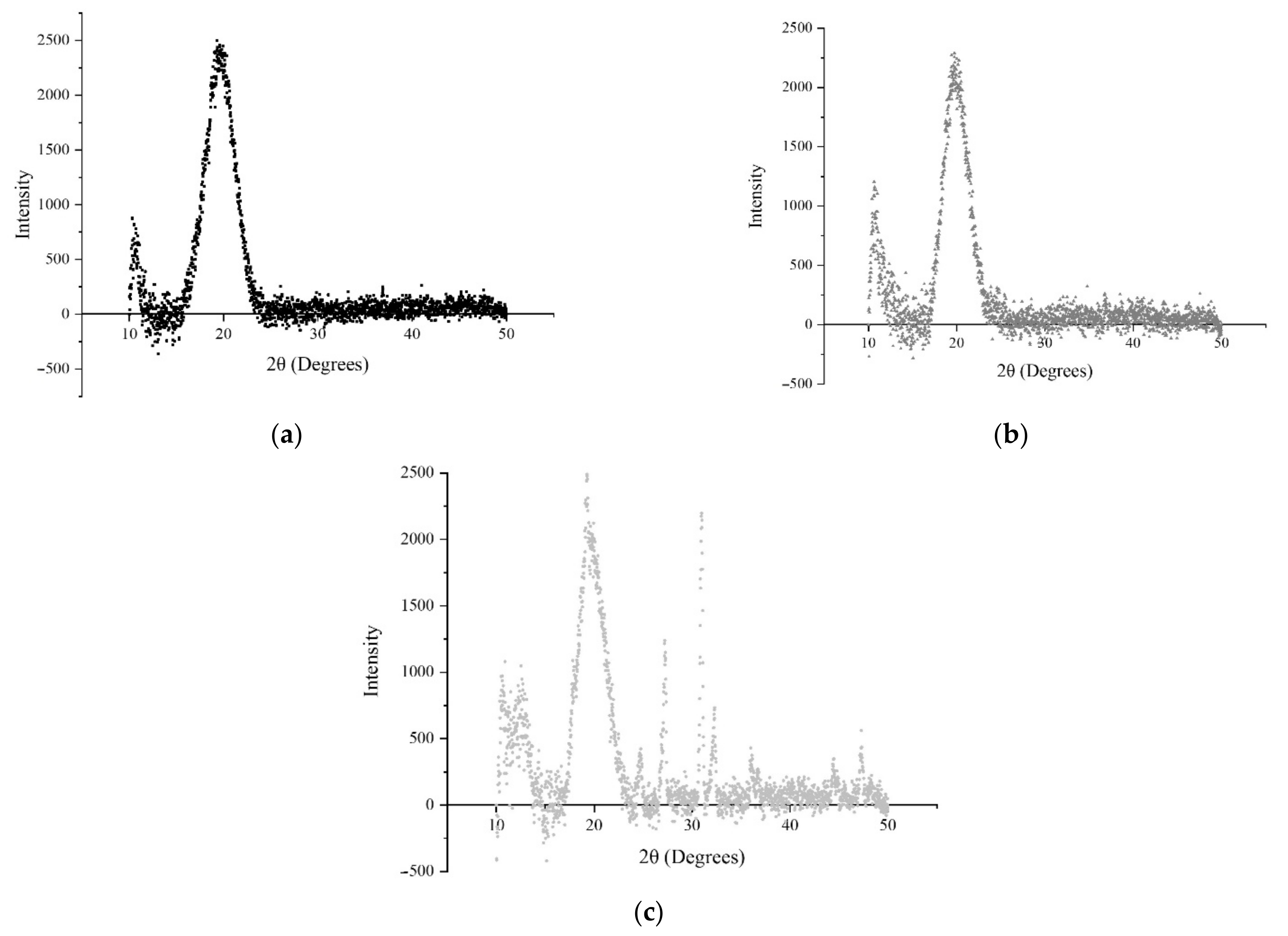
3.3. Morphological and Chemical Characterization
3.3.1. Transmission Electron Microscopy (TEM)
3.3.2. FTIR and XRD Analysis
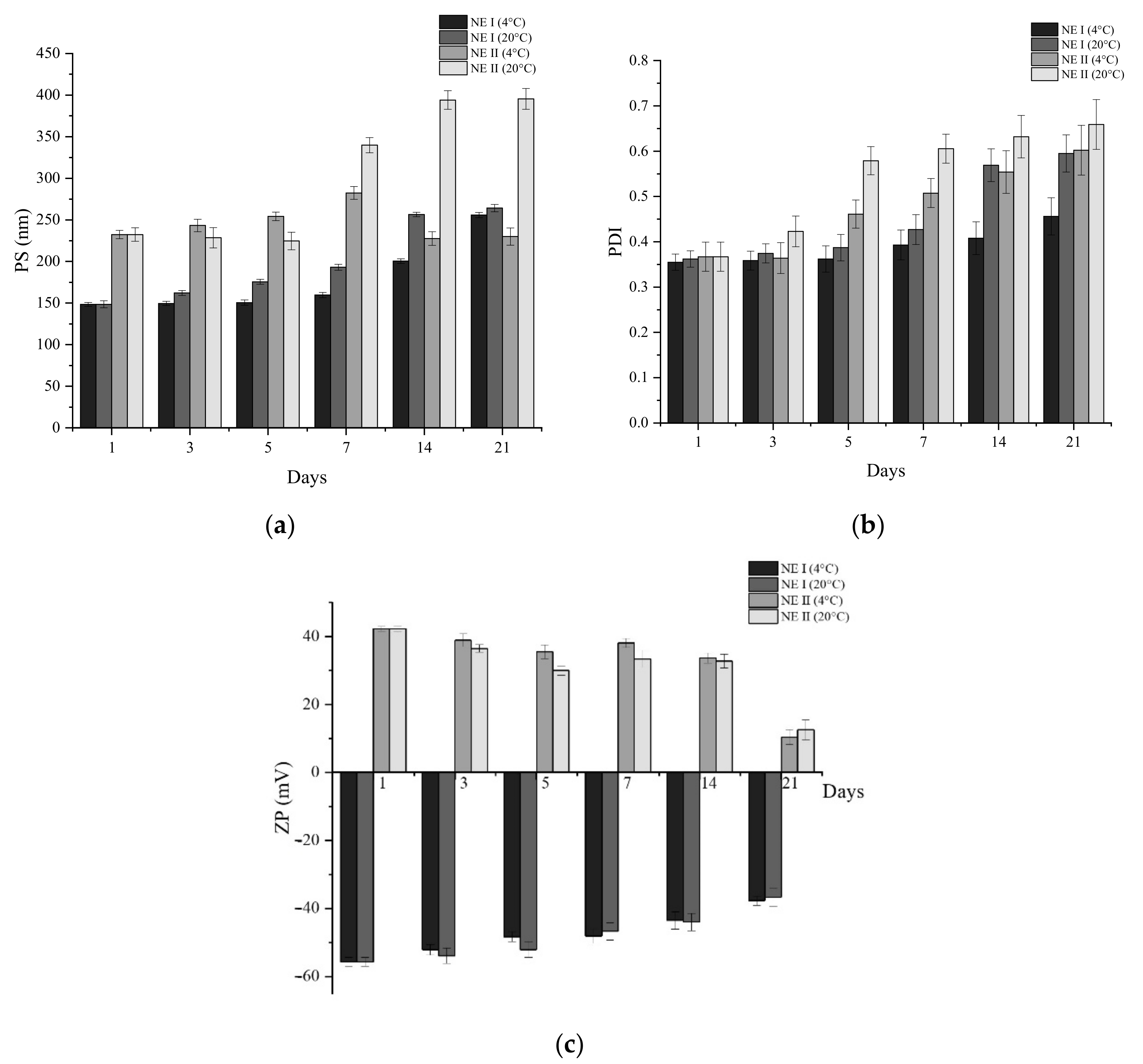
3.4. Stability of OEO Loaded-NE
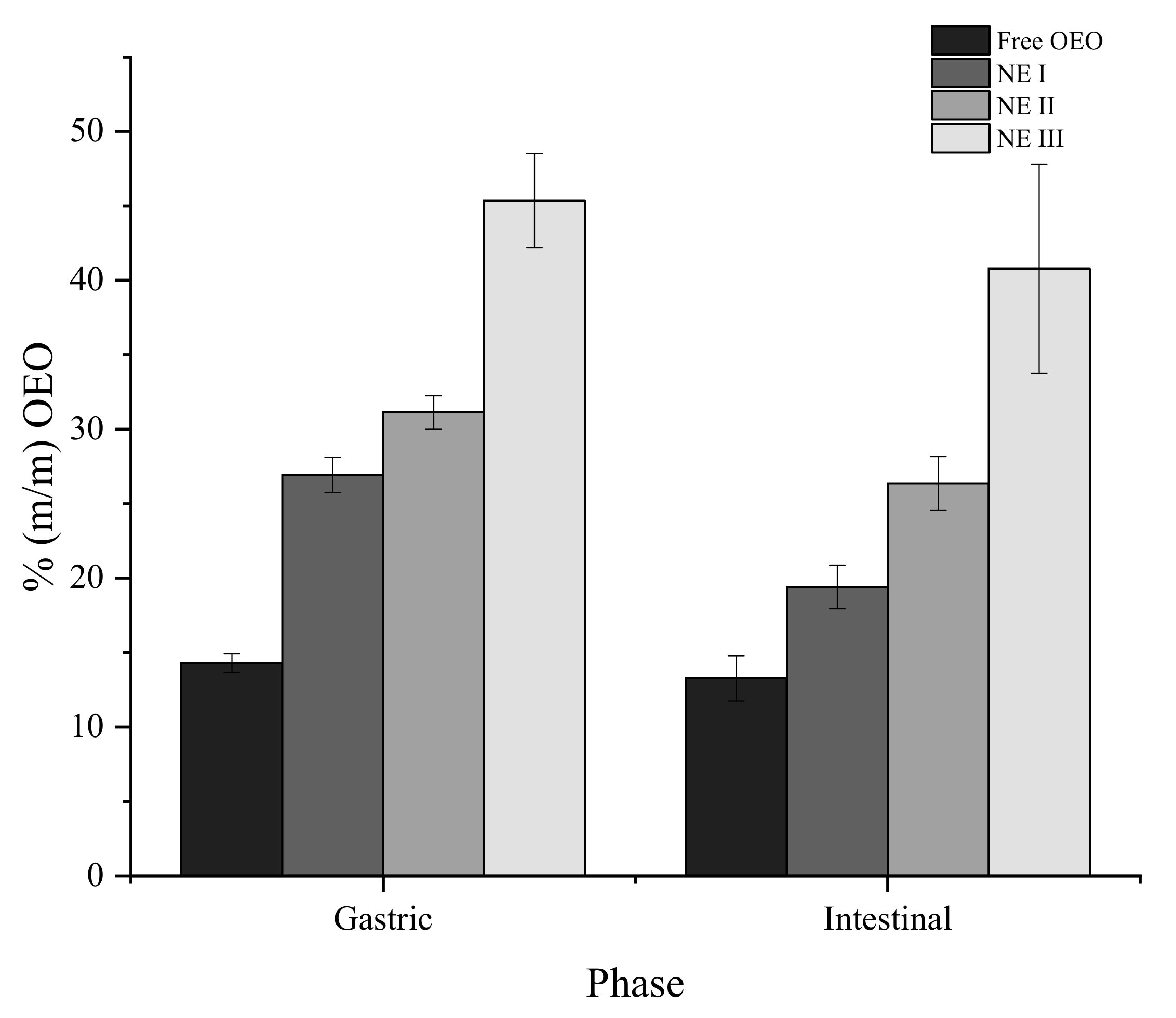
3.5. Release of OEO under In Vitro Gastric and Intestinal Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.M.; Paximada, P.; Mandala, I.; Assadpour, E.; Mehrnia, M.A. Encapsulation by nanoemulsions. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128094365. [Google Scholar]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012. [Google Scholar] [CrossRef]
- Jafari, S.M.; McClements, D.J. Nanoemulsions: Formulation, Applications, and Characterization; Academic Press: Cambridge, MA, USA, 2018; ISBN 9780128118399. [Google Scholar]
- Liang, R.; Shoemaker, C.F.; Yang, X.; Zhong, F.; Huang, Q. Stability and bioaccessibility of β-carotene in nanoemulsions stabilized by modified starches. J. Agric. Food Chem. 2013, 61, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Comparison of biopolymer emulsifier performance in formation and stabilization of orange oil-in-water emulsions. JAOCS J. Am. Oil Chem. Soc. 2011, 88, 47–55. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef]
- Sonia, T.A.; Sharma, C.P. Chitosan and its derivatives for drug delivery perspective. In Chitosan for Biomaterials I. Advances in Polymer Science; Jayakumar, R., Prabaharan, M., Muzzarelli, R., Eds.; Springer: Berlin, Heidelberg, 2011; Volume 243, pp. 23–53. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.; Liang, J.; Li, X.; Liang, W.; He, S.; Zhu, C.; Mao, L. Buildup mechanism of carboxymethyl cellulose and chitosan self-assembled films. Cellulose 2013, 20, 1135–1143. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.A.; Ortega-Ramirez, L.A.; Leyva, J.M.; Siddiqui, M.W.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Braga, M.A.; Cesar, P.H.S.; Trento, M.V.C.; Espósito, M.A.; Silva, L.F.; Marcussi, S. Anticancer Properties of Essential Oils: An Overview. Curr. Cancer Drug Targets 2018, 18, 957–966. [Google Scholar] [CrossRef]
- Beirão-da-Costa, S.; Duarte, C.; Bourbon, A.I.; Pinheiro, A.C.; Januário, M.I.N.; Vicente, A.A.; Beirão-da-Costa, M.L.; Delgadillo, I. Inulin potential for encapsulation and controlled delivery of Oregano essential oil. Food Hydrocoll. 2013, 33, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Abbas, S.; Bashari, M.; Akhtar, W.; Li, W.W.; Zhang, X. Process optimization of ultrasound-assisted curcumin nanoemulsions stabilized by OSA-modified starch. Ultrason. Sonochem. 2014, 21, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- El Kinawy, O.S.; Petersen, S.; Ulrich, J. Technological Aspects of Nanoemulsion Formation of Low-Fat Foods Enriched with Vitamin E by High-Pressure Homogenization. Chem. Eng. Technol. 2012, 35, 937–940. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Optimization of nano-emulsions production by microfluidization. Eur. Food Res. Technol. 2007, 225, 733–741. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, C.; Yi, J.; Cui, L.; Liu, N.; Cao, Y.; Decker, E.A. Ultrasound improving the physical stability of oil-in-water emulsions stabilized by almond proteins. J. Sci. Food Agric. 2018, 98, 4323–4330. [Google Scholar] [CrossRef]
- Kaltsa, O.; Michon, C.; Yanniotis, S.; Mandala, I. Ultrasonic energy input influence on the production of sub-micron o/w emulsions containing whey protein and common stabilizers. Ultrason. Sonochem. 2013, 20, 881–891. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason. Sonochem. 2013, 20, 338–344. [Google Scholar] [CrossRef]
- Kentish, S.; Wooster, T.J.; Ashokkumar, M.; Balachandran, S.; Mawson, R.; Simons, L. The use of ultrasonics for nanoemulsion preparation. Innov. Food Sci. Emerg. Technol. 2008, 9, 170–175. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Xing, L.; Zhang, W. Applications and effects of ultrasound assisted emulsification in the production of food emulsions: A review. Trends Food Sci. Technol. 2021, 110, 493–512. [Google Scholar] [CrossRef]
- Koshani, R.; Jafari, S.M. Ultrasound-assisted preparation of different nanocarriers loaded with food bioactive ingredients. Adv. Colloid Interface Sci. 2019, 270, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Erazo, E.M.; Consoli, L.; Hubinger, M.D. Mono and double-layer emulsions of chia oil produced with ultrasound mediation. Food Bioprod. Process. 2018, 112, 108–118. [Google Scholar] [CrossRef]
- Carpenter, J.; George, S.; Saharan, V.K. A comparative study of batch and recirculating flow ultrasonication system for preparation of multilayer olive oil in water emulsion stabilized with whey protein isolate and sodium alginate. Chem. Eng. Process. Process Intensif. 2018, 125, 139–149. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, S.S.; Lim, S.T. Preparation, characterization and utilization of starch nanoparticles. Colloids Surf. B Biointerfaces 2015, 126, 607–620. [Google Scholar] [CrossRef]
- Abbas, S.; Hayat, K.; Karangwa, E.; Bashari, M.; Zhang, X. An Overview of Ultrasound-Assisted Food-Grade Nanoemulsions. Food Eng. Rev. 2013, 5, 139–157. [Google Scholar] [CrossRef]
- Abbas, S.; Karangwa, E.; Bashari, M.; Hayat, K.; Hong, X.; Sharif, H.R.; Zhang, X. Fabrication of polymeric nanocapsules from curcumin-loaded nanoemulsion templates by self-assembly. Ultrason. Sonochem. 2015, 23, 81–92. [Google Scholar] [CrossRef]
- Guzey, D.; McClements, D.J. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 2006, 128–130, 227–248. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.J.; Pietrangelo, C.; MacNaughton, A. Influence of simulated upper intestinal parameters on the efficiency of beta carotene micellarisation using an in vitro model of digestion. Food Chem. 2008, 107, 1253–1260. [Google Scholar] [CrossRef]
- Esmaeilzadeh-Gharedaghi, E.; Faramarzi, M.A.; Amini, M.A.; Rouholamini Najafabadi, A.; Rezayat, S.M.; Amani, A. Effects of processing parameters on particle size of ultrasound prepared chitosan nanoparticles: An Artificial Neural Networks Study. Pharm. Dev. Technol. 2012, 17, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Hon, M.H. The effect of the molecular weight of chitosan nanoparticles and its application on drug delivery. Microchem. J. 2009, 92, 87–91. [Google Scholar] [CrossRef]
- Terenina, M.B.; Misharina, T.A.; Krikunova, N.I.; Alinkina, E.S.; Fatkulina, L.D.; Vorob’yova, A.K. Oregano essential oil as an inhibitor of higher fatty acid oxidation. Appl. Biochem. Microbiol. 2011, 47, 445–449. [Google Scholar] [CrossRef]
- Matos, M.; Marefati, A.; Gutiérrez, G.; Wahlgren, M.; Rayner, M. Comparative emulsifying properties of octenyl succinic anhydride (OSA)-modified starch: Granular form vs dissolved state. PLoS ONE 2016, 11, e0160140. [Google Scholar] [CrossRef]
- Taylor, P. Ostwald ripening in emulsions. Adv. Colloid Interface Sci. 1998, 75, 107–163. [Google Scholar] [CrossRef]
- Ziani, K.; Chang, Y.; McLandsborough, L.; McClements, D.J. Influence of surfactant charge on antimicrobial efficacy of surfactant-stabilized thyme oil nanoemulsions. J. Agric. Food Chem. 2011, 59, 6247–6255. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Yoksan, R.; Jirawutthiwongchai, J.; Arpo, K. Encapsulation of ascorbyl palmitate in chitosan nanoparticles by oil-in-water emulsion and ionic gelation processes. Colloids Surf. B Biointerfaces 2010, 76, 292–297. [Google Scholar] [CrossRef]
- Rashed, M.M.A.; Mahdi, A.A.; Ghaleb, A.D.S.; Zhang, F.R.; YongHua, D.; Qin, W.; WanHai, Z. Synergistic effects of amorphous OSA-modified starch, unsaturated lipid-carrier, and sonocavitation treatment in fabricating of Lavandula angustifolia essential oil nanoparticles. Int. J. Biol. Macromol. 2020, 151, 702–712. [Google Scholar] [CrossRef]
- Ramli, S.; Ja’afar, S.M.; Sisak, M.A.A.; Zainuddin, N.; Rahman, I.A. Formulation and physical characterization of microemulsions based carboxymethyl cellulose as vitamin c carrier. Malaysian J. Anal. Sci. 2015, 19, 275–283. [Google Scholar]
- Li, D.; Li, L.; Xiao, N.; Li, M.; Xie, X. Physical properties of oil-in-water nanoemulsions stabilized by OSA-modified starch for the encapsulation of lycopene. Colloids Surf. A Physicochem. Eng. Asp. 2018, 552, 59–66. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Sugumar, S.; Mukherjee, A.; Chandrasekaran, N. Eucalyptus oil nanoemulsion-impregnated chitosan film: Antibacterial effects against a clinical pathogen, Staphylococcus aureus, in vitro. Int. J. Nanomedicine 2015, 10 (Suppl. 1), 67. [Google Scholar] [CrossRef] [Green Version]
- Zhigang, L.; Linrong, S.; Meina, Z. Preparation of Starch Nanoparticles in a New Ionic Liquid-in-Oil Microemulsion. J. Formul. Sci. Bioavailab. 2018. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Chen, M.; Xu, F.; Zhong, F. Stabilizing Oil-in-Water Emulsion with Amorphous and Granular Octenyl Succinic Anhydride Modified Starches. J. Agric. Food Chem. 2018, 66, 9301–9308. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Li, L.; Chen, L.; Li, X. Nano-structure of octenyl succinic anhydride modified starch micelle. Food Hydrocoll. 2013, 32, 1–8. [Google Scholar] [CrossRef]
- Krstonošić, V.; Dokić, L.; Milanović, J. Micellar properties of OSA starch and interaction with xanthan gum in aqueous solution. Food Hydrocoll. 2011, 25, 361–367. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Thermomechanical-induced polyelectrolyte complexation between chitosan and carboxymethyl cellulose enabling unexpected hydrolytic stability. Compos. Sci. Technol. 2020, 189, 108031. [Google Scholar] [CrossRef]
- Rosca, C.; Popa, M.I.; Lisa, G.; Chitanu, G.C. Interaction of chitosan with natural or synthetic anionic polyelectrolytes. 1. The chitosan-carboxymethylcellulose complex. Carbohydr. Polym. 2005, 62, 35–41. [Google Scholar] [CrossRef]
- Trabelsi, S.; Albouy, P.A.; Impéror-Clerc, M.; Guillot, S.; Langevin, D. X-ray diffraction study of the structure of carboxymethylcellulose-cationic surfactant complexes. ChemPhysChem 2007, 8, 2379–2385. [Google Scholar] [CrossRef]
- Guerra-Rosas, M.I.; Morales-Castro, J.; Ochoa-Martínez, L.A.; Salvia-Trujillo, L.; Martín-Belloso, O. Long-term stability of food-grade nanoemulsions from high methoxyl pectin containing essential oils. Food Hydrocoll. 2016, 52, 438–446. [Google Scholar] [CrossRef]
- Terjung, N.; Löffler, M.; Gibis, M.; Hinrichs, J.; Weiss, J. Influence of droplet size on the efficacy of oil-in-water emulsions loaded with phenolic antimicrobials. Food Funct. 2012, 3, 290–301. [Google Scholar] [CrossRef]
- Klang, V.; Matsko, N.B.; Valenta, C.; Hofer, F. Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 2012, 43, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Samadi, F.; Jafari, S.M.; Ramezanpour, S.; Shams Shargh, M. Ultrasound-assisted preparation of flaxseed oil nanoemulsions coated with alginate-whey protein for targeted delivery of omega-3 fatty acids into the lower sections of gastrointestinal tract to enrich broiler meat. Ultrason. Sonochem. 2019, 50, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.C.; Yu, H.; Zhu, J.; De Lange, K.; Yin, Y.; Wang, Q.; Gong, J. Evaluation of alginate-whey protein microcapsules for intestinal delivery of lipophilic compounds in pigs. J. Sci. Food Agric. 2016, 96, 2674–2681. [Google Scholar] [CrossRef] [PubMed]

| OEO Concentration (%) | ZP (mV) 1 | ||
|---|---|---|---|
| NE I | NE II | NE III | |
| 0 | −42.02 ± 1.32 | 44.91 ± 0.71 | −6.43 ± 0.58 |
| 1 | −36.15 ± 0.17 | 42.31 ± 0.31 | −0.92 ± 3.43 |
| 5 | −39.70 ± 0.36 | 38.75 ± 0.53 | −3.54 ± 2.52 |
| 10 | −44.14 ± 0.90 | 38.54 ± 0.27 | −7.62 ± 2.53 |
| 15 | −46.66 ± 0.54 | 38.43 ± 0.96 | −9.16 ± 0.61 |
| 20 | −45.68 ± 0.67 | 37.81 ± 0.26 | −11.43 ± 0.51 |
| 25 | −48.74 ± 0.62 | 36.22 ± 0.27 | −13.98 ± 1.61 |
| 50 | −47.40 ± 0.97 | 34.88 ± 0.23 | −17.65 ± 4.44 |
| 75 | −48.64 ± 0.55 | 27.61 ± 0.24 | −18.82 ± 0.92 |
| p-value | 5.35 × 10−2 | 5.96 × 10−2 | 5.55 × 10−2 |
| OEO Concentration % (v/v) | PS (nm) 1 | PDI 1 | ||||
|---|---|---|---|---|---|---|
| NE I | NE II | NE III | NE I | NE II | NE III | |
| 0 | 180.98 ± 0.94 | 227.98 ± 11.39 | 306.18 ± 92.70 | 0.25 ± 0.03 | 0.27 ± 0.48 | 0.76 ± 0.09 |
| 1 | 180.96 ± 0.64 | 252.57 ± 56.79 | 264.51 ± 81.20 | 0.27 ± 0.02 | 0.27 ± 0.04 | 0.64 ± 0.04 |
| 5 | 182.72 ± 0.38 | 248.64 ± 33.69 | >1000 | 0.27 ± 0.06 | 0.26 ± 0.10 | >1 |
| 10 | 182.72 ± 0.61 | 236.81 ± 55.83 | >1000 | 0.24 ± 0.02 | 0.24 ± 0.15 | >1 |
| 15 | 181.84 ± 0.15 | 232.35 ± 55.83 | >1000 | 0.19 ± 0.05 | 0.29 ± 0.05 | >100 |
| 20 | 181.60 ± 0.23 | 229.38 ± 5.66 | >1000 | 0.20 ± 0.03 | 0.29 ± 0.06 | >100 |
| 25 | 181.50 ± 0.20 | 220.72 ± 1.55 | >1000 | 0.22 ± 0.02 | 0.29 ± 0.06 | >1000 |
| 50 | 160.70 ± 0.35 | 215.84 ± 41.74 | >1000 | 0.23 ± 0.04 | 0.24 ± 0.07 | >1000 |
| 75 | 139.82 ± 28.83 | 212.45 ± 67.62 | >1000 | 0.23 ± 0.05 | 0.33 ± 0.10 | >1000 |
| p-value | 5.49 × 10−2 | 8.55 × 10−2 | - | 1.53 × 10−3 | 1.16 × 10−3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Sandoval, L.; Ochoa-Martínez, C.; Ayala-Aponte, A.; Pastrana, L.; Gonçalves, C.; Cerqueira, M.A. Polysaccharide-Based Multilayer Nano-Emulsions Loaded with Oregano Oil: Production, Characterization, and In Vitro Digestion Assessment. Nanomaterials 2021, 11, 878. https://doi.org/10.3390/nano11040878
Espinosa-Sandoval L, Ochoa-Martínez C, Ayala-Aponte A, Pastrana L, Gonçalves C, Cerqueira MA. Polysaccharide-Based Multilayer Nano-Emulsions Loaded with Oregano Oil: Production, Characterization, and In Vitro Digestion Assessment. Nanomaterials. 2021; 11(4):878. https://doi.org/10.3390/nano11040878
Chicago/Turabian StyleEspinosa-Sandoval, Luz, Claudia Ochoa-Martínez, Alfredo Ayala-Aponte, Lorenzo Pastrana, Catarina Gonçalves, and Miguel A. Cerqueira. 2021. "Polysaccharide-Based Multilayer Nano-Emulsions Loaded with Oregano Oil: Production, Characterization, and In Vitro Digestion Assessment" Nanomaterials 11, no. 4: 878. https://doi.org/10.3390/nano11040878
APA StyleEspinosa-Sandoval, L., Ochoa-Martínez, C., Ayala-Aponte, A., Pastrana, L., Gonçalves, C., & Cerqueira, M. A. (2021). Polysaccharide-Based Multilayer Nano-Emulsions Loaded with Oregano Oil: Production, Characterization, and In Vitro Digestion Assessment. Nanomaterials, 11(4), 878. https://doi.org/10.3390/nano11040878








