Study on Performance Improvements in Perovskite-Based Ultraviolet Sensors Prepared Using Toluene Antisolvent and CH3NH3Cl
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ITO
2.3. Synthesis of Perovskite
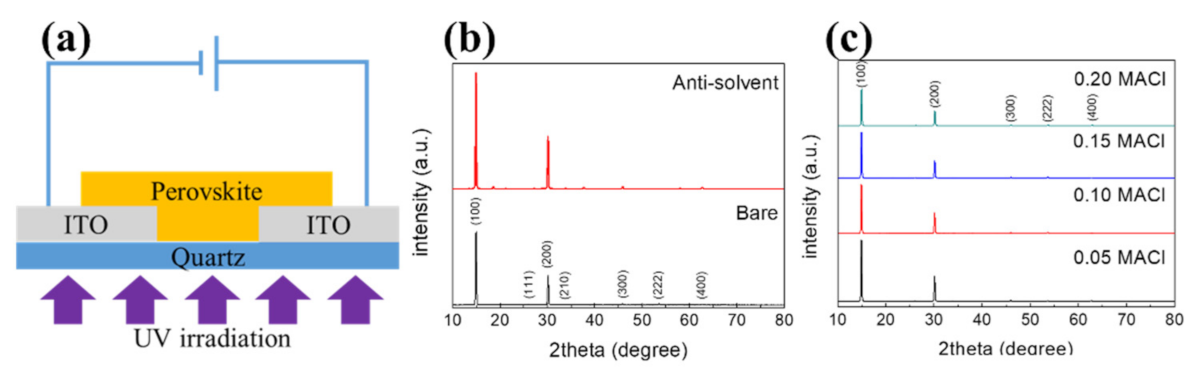
2.4. Preparation of a UV Sensor Based on Perovskite Films
2.5. Characterization and Device Measurement
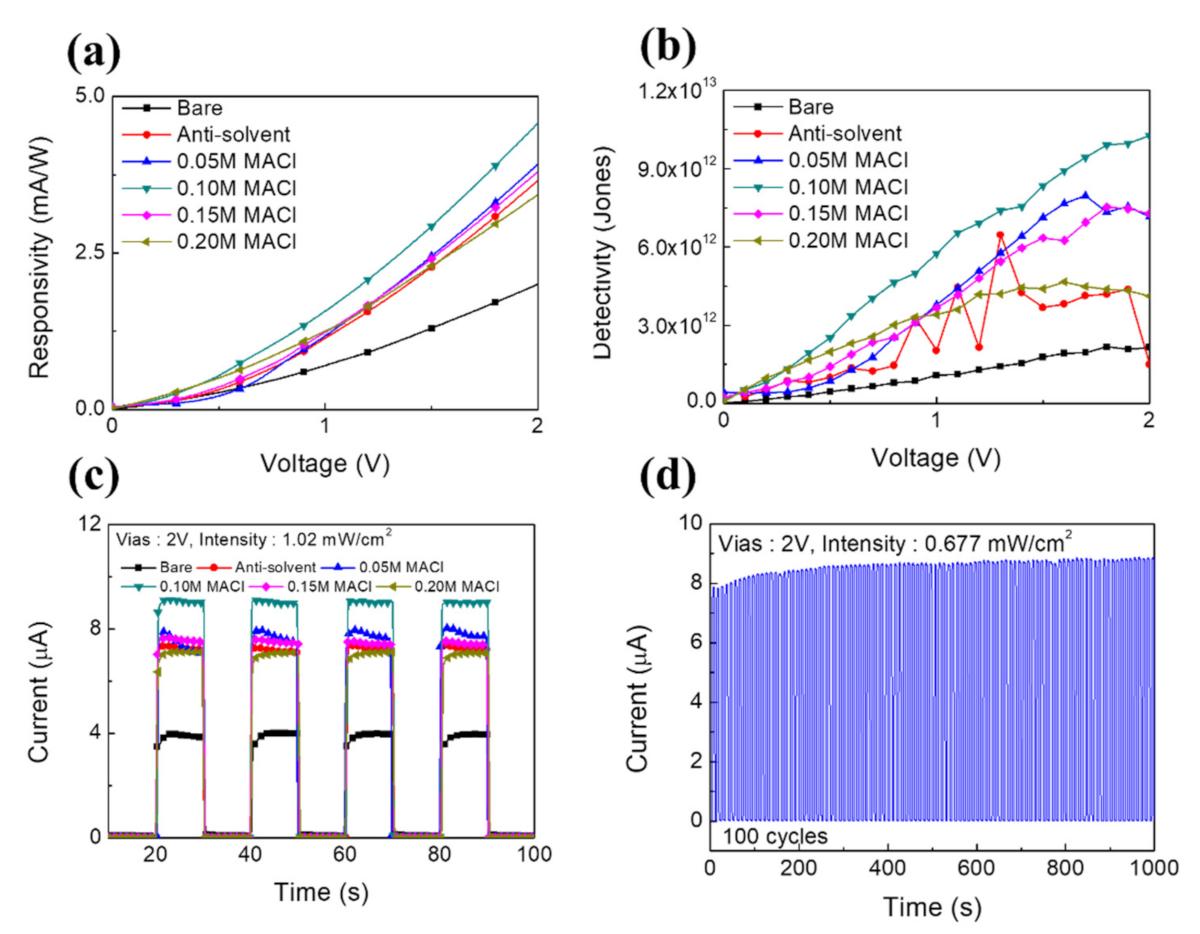
3. Results and Discussion
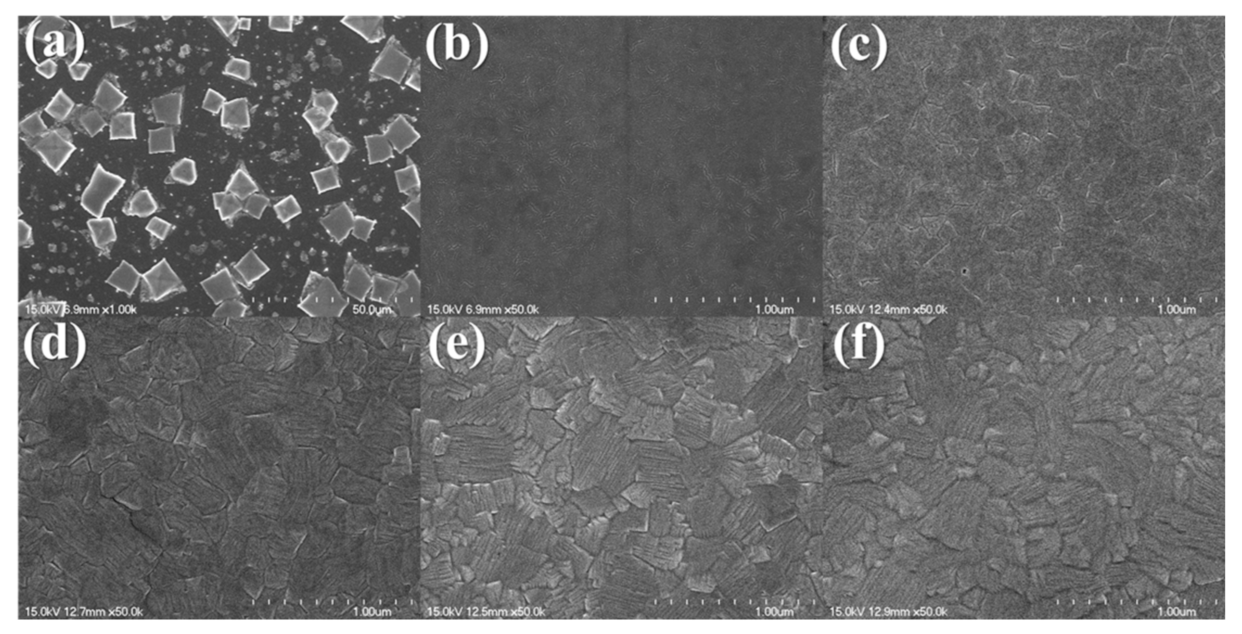
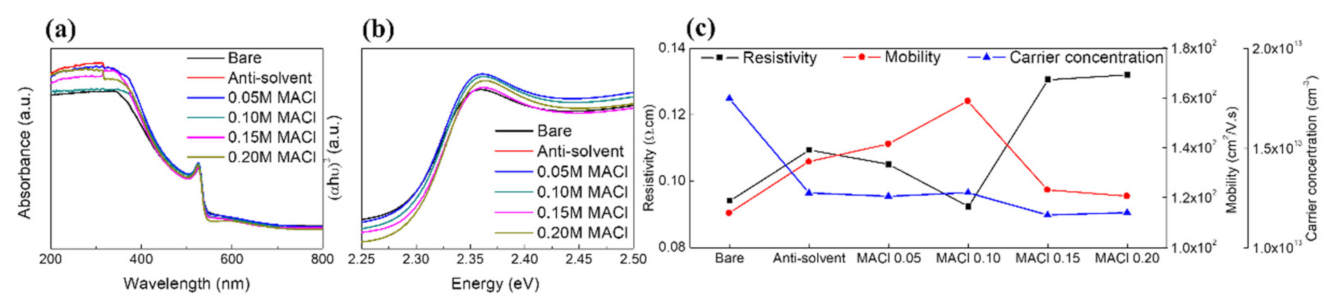
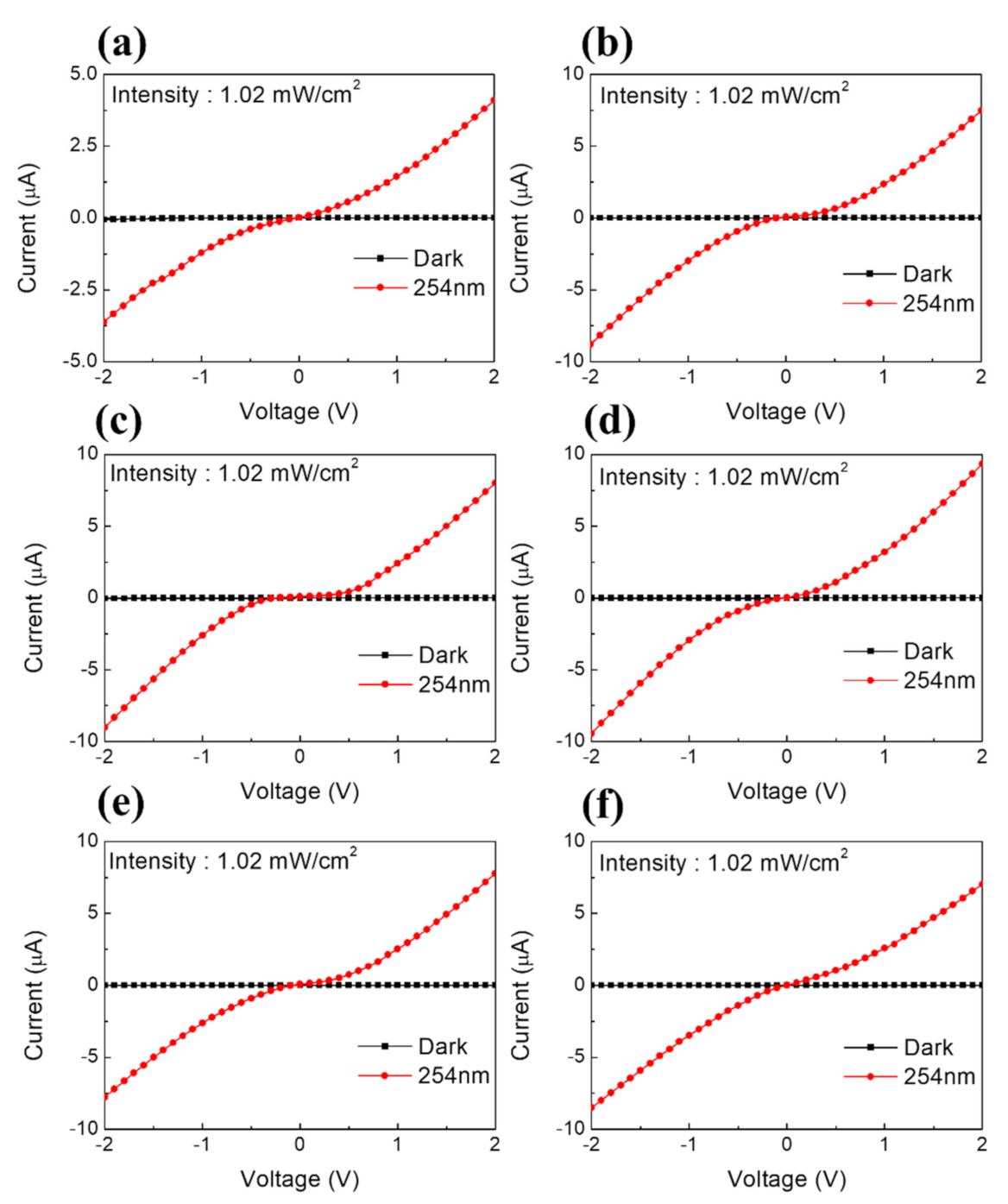
Characteristics of the Prepared Perovskite Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boggess, A.; Carr, F.A.; Evans, D.C.; Fischel, D.; Freeman, H.R.; Fuechsel, C.F.; Klinglesmith, D.A.; Krueger, V.L.; Longanecker, G.W.; Moore, J.V.; et al. The IUE spacecraft and instrumentation. Nat. Cell Biol. 1978, 275, 372–377. [Google Scholar] [CrossRef]
- Xu, Z.; Ding, H.; Sadler, B.M.; Chen, G. Analytical performance study of solar blind non-line-of-sight ultraviolet short-range communication links. Opt. Lett. 2008, 33, 1860–1862. [Google Scholar] [CrossRef]
- Lin, R.; Zheng, W.; Zhang, D.; Zhang, Z.; Liao, Q.; Yang, L.; Huang, F. High-performance graphene/β-Ga2O3 heterojunction deep-ultraviolet photodetector with hot-electron excited carrier multiplication. ACS Appl. Mater. Interfaces 2018, 10, 22419–22426. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lin, R.; Ran, J.; Zhang, Z.; Ji, X.; Huang, F. Vacuum-Ultraviolet Photovoltaic Detector. ACS Nano 2018, 12, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lin, R.; Zhang, Z.; Huang, F. Vacuum-Ultraviolet Photodetection in Few-Layered h-BN. ACS Appl. Mater. Interfaces 2018, 10, 27116–27123. [Google Scholar] [CrossRef] [PubMed]
- Gökkavas, M.; Butun, S.; Caban, P.; Strupinski, W.; Ozbay, E. Integrated AlGaN quadruple-band ultraviolet photodetectors. Semicond. Sci. Technol. 2012, 27, 065004. [Google Scholar] [CrossRef]
- Ju, Z.G.; Shan, C.X.; Jiang, D.Y.; Zhang, J.Y.; Yao, B.; Zhao, D.X.; Shen, D.Z.; Fan, X.W. MgxZn1−xO-based photodetectors covering the whole solar-blind spectrum range. Appl. Phys. Lett. 2008, 93, 173505. [Google Scholar] [CrossRef]
- Mendoza, F.; Makarov, V.; Weiner, B.R.; Morell, G. Solar-blind field-emission diamond ultraviolet detector. Appl. Phys. Lett. 2015, 107, 201605. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Zhang, Z.; Hui, L.; Fang, X. Nanostructured Photodetectors: From Ultraviolet to Terahertz. Adv. Mater. 2016, 28, 403–433. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, W.; Lin, R.; Li, T.; Zhang, Z.; Huang, F. High quality β-Ga2O3 film grown with N2O for high sensitivity solar-blind-ultraviolet photodetector with fast response speed. J. Alloys Compd. 2018, 735, 150–154. [Google Scholar] [CrossRef]
- Zhumekenov, A.A.; Burlakov, V.M.; Saidaminov, M.I.; Alofi, A.; Haque, A.; Turedi, B.; Davaasuren, B.; Dursun, I.; Cho, N.; El-Zohry, A.M.; et al. The Role of Surface Tension in the Crystallization of Metal Halide Perovskites. ACS Energy Lett. 2017, 2, 1782–1788. [Google Scholar] [CrossRef]
- Tisdale, J.T.; Smith, T.; Salasin, J.R.; Ahmadi, M.; Johnson, N.; Ievlev, A.V.; Koehler, M.; Rawn, C.J.; Lukosi, E.; Hu, B. Precursor purity effects on solution-based growth of MAPbBr3 single crystals towards efficient radiation sensing. CrystEngComm 2018, 20, 7818–7825. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Abdelhady, A.L.; Murali, B.; Alarousu, E.; Burlakov, V.M.; Peng, W.; Dursun, I.; Wang, L.; He, Y.; Maculan, G.; et al. High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun. 2015, 6, 7586. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, G.D.; Duan, R.F.; Huang, F.; Wei, T.B.; Liu, Z.Q.; Wang, J.X.; Li, J.M. Tunable bandgap in hybrid perovskite CH3NH3Pb(Br3−yXy) single crystals and photodetector applications. AIP Adv. 2016, 6, 045115. [Google Scholar] [CrossRef]
- Senocrate, A.; Kim, G.Y.; Grätzel, M.; Maier, J. Thermochemical stability of hybrid halide perovskites. ACS Energy Lett. 2019, 4, 2859–2870. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, H.; Yuan, S.; Wang, J.; Zhan, Y.; Zheng, L. An optical dynamic study of MAPbBr3 single crystals passivated with MAPbCl3/I3-MAPbBr3 heterojunctions. Phys. Chem. Chem. Phys. 2017, 19, 4516–4521. [Google Scholar] [CrossRef]
- Mhamdi, A.; Mehdi, H.; Bouazizi, A.; Garcia-Belmonte, G. One-step methylammonium lead bromide films: Effect of annealing treatment. J. Mol. Struct. 2019, 1192, 1–6. [Google Scholar] [CrossRef]
- Tidhar, Y.; Edri, E.; Weissman, H.; Zohar, D.; Hodes, G.; Cahen, D.; Rybtchinski, B.; Kirmayer, S. Crystallization of methyl ammonium lead halide perovskites: Implications for photovoltaic applications. J. Am. Chem. Soc. 2014, 136, 13249–13256. [Google Scholar] [CrossRef] [PubMed]
- Dualeh, A.; Tétreault, N.; Moehl, T.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Effect of Annealing Temperature on Film Morphology of Organic-Inorganic Hybrid Pervoskite Solid-State Solar Cells. Adv. Funct. Mater. 2014, 24, 3250–3258. [Google Scholar] [CrossRef]
- Eperon, G.E.; Burlakov, V.M.; Docampo, P.; Goriely, A.; Snaith, H.J. Morphological Control for High Performance, Solution-Processed Planar Heterojunction Perovskite Solar Cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar] [CrossRef]
- Mehdi, H.; Mhamdi, A.; Hannachi, R.; Bouazizi, A. MAPbBr3 perovskite solar cells via a two-step deposition process. RSC Adv. 2019, 9, 12906–12912. [Google Scholar] [CrossRef]
- Chen, L.C.; Wu, J.R.; Tseng, Z.L.; Chen, C.C.; Chang, S.H.; Huang, J.K.; Lee, K.L.; Cheng, H.M. Annealing effect on (FAPbI3)1−x (MAPbBr3)x perovskite films in inverted-type perovskite solar cells. Materials 2016, 9, 747. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status solidi (b) 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Davis, E.A.; Mott, N. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 0903–0922. [Google Scholar] [CrossRef]
- Wang, K.-H.; Li, L.-C.; Shellaiah, M.; Sun, K.W. Structural and Photophysical Properties of Methylammonium Lead Tribromide (MAPbBr3) Single Crystals. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Kong, L.; Liu, G.; Gong, J.; Hu, Q.; Schaller, R.D.; Dera, P.; Tang, Y. Simultaneous band-gap narrowing and carrier-lifetime prolongation of organic–inorganic trihalide perovskites. Proc. Natl. Acad. Sci. USA 2016, 113, 8910–8915. [Google Scholar] [CrossRef]
- Singh, M.; Goyal, M.; Devlal, K. Size and shape effects on the band gap of semiconductor compound nanomaterials. J. Taibah Univ. Sci. 2018, 12, 470–475. [Google Scholar] [CrossRef]
- Xie, F.X.; Su, H.; Mao, J.; Wong, K.S.; Choy, W.C.H. Evolution of Diffusion Length and Trap State Induced by Chloride in Perovskite Solar Cell. J. Phys. Chem. C 2016, 120, 21248–21253. [Google Scholar] [CrossRef]
- Yu, J.; Javaid, K.; Liang, L.; Wu, W.; Liang, Y.; Song, A.; Cao, H. High-performance visible-blind ultraviolet photodetector based on IGZO TFT coupled with p–n heterojunction. ACS Appl. Mater. Interfaces 2018, 10, 8102–8109. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, X.; Hong, R.; Yang, W.; Wu, Z. High-performance 4H-SiC-based pin ultraviolet photodiode and investigation of its capacitance characteristics. Opt. Commun. 2014, 333, 182–186. [Google Scholar] [CrossRef]
- Inamdar, S.; Ganbavle, V.; Shaikh, S.; Rajpure, K. Effect of the buffer layer on the metal-semiconductor-metal UV photodetector based on Al-doped and undoped ZnO thin films with different device structures. Phys. Status Solidi (A) 2015, 212, 1704–1712. [Google Scholar] [CrossRef]
- Singh, S. Al doped ZnO based metal–semiconductor–metal and metal–insulator–semiconductor–insulator–metal UV sensors. Optik 2016, 127, 3523–3526. [Google Scholar] [CrossRef]
- Li, S.; Yan, Z.; Liu, Z.; Chen, J.; Zhi, Y.; Guo, D.; Li, P.; Wu, Z.; Tang, W. A self-powered solar-blind photodetector with large V oc enhancing performance based on the PEDOT:PSS/Ga2O3 organic–inorganic hybrid heterojunction. J. Mater. Chem. C 2020, 8, 1292–1300. [Google Scholar] [CrossRef]
- Kim, J.O.; Sengupta, S.; Barve, A.V.; Sharma, Y.D.; Adhikary, S.; Lee, S.J.; Noh, S.K.; Allen, M.S.; Allen, J.W.; Chakrabarti, S.; et al. Multi-stack InAs/InGaAs sub-monolayer quantum dots infrared photodetectors. Appl. Phys. Lett. 2013, 102, 011131. [Google Scholar] [CrossRef]
- Chen, J.-H.; Jing, Q.; Xu, F.; Lu, Z.-D.; Lu, Y.-Q. High-sensitivity optical-fiber-compatible photodetector with an integrated CsPbBr_3–graphene hybrid structure. Optica 2017, 4, 835–838. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, W.; Lin, R.; Huang, F. High-sensitive and fast response to 255 nm deep-UV light of CH3NH3PbX3 (X = Cl, Br, I) bulk crystals. R. Soc. Open Sci. 2018, 5, 180905. [Google Scholar] [CrossRef]
- Maculan, G.; Sheikh, A.D.; Abdelhady, A.L.; Saidaminov, M.I.; Haque, A.; Murali, B.; Alarousu, E.; Mohammed, O.F.; Wu, T.; Bakr, O.M. CH3NH3PbCl3 Single Crystals: Inverse Temperature Crystallization and Visible-Blind UV-Photodetector. J. Phys. Chem. Lett. 2015, 6, 3781–3786. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.C.; Tsai, D.S.; Ravadgar, P.; Ke, J.J.; Tsai, M.L.; Lien, D.H.; Huang, C.Y.; Horng, R.H.; He, J.H. See-Through Ga2O3 Solar-Blind Photodetectors for Use in Harsh Environments. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 112–117. [Google Scholar]
- Tong, G.; Li, H.; Zhu, Z.; Zhang, Y.; Yu, L.; Xu, J.; Jiang, Y. Enhancing Hybrid Perovskite Detectability in the Deep Ultraviolet Region with Down-Conversion Dual-Phase (CsPbBr3–Cs4PbBr6) Films. J. Phys. Chem. Lett. 2018, 9, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Zheng, E.; Yuh, B.; Tosado, G.A.; Yu, Q. Solution-processed visible-blind UV-A photodetectors based on CH3NH3PbCl3 perovskite thin films. J. Mater. Chem. C 2017, 5, 3796–3806. [Google Scholar] [CrossRef]
| Materials | Light (nm) | Method | Voltage (V) | Responsivity (mA/W) | Detectivity (Jones) | EQE (%) |
|---|---|---|---|---|---|---|
| CH3NH3PbBr3 [this study] | 254 | Solution | 2 | 4.57 | 1.02 × 1013 | 22.2 |
| CH3NH3PbCl3 [36] | 255 | Single crystals | 5 | 450 | - | 219 |
| CH3NH3PbBr3 [36] | 255 | Single crystals | 5 | 300 | - | 146 |
| CH3NH3PbI3 [36] | 255 | Single crystals | 5 | 120 | - | 58 |
| CH3NH3PbCl3 [37] | 365 | Single crystals | 15 | 46.90 | 1.2 × 1010 | - |
| Ga2O3 [38] | 185 | MOCVD * | 10 | 0.3 | 2.8 × 1010 | 0.2 |
| CsPbBr3-Cs4PbBr6 [39] | 254 | Vapor | 0 | 49.40 | 1.2 × 1012 | 31 |
| CH3NH3PbCl3 [40] | 398 | Solution | −1 | 71 | 1.2 × 1010 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.G.; Bark, C.W.; Choi, H.W. Study on Performance Improvements in Perovskite-Based Ultraviolet Sensors Prepared Using Toluene Antisolvent and CH3NH3Cl. Nanomaterials 2021, 11, 1000. https://doi.org/10.3390/nano11041000
Shin SG, Bark CW, Choi HW. Study on Performance Improvements in Perovskite-Based Ultraviolet Sensors Prepared Using Toluene Antisolvent and CH3NH3Cl. Nanomaterials. 2021; 11(4):1000. https://doi.org/10.3390/nano11041000
Chicago/Turabian StyleShin, Seong Gwan, Chung Wung Bark, and Hyung Wook Choi. 2021. "Study on Performance Improvements in Perovskite-Based Ultraviolet Sensors Prepared Using Toluene Antisolvent and CH3NH3Cl" Nanomaterials 11, no. 4: 1000. https://doi.org/10.3390/nano11041000
APA StyleShin, S. G., Bark, C. W., & Choi, H. W. (2021). Study on Performance Improvements in Perovskite-Based Ultraviolet Sensors Prepared Using Toluene Antisolvent and CH3NH3Cl. Nanomaterials, 11(4), 1000. https://doi.org/10.3390/nano11041000






