Kinematic Viscosity ofMulticomponent FeCuNbSiB-BasedMelts
Abstract
:1. Introduction
2. Materials and Methods
3. Consequences of Arrhenius Equation
4. Simple Liquid Metals
5. Multicomponent Melts
6. Conclusions
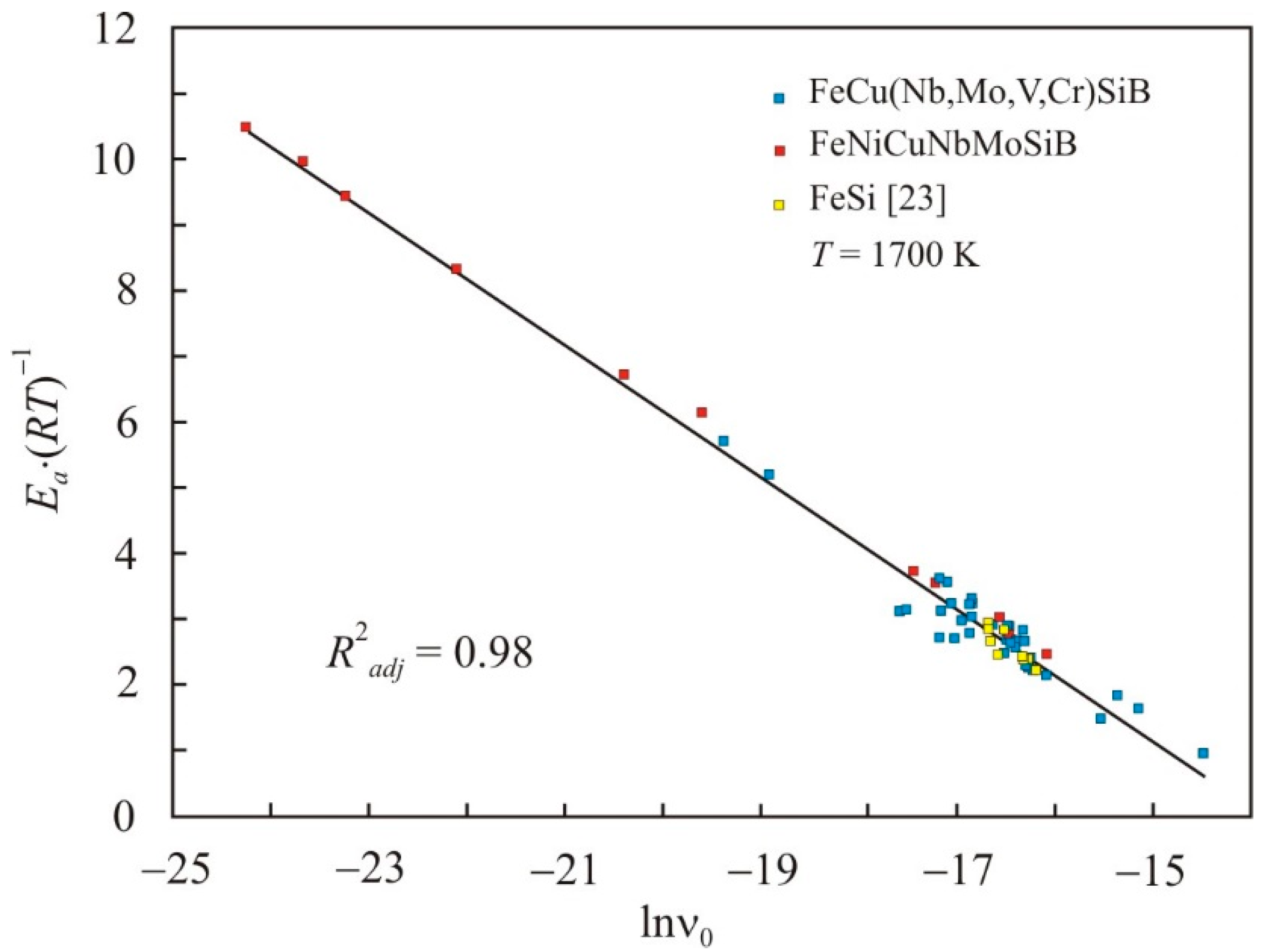
- There is a relationship between the reduced activation energy of viscous flow Ea·(RT)−1 and the pre-exponential factor ν0, which can be expressed by the relation:where C is a constant that generally depends on temperature. This relationship is universal for all quantities, the temperature dependence of which is expressed by the Arrhenius equation.
- The activation energy of viscous flow is linearly related to the cluster size on a natural logarithmic scale.
- Melt viscosity increases with decreasing cluster size.
- The change in the Arrhenius plot in the anomalous zone can be interpreted as a liquid–liquid structure transition, which begins with the disintegration of clusters and ends with the formation of a new cluster structure.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshizawa, Y.; Oguma, S.; Yamauchi, K. New Fe-based soft magnetic alloys composed of ultrafine grain structure. J. Appl. Phys. 1988, 64, 6044–6046. [Google Scholar] [CrossRef]
- Tsepelev, V.S.; Starodubtsev, Y.N. Nanocrystalline soft magnetic iron-based materials from liquid state to ready product. Nanomatarials 2021, 11, 108. [Google Scholar] [CrossRef]
- Calvo-Dahlborg, M.; Popel, P.S.; Kramer, M.J.; Besser, M.; Morris, J.R.; Dahlborg, U. Superheat-dependent microstructure of molten Al-Si alloys of different compositions studied by small angle neutron scattering. J. Alloys Compd. 2013, 550, 9–22. [Google Scholar] [CrossRef]
- Manov, V.P.; Popel, S.I.; Buler, P.I.; Manukhin, A.B.; Komlev, D.G. The influence of quenching temperature on the structure and properties of amorphous alloys. Mater. Sci. Eng. A 1991, 133, 535–540. [Google Scholar] [CrossRef]
- Tsepelev, V.; Starodubtsev, Y.; Konashkov, V. Melt viscosity of the soft magnetic nanocrystalline Fe72.5Cu1Nb2Mo1.5Si14B9 alloy. EPJ Web Conf. 2017, 151, 040062017. [Google Scholar] [CrossRef] [Green Version]
- Starodubtsev, Y.N.; Son, L.D.; Tsepelev, V.S.; Tyagunov, G.V.; Tishkin, A.P.; Korobka, O.B. Influence of the melt heating temperature on the mechanical and magnetic properties of an amorphous ribbon. Rasplavy 1992, 25, 76–79. [Google Scholar]
- Bel’tyukov, A.L.; Lad’yanov, V.I.; Shishmarin, A.I.; Menshikov, S.G. Viscosity of liquid amorphizing alloys of iron with boron and silicon. J. Non Cryst. Solids 2014, 401, 245–249. [Google Scholar] [CrossRef]
- Dong, B.S.; Zhou, S.X.; Wang, Y.G.; Li, Y.; Qin, J.Y.; Li, G.Z. Revealing a structure transition in typical Fe-based glass-forming alloy. J. Non Cryst. Solids 2018, 498, 305–308. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Zheng, H.; Tian, Z.; Hu, L. The role of liquid-liquid transition in glass formation of CuZr alloys. Phys. Chem. Chem. Phys. 2018, 19, 15962–15972. [Google Scholar] [CrossRef] [PubMed]
- Stiller, W. Arrhenius Equation and Non-Equlibrium Kinetics; Mir: Moscow, Russia, 2000; pp. 9–24. [Google Scholar]
- Frenkel, J. Kinetic Theory of Liquids; Nauka: Leningrad, Russia, 1975; pp. 224–228. [Google Scholar]
- Ward, A.G. The viscosity of pure liquids. Trans. Faraday Soc. 1937, 33, 88–97. [Google Scholar] [CrossRef]
- Chikova, O.A.; Tkachuk, G.A.; V’yukhin, V.V. Viscosity of Cu-Ni melts. Rus. J. Phys. Chem. A 2019, 93, 198–2003. [Google Scholar] [CrossRef]
- Tanaka, H. General view of a liquid-liquid phase transition. Phys. Rev. E 2000, 62, 6968–6976. [Google Scholar] [CrossRef] [PubMed]
- Vasin, M.G.; Lad’yanov, V.I. Structural transitions and nonmonotonic relaxation processes in liquid metals. Phys. Rev. E 2003, 68, 051202. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Li, J.; Wang, J.; Kou, H.; Beagunon, E. Liquid-liquid structure transition and nucleation in undercooled Co-B eutectic alloys. Appl. Phys. A 2017, 123, 391. [Google Scholar] [CrossRef]
- Iida, T.; Guthrie, R.I.L. The Thermophysical Properties of Metallic Liquids; Oxford University Press: Oxford, UK, 2015; pp. 497–543. [Google Scholar]
- Konashkov, V.V.; Tsepelev, V.S.; Belozerov, V.Y.; Starodubtsev, Y.N. Influence of smelting technology on properties of amorphizing Fe-S-B melts. Steel Transl. 2012, 42, 679–681. [Google Scholar] [CrossRef]
- Tsepelev, V.; Starodubtsev, Y.; Konashkov, V.; Wu, K.; Wang, R. Melt viscosity of nanocrystalline alloys in the model of free volume. J. Alloys Compounds 2019, 790, 547–550. [Google Scholar] [CrossRef]
- Tsepelev, V.S.; Starodubtsev, Y.N.; Wu, K.M.; Kochetkova, Y.A. Nanoparticles size in Fe73.5Cu1Mo3Si13.5B9 melt. Key Eng. Mater. 2020, 861, 107–112. [Google Scholar] [CrossRef]
- Kochetkova, Y.A.; Starodubtsev, Y.N.; Tsepelev, V.S. Kinematic viscosity of melt prepared from an amorphous Fe72.5Cu1Nb2Mo1.5Si14B9 ribbon. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 969, p. 012027. [Google Scholar] [CrossRef]
- Bel’tyukov, A.L.; Lad’yanov, V.I.; Shishmarin, A.I. Viscosity of Fe-Si melts with silicon content up to 45 at%. High. Temp. 2014, 52, 185–191. [Google Scholar] [CrossRef]
- Tsepelev, V.S.; Starodubtsev, Y.N.; Wu, K.M. Influence of Ni on crystallization and magnetic properties of Fe72.5-xNixCu1Nb2Mo1.5Si14B9 alloys. J. Cryst. Growth 2019, 528, 125256. [Google Scholar] [CrossRef]
- Tsepelev, V.; Konashkov, V.; Starodubtsev, Y.; Belozerov, Y.; Gaipisherov, D. Optimum regime of heat treatment of soft magnetic amorphous materials. IEEE Trans. Magn. 2012, 48, 1327–1330. [Google Scholar] [CrossRef]
- De With, G. Liquid-State Physical Chemistry. Fundamentals, Modeling, and Applications; Wiley-VCH: Weinheim, Germany, 2013; pp. 187–191. [Google Scholar]
- Glasstone, S.; Laidler, K.; Eyring, H. The Theory of Rate Processes. The Kinetics of Chemical Reactions, Viscosity, Diffusion and Electrochemical Phenomena; McGraw Hill: New York, NY, USA; London, UK, 1941; pp. 477–551. [Google Scholar]
- Lindemann, F.A. Über die Berechnung molekularer Eigenfrequenzen. Phys. Z. 1910, 11, 609–612. [Google Scholar]
- Zhai, Q.; Luo, J.; Zhao, P. Effect of thermal cycle on liquid structure of pure iron at just above its melting point. ISIJ Int. 2004, 8, 1279–1282. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Miyama, M.; Nishio, K.; Tsarkov, A.A.; Greer, A.L. Vitrification and nanocrystallization of pure liquid Ni studied molecular-dynamics simulation. J. Chem. Phys. 2019, 151, 124502. [Google Scholar] [CrossRef]
- Song, L.; Tian, X.; Yang, Y.; Qin, J.; Li, H.; Lin, X. Probing the microstructure in pure Al & Cu melts: Theory meets experiment. Front. Chem. 2020, 8, 00607. [Google Scholar] [CrossRef]
- Smirnov, B.M. The properties of fractal clusters. Phys. Rep. 1990, 188, 1–78. [Google Scholar] [CrossRef]
- Yang, M.H.; Li, J.H.; Liu, B.X. Fractal analysis on the cluster network in metallic liquid and glass. J. Alloys Comp. 2018, 757, 228–232. [Google Scholar] [CrossRef]
- Baum, B.A. Metal Liquids; Nauka: Moscow, Russia, 1979; pp. 67–80. [Google Scholar]
- Koca, H.D.; Doganay, S.; Turgut, A.; Tavman, I.H.; Saidur, R.; Mahbubul, I.M. Effect of particles size on viscosity of nanofluids: A review. Renew. Sustain. Energy Rev. 2018, 82, 1664–1674. [Google Scholar] [CrossRef] [Green Version]
- Dahlborg, U.; Calvo-Dahlborg, M.; Popel, P.S.; Sidorov, V.E. Structure and properties of som glass-forming liquid alloys. Eur. Phys. J. B 2000, 14, 639–648. [Google Scholar] [CrossRef]
- Chikova, O.; Sinitsin, N.; Vyukhin, V.; Chezganov, D. Microheterogeneity and crystallization conditions of Fe-Mn melts. J. Cryst. Growth 2019, 527, 125239. [Google Scholar] [CrossRef]
- Beľtyukov, A.L.; Goncharov, O.Y.; Laďyanov, V.I. Features of polytherms of the viscosity of Fe-B melts. Rus. J. Phys. Chem. 2017, 91, 1919–1924. [Google Scholar] [CrossRef]
- Dong, B.; Zhou, S.; Qin, J.; Li, Y.; Chen, H.; Wang, Y. The hidden disintegration of cluster heterogeneity in Fe-based glass-forming. Prog. Nat. Sci. Mater. 2018, 28, 696–703. [Google Scholar] [CrossRef]





| Ni Content (at%) | Heating | Cooling | ||||||
|---|---|---|---|---|---|---|---|---|
| T > 1700 K | T < 1700 K | T > 1700 K | T < 1700 K | |||||
| Ea kJ·mol−1 | ν0 × 10−8 m2·s−1 | Ea kJ·mol−1 | ν0 × 10−8 m2·s−1 | Ea kJ·mol−1 | ν0 × 10−8 m2·s−1 | Ea kJ·mol−1 | ν0 × 10−8 m2·s−1 | |
| 2.5 | 141 | 0.0052 | 35 | 10.3 | 86.8 | 0.307 | 42.6 | 6.38 |
| 6.3 | 134 | 0.0081 | 52.6 | 2.66 | 95 | 0.139 | 39.1 | 7.04 |
| 12.7 | 148 | 0.0029 | – | – | 118 | 0.025 | 50 | 3.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starodubtsev, Y.N.; Tsepelev, V.S.; Tsepeleva, N.P. Kinematic Viscosity ofMulticomponent FeCuNbSiB-BasedMelts. Nanomaterials 2021, 11, 1042. https://doi.org/10.3390/nano11041042
Starodubtsev YN, Tsepelev VS, Tsepeleva NP. Kinematic Viscosity ofMulticomponent FeCuNbSiB-BasedMelts. Nanomaterials. 2021; 11(4):1042. https://doi.org/10.3390/nano11041042
Chicago/Turabian StyleStarodubtsev, Yuri N., Vladimir S. Tsepelev, and Nadezhda P. Tsepeleva. 2021. "Kinematic Viscosity ofMulticomponent FeCuNbSiB-BasedMelts" Nanomaterials 11, no. 4: 1042. https://doi.org/10.3390/nano11041042
APA StyleStarodubtsev, Y. N., Tsepelev, V. S., & Tsepeleva, N. P. (2021). Kinematic Viscosity ofMulticomponent FeCuNbSiB-BasedMelts. Nanomaterials, 11(4), 1042. https://doi.org/10.3390/nano11041042







