Size-Dependent Alloying Ability of Immiscible W-Cu Bimetallic Nanoparticles: A Theoretical and Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Theoretical Calculation Procedures
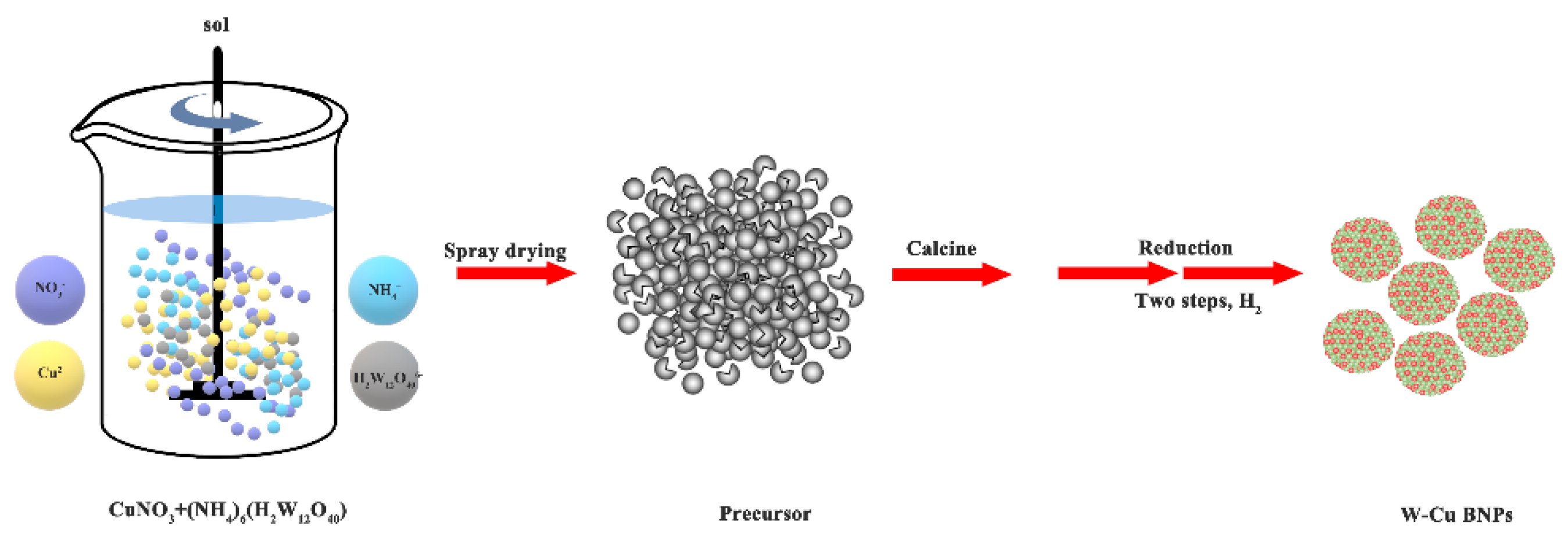
2.2. Experimental Procedures
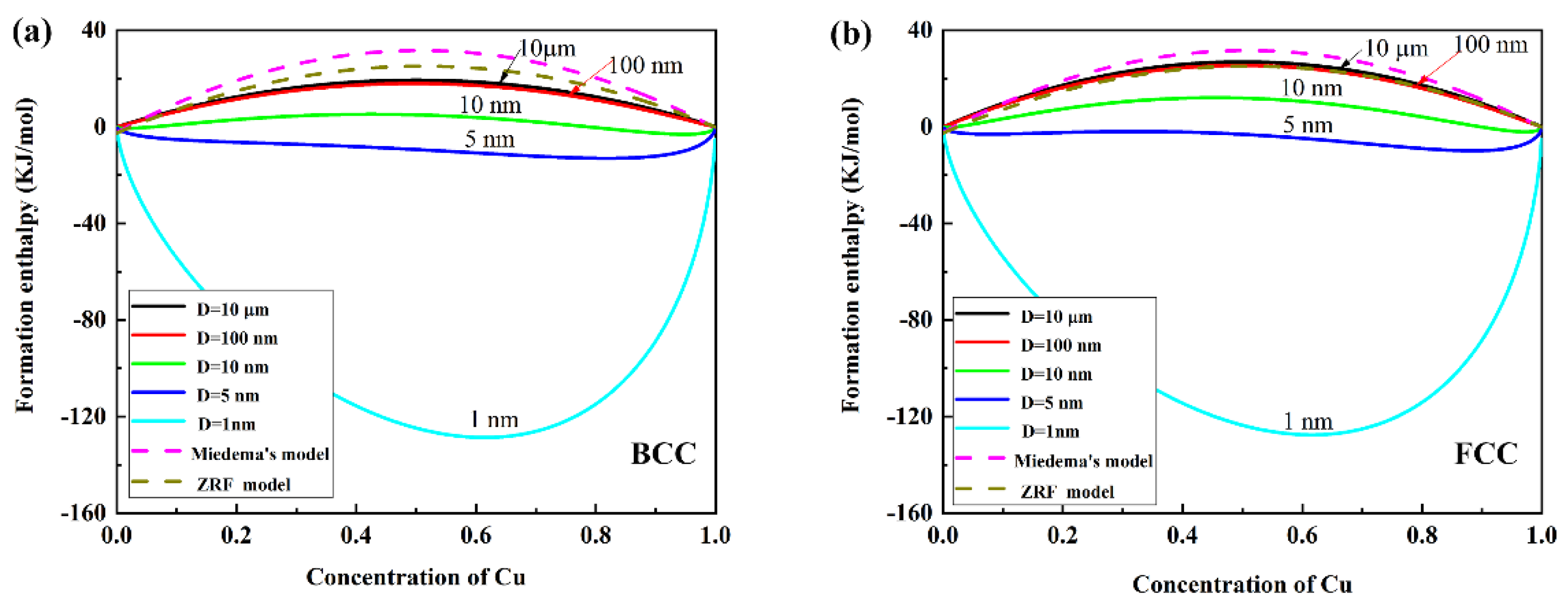
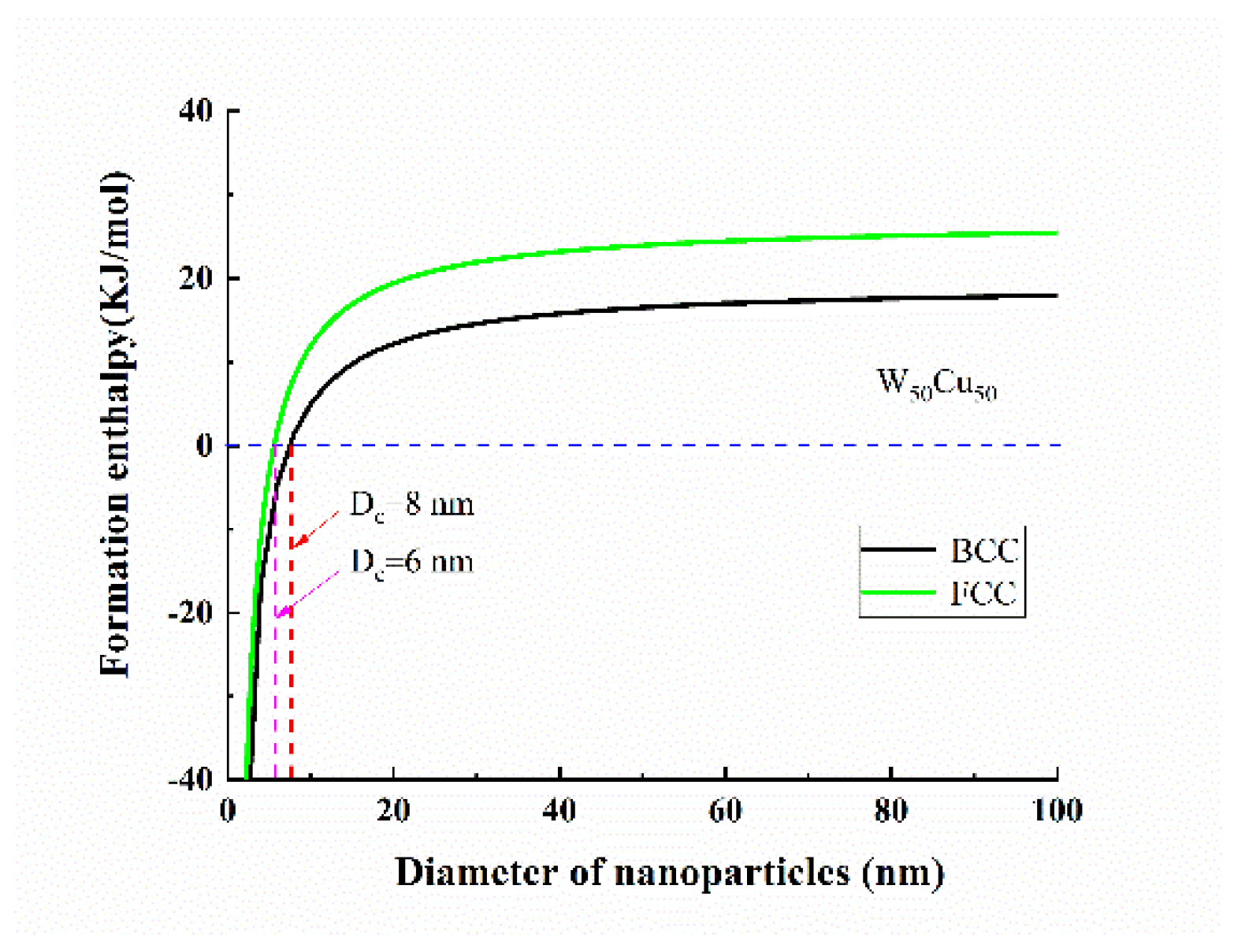
3. Results and Discussion
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y.N. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y. Bimetallic nanocrystals: Liquid-phase synthesis and catalytic applications. Adv. Mater. 2011, 23, 1044–1060. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold-copper bimetallic nanoparticles. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.G.; Huang, Z.N.; Xie, P.F.; Lacey, S.D.; Jacob, R.J.; Xie, H.; Chen, F.J.; Nie, A.M.; Pu, T.C.; Rehwoldt, M.; et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 2018, 359, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ko, B.H.; Hwang, S.; Liu, Z.; Yao, Y.; Luc, W.; Cui, M.; Malkani, A.S.; Li, T.; Wang, X.; et al. Overcoming immiscibility toward bimetallic catalyst library. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Ma, E. Alloys created between immiscible elements. Prog. Mater. Sci. 2005, 50, 413–509. [Google Scholar] [CrossRef]
- Miedema, A.R.; Chatel, P.F.D.; Boer, F.R.D. Cohesion in alloys—fundamentals of a semi-empirical model. Phys. B + C 1980, 100, 1–28. [Google Scholar] [CrossRef]
- Qi, W. Nanoscopic thermodynamics. Acc. Chem. Res. 2016, 49, 1587–1595. [Google Scholar] [CrossRef]
- Xiong, S.; Qi, W.; Huang, B.; Wang, M. Size-, shape-and composition-dependent alloying ability of bimetallic nanoparticles. ChemPhysChem 2011, 12, 1317–1324. [Google Scholar] [CrossRef]
- Zhao, M.; Issa, I.; Pfeifenberger, M.J.; Wurmshuber, M.; Kiener, D. Tailoring ultra-strong nanocrystalline tungsten nanofoams by reverse phase dissolution. Acta Mater. 2020, 182, 215–225. [Google Scholar] [CrossRef]
- Wu, W.; Hou, C.; Cao, L.; Liu, X.; Wang, H.; Lu, H.; Song, X. High hardness and wear resistance of W-Cu composites achieved by elemental dissolution and interpenetrating nanostructure. Nanotechnology 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Ahangarkani, M.; Zangeneh-Madar, K.; Borji, S.; Valefi, Z. The effect of post-sintering annealing on the erosion resistance of infiltrated W-Cu composites. Mater. Lett. 2017, 209, 566–570. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, S.Y.; Ham, H.J. Thermal conductivity of tungsten-copper composites. Thermochim. Acta 2012, 542, 2–5. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, J.; Zhao, S.; Zhang, H.; Yin, F.; Han, Y.; Liu, T. Microstructure and magnetic properties of MnZn ferrite powders prepared by nano-in-situ composite method. J. Alloy. Compd. 2020, 835. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, J.; Han, Y.; Liu, T.; Zhang, H.; Song, K.; Zhang, C. Preparation and characterization of Mn-Zn ferrites via nano-in-situ composite method. Solid State Sci. 2019, 98. [Google Scholar] [CrossRef]
- Lv, Y.; Fan, J.; Han, Y.; Liu, T.; Li, P.; Yan, H. The influence of modification route on the properties of W-0.3 wt% Y2O3 powder and alloy prepared by nano-in-situ composite method. J. Alloy. Compd. 2019, 774, 1140–1150. [Google Scholar] [CrossRef]
- Qi, W.H.; Huang, B.Y.; Wang, M.P. Size and shape-dependent formation enthalpy of binary alloy nanoparticles. Phys. B 2009, 404, 1761–1765. [Google Scholar] [CrossRef]
- Boer, F.R.D.; Boom, R.; Niessen, A.K.; Mattens, W.C.M.; Miedema, R. Cohesion in Metals: Transition Metal Alloys; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Fang, F.; Shu, X.L.; Deng, H.Q.; Hu, W.Y.; Zhu, M. Modified analytic EAM potentials for the binary immiscible alloy systems. Mater. Sci. Eng. A 2003, 355, 357–367. [Google Scholar] [CrossRef]
- Yang, J.Y.; Hu, W.Y.; Deng, H.Q.; Zhao, D.L. Temperature dependence of atomic relaxation and vibrations for the vicinal Ni(977) surface: A molecular dynamics study. Surf. Sci. 2004, 572, 439–448. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, W.; Han, S. Theoretical calculation of thermodynamic data for gold-rare earth alloys with the embedded-atom method. J. Alloy. Compd. 2006, 420, 83–93. [Google Scholar] [CrossRef]
- Zhang, R.F.; Liu, B.X. Proposed model for calculating the standard formation enthalpy of binary transition-metal systems. Appl. Phys. Lett. 2002, 81, 1219–1221. [Google Scholar] [CrossRef]
- Charles, K. Introduction to Solid State Physics; Wiley: Hoboken, NJ, USA, 1971. [Google Scholar]
- Shibata, T.; Bunker, B.A.; Zhang, Z.; Meisel, D.; Vardeman, C.F.; Gezelter, J.D. Size-dependent spontaneous alloying of Au-Ag nanoparticles. J. Am. Chem. Soc. 2002, 124, 11989–11996. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.K.; Jiang, P.; Che, J.G. Surface alloying of immiscible metals induced by surface state shift. Surf. Sci. 2003, 545, 199–210. [Google Scholar] [CrossRef]
- Yavari, A.R.; Desré, P.; Benameur, T. Mechanically driven alloying of immiscible elements. Phys. Rev. Lett. 1992, 68, 2235–2238. [Google Scholar] [CrossRef]
- Rizzo, H.F.; Massalski, T.B.; Nastasi, M. Metastable crystalline and amorphous structures formed in the Cu-W system by vapor deposition. Metall. Trans. A 1993, 24, 1027–1037. [Google Scholar] [CrossRef]
- Ding, F.; Rosen, A.; Bolton, K. Size dependence of the coalescence and melting of iron clusters: A molecular-dynamics study. Phys. Rev. B 2004, 70. [Google Scholar] [CrossRef]
- Sun, C.Q. Size dependence of nanostructures: Impact of bond order deficiency. Prog. Solid State Chem. 2007, 35, 1–159. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, T.; Zhao, S.; Xu, Z.; Lv, Y.; Fan, J.; Han, Y. Size-Dependent Alloying Ability of Immiscible W-Cu Bimetallic Nanoparticles: A Theoretical and Experimental Study. Nanomaterials 2021, 11, 1047. https://doi.org/10.3390/nano11041047
Zhang H, Liu T, Zhao S, Xu Z, Lv Y, Fan J, Han Y. Size-Dependent Alloying Ability of Immiscible W-Cu Bimetallic Nanoparticles: A Theoretical and Experimental Study. Nanomaterials. 2021; 11(4):1047. https://doi.org/10.3390/nano11041047
Chicago/Turabian StyleZhang, Hongbo, Tao Liu, Siqi Zhao, Zhanyuan Xu, Yaozha Lv, Jinglian Fan, and Yong Han. 2021. "Size-Dependent Alloying Ability of Immiscible W-Cu Bimetallic Nanoparticles: A Theoretical and Experimental Study" Nanomaterials 11, no. 4: 1047. https://doi.org/10.3390/nano11041047
APA StyleZhang, H., Liu, T., Zhao, S., Xu, Z., Lv, Y., Fan, J., & Han, Y. (2021). Size-Dependent Alloying Ability of Immiscible W-Cu Bimetallic Nanoparticles: A Theoretical and Experimental Study. Nanomaterials, 11(4), 1047. https://doi.org/10.3390/nano11041047





