Liquid Crystal Elastomers for Biological Applications
Abstract
1. Introduction
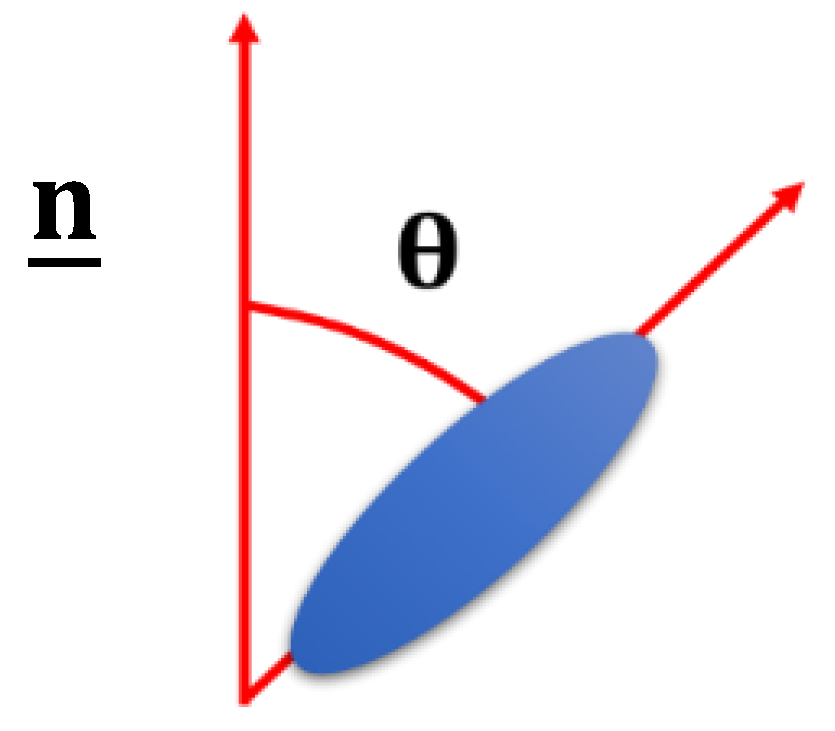
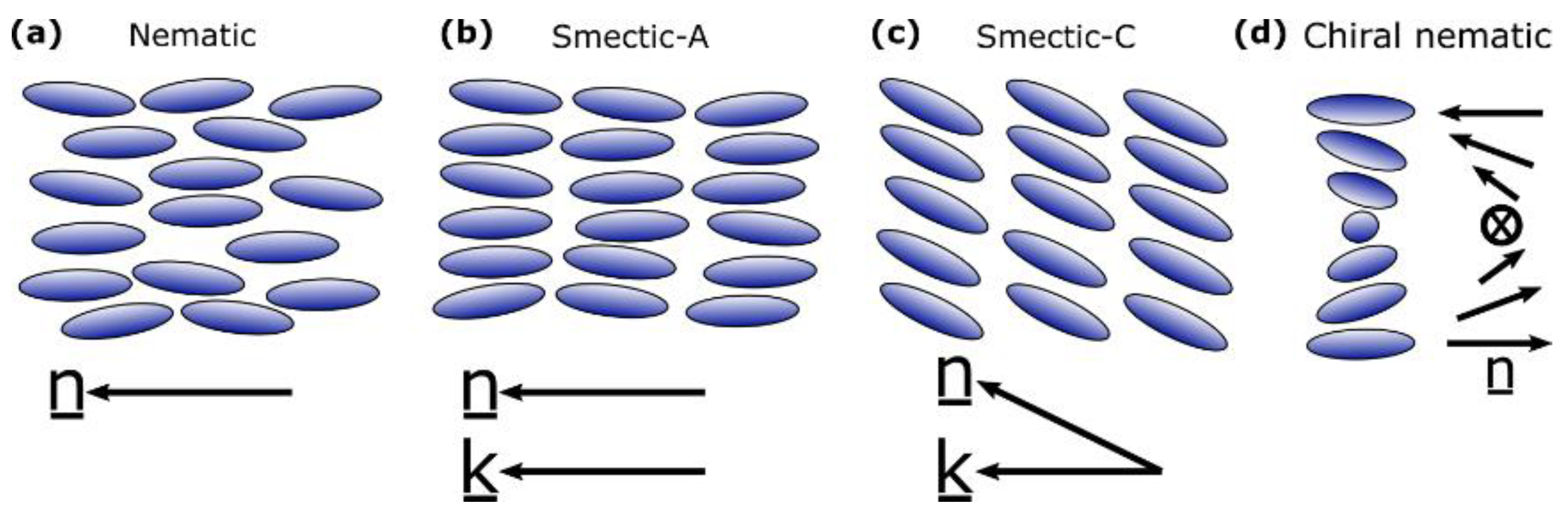
2. Background to Liquid Crystal Elastomers and Liquid Crystals
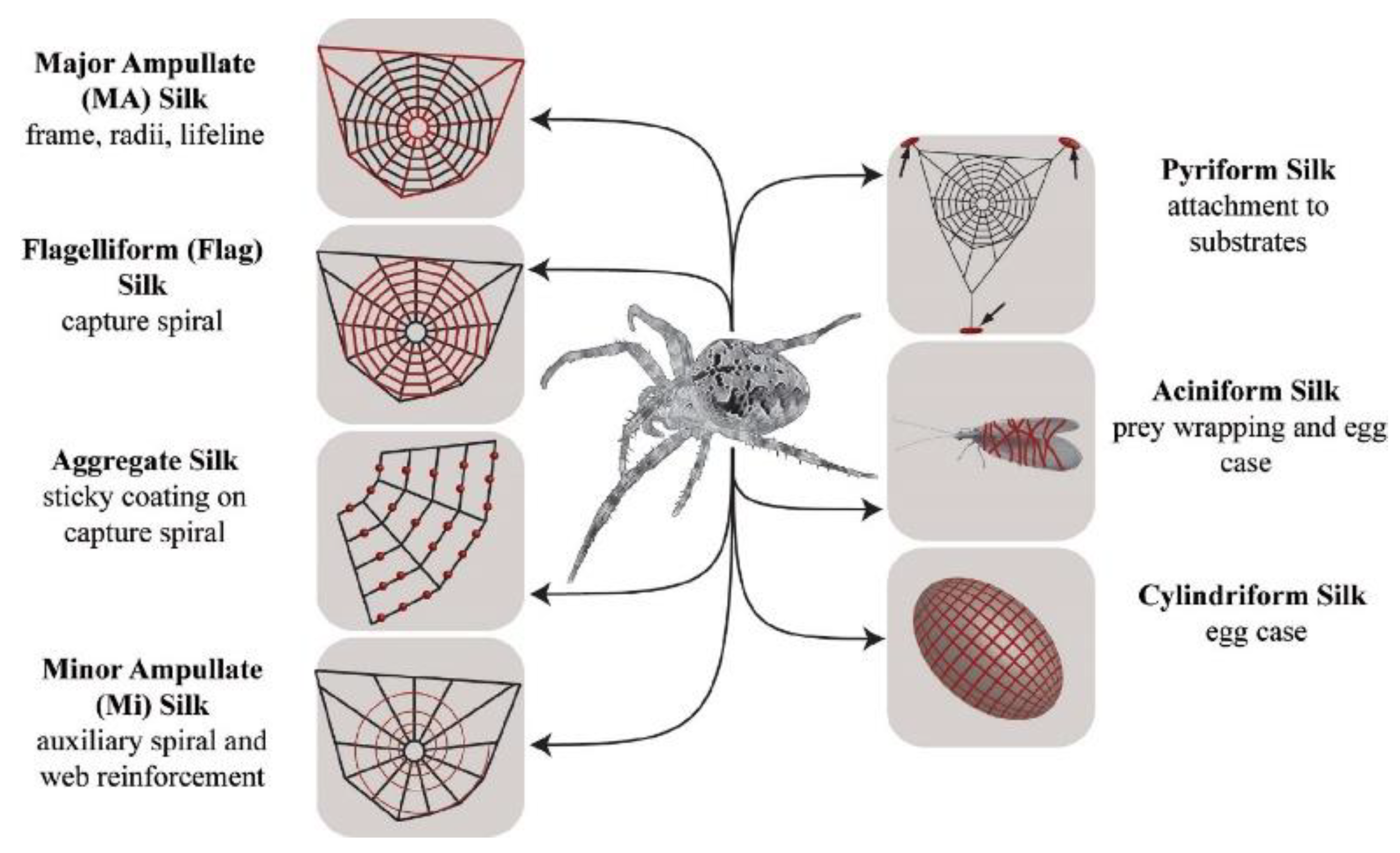
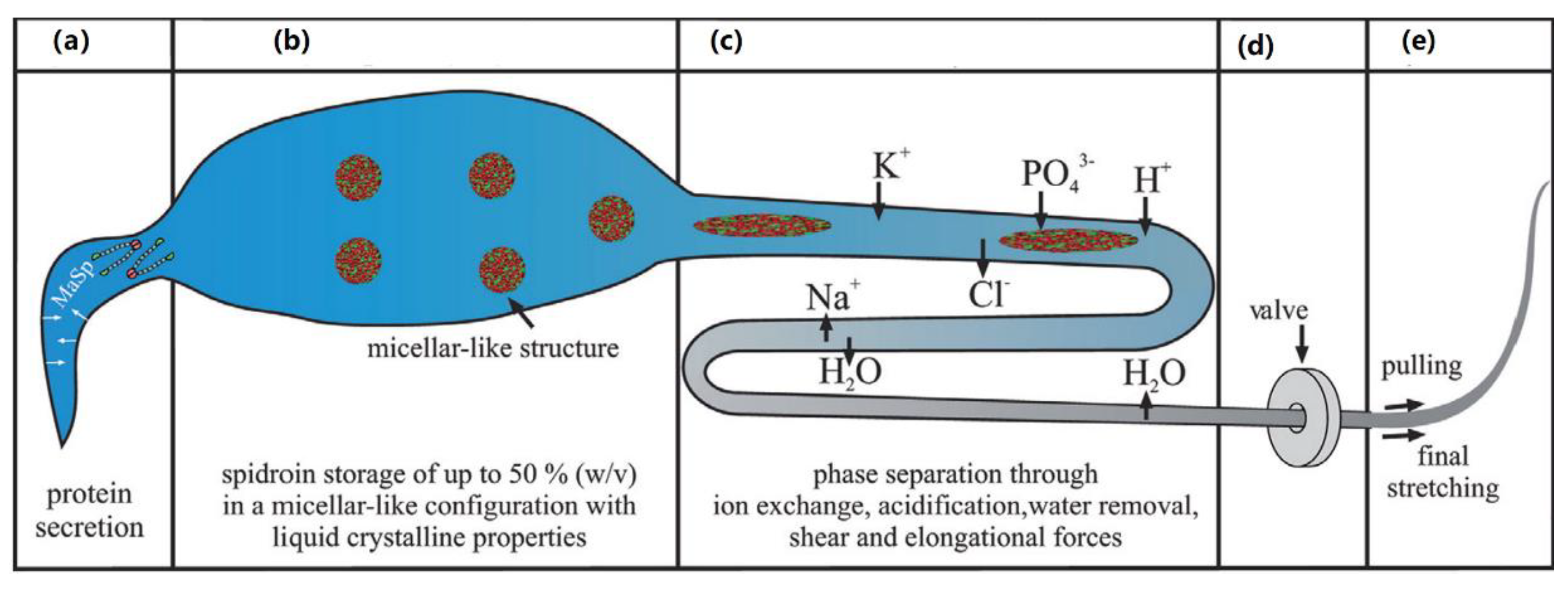
3. Liquid Crystal Elastomers in Nature
4. Biomedical Advances Inspired by LCEs
5. The Significance of Liquid Crystal Elastomers for Understanding Biological Systems
6. Liquid Crystal Elastomers for Biomedical Applications
7. Liquid Crystal Elastomers in Tissue Engineering
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Warner, M.; Terentjev, E. Liquid Crystal Elastomers; Clarendon Press: Oxford, UK, 2007; Volume 1, ISBN 9788578110796. [Google Scholar]
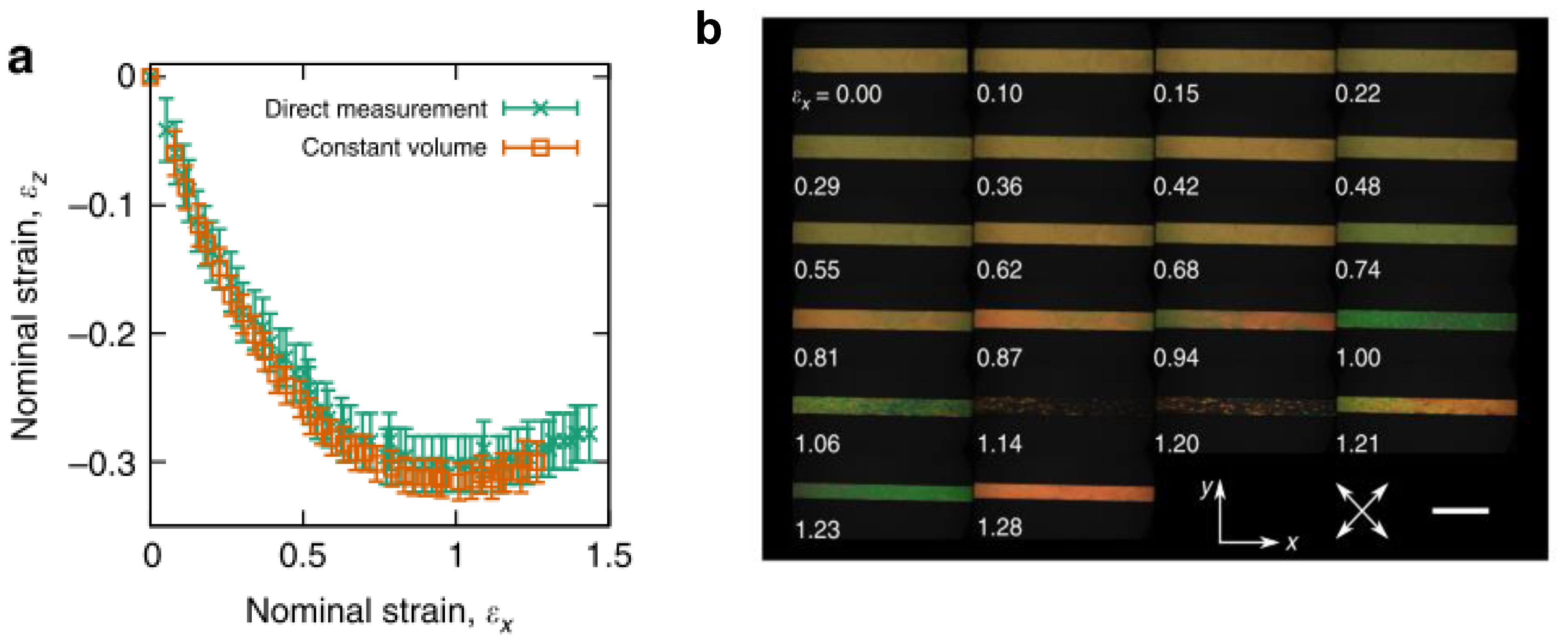
- Mistry, D.; Connell, S.D.; Mickthwaite, S.L.; Morgan, P.B.; Clamp, J.H.; Gleeson, H.F. Coincident molecular auxeticity and negative order parameter in a liquid crystal elastomer. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Prévôt, M.E.; Ustunel, S.; Hegmann, E. Liquid crystal elastomers-A path to biocompatible and biodegradable 3D-LCE scaffolds for tissue regeneration. Materials 2018, 11, 377. [Google Scholar] [CrossRef]
- Steinsträsser, R.; Pohl, L. Chemistry and Applications of Liquid Crystals. Angew. Chem. Int. Ed. 1973, 12, 617–630. [Google Scholar] [CrossRef]
- Gin, D.L.; Pecinovsky, C.S.; Bara, J.E.; Kerr, R.L. Functional lyotropic liquid crystal materials. Struct. Bond. 2007, 128, 181–222. [Google Scholar]
- Luckhurst, G.R. Orientational order in liquid crystals. Experiment and Plenary lecture. Ber. Bunsenges. Phys. Chem. Chem. Phys. 1993, 97, 1169–1187. [Google Scholar] [CrossRef]
- Oswald, P.; Pieranski, P. Nematic and Cholesteric Liquid Crystals; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780415321402. [Google Scholar]
- Dumanli, A.G.; Savin, T. Recent advances in the biomimicry of structural colours. Chem. Soc. Rev. 2016, 45, 6698–6724. [Google Scholar] [CrossRef] [PubMed]
- Finkelmann, H.; Koch, H.; Rehage, G. Investigations on liquid crystalline polysiloxanes 3†. Liquid crystalline elastomers—A new type of liquid crystalline material. Macromol. Rapid Commun. 1981, 2, 317–322. [Google Scholar] [CrossRef]
- Kularatne, R.S.; Kim, H.; Boothby, J.M.; Ware, T.H. Liquid crystal elastomer actuators: Synthesis, alignment, and applications. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 395–411. [Google Scholar] [CrossRef]
- Ula, S.W.; Traugutt, N.A.; Volpe, R.H.; Patel, R.R.; Yu, K.; Yakacki, C.M. Liquid crystal elastomers: An introduction and review of emerging technologies. Liq. Cryst. Rev. 2018, 6, 78–107. [Google Scholar] [CrossRef]
- Mistry, D.A. Developing the Mechanical Applications of Liquid Crystal Elastomers. Doctoral Thesis, University of Leeds, Leeds, UK, 2018. [Google Scholar]
- Mitchell, G.; Davis, F.; Guo, W. Strain-induced transitions in liquid-crystal elastomers. Phys. Rev. Lett. 1993, 21, 2947. [Google Scholar] [CrossRef]
- Mistry, D.; Morgan, P.B.; Clamp, J.H.; Gleeson, H.F. New insights into the nature of semi-soft elasticity and “mechanical-Fréedericksz transitions” in liquid crystal elastomers. Soft Matter 2018, 14, 1301–1310. [Google Scholar] [CrossRef]
- Ware, T.H.; Biggins, J.S.; Shick, A.F.; Warner, M.; White, T.J. Localized soft elasticity in liquid crystal elastomers. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Agrawal, A.; Chipara, A.C.; Shamoo, Y.; Patra, P.K.; Carey, B.J.; Ajayan, P.M.; Chapman, W.G.; Verduzco, R. Dynamic self-stiffening in liquid crystal elastomers. Nat. Commun. 2013, 4, 1739. [Google Scholar] [CrossRef]
- Chung, S.W.; Kwak, J.B. A novel evaluation of shock absorption and adhesive strength under shear impact loading. J. Adhes. 2020, 1–17. [Google Scholar] [CrossRef]
- Merkel, D.R.; Shaha, R.K.; Yakacki, C.M.; Frick, C.P. Mechanical energy dissipation in polydomain nematic liquid crystal elastomers in response to oscillating loading. Polymer 2019, 166, 148–154. [Google Scholar] [CrossRef]
- Shaha, R.K.; Merkel, D.R.; Anderson, M.P.; Devereaux, E.J.; Patel, R.R.; Torbati, A.H.; Willett, N.; Yakacki, C.M.; Frick, C.P. Biocompatible liquid-crystal elastomers mimic the intervertebral disc. J. Mech. Behav. Biomed. Mater. 2020, 107, 103757. [Google Scholar] [CrossRef] [PubMed]
- De Gennes, P. Physique moléculaire—Réflexions sur un type de polymères nématiques. CR Acad. Sci. Ser. B 1975, 271, 101–103. [Google Scholar]
- Kim, D.Y.; Jeong, K.U. Light responsive liquid crystal soft matters: Structures, properties, and applications. Liq. Cryst. Today 2019, 28, 34–45. [Google Scholar] [CrossRef]
- Ganicz, T.; Stańczyk, W. Side-Chain Liquid Crystal Polymers (SCLCP): Methods and Materials. An Overview. Materials 2009, 2, 95–128. [Google Scholar] [CrossRef]
- Rogez, D.; Krause, S.; Martinoty, P. Main-chain liquid-crystal elastomers: Versus side-chain liquid-crystal elastomers: Similarities and differences in their mechanical properties. Soft Matter 2018, 14, 6449–6462. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, E.K.; Forst, F.R.; Köder, K.; Kapernaum, N.; Zentel, R. Microactuators from a main-chain liquid crystalline elastomer via thiol-ene “click” chemistry. J. Mater. Chem. C 2013, 1, 5885–5891. [Google Scholar] [CrossRef]
- Beyer, P.; Terentjev, E.M.; Zentel, R. Monodomain liquid crystal main chain elastomers by photocrosslinking. Macromol. Rapid Commun. 2007, 28, 1485–1490. [Google Scholar] [CrossRef]
- Xie, W.; Ouyang, R.; Wang, H.; Li, N.; Zhou, C. Synthesis and cytotoxicity of novel elastomers based on cholesteric liquid crystals. Liq. Cryst. 2020, 47, 449–464. [Google Scholar] [CrossRef]
- Martella, D.; Paoli, P.; Pioner, J.M.; Sacconi, L.; Coppini, R.; Santini, L.; Lulli, M.; Cerbai, E.; Wiersma, D.S.; Poggesi, C.; et al. Liquid Crystalline Networks toward Regenerative Medicine and Tissue Repair. Small 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, L.; Yu, M.; Zhao, D.; Song, P.; Chi, H.; Guo, L.; Yang, H. Liquid Crystal Elastomer Actuators from Anisotropic Porous Polymer Template. Macromol. Rapid Commun. 2017, 38, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Petr, M.; Katzman, B.A.; Dinatale, W.; Hammond, P.T. Synthesis of a new, low- T g siloxane thermoplastic elastomer with a functionalizable backbone and its use as a rapid, room temperature photoactuator. Macromolecules 2013, 46, 2823–2832. [Google Scholar] [CrossRef]
- Mohana, K.; Umadevi, S. Side-chain polysiloxane liquid crystalline elastomers from non-mesogenic components. New J. Chem. 2019, 43, 15968–15978. [Google Scholar] [CrossRef]
- Jia, Y.G.; Zhang, B.Y.; Tian, M.; Pan, W. Synthesis and structure of polysiloxane liquid crystalline elastomers with a mesogenic crosslinking agent. J. Appl. Polym. Sci. 2004, 93, 1736–1742. [Google Scholar] [CrossRef]
- Thomsen, D.L.; Keller, P.; Naciri, J.; Pink, R.; Jeon, H.; Shenoy, D.; Ratna, B.R. Liquid crystal elastomers with mechanical properties of a muscle. Macromolecules 2001, 34, 5868–5875. [Google Scholar] [CrossRef]
- Legge, C.; Davis, F.; Mitchell, G. Memory effects in liquid crystal elastomers. J. Phys. II Fr. 1991, 1, 1253–1261. [Google Scholar] [CrossRef]
- Chambers, M.; Finkelmann, H.; Remkar, M.; Sánchez-Ferrer, A.; Zalar, B.; Umer, S. Liquid crystal elastomer-nanoparticle systems for actuation. J. Mater. Chem. 2009, 19, 1524–1531. [Google Scholar] [CrossRef]
- Urayama, K.; Honda, S.; Takigawa, T. Deformation coupled to director rotation in swollen nematic elastomers under electric fields. Macromolecules 2006, 39, 1943–1949. [Google Scholar] [CrossRef]
- Seki, T. New strategies and implications for the photoalignment of liquid crystalline polymers. Polym. J. 2014, 46, 751–768. [Google Scholar] [CrossRef]
- Davidson, Z.S.; Shahsavan, H.; Aghakhani, A.; Guo, Y.; Hines, L.; Xia, Y.; Yang, S.; Sitti, M. Monolithic shape-programmable dielectric liquid crystal elastomer actuators. Sci. Adv. 2019, 5, eaay0855. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Saed, M.; Nair, D.P.; Gong, T.; Reed, S.M.; Bowman, C.N. Tailorable and programmable liquid-crystalline elastomers using a two-stage thiol-acrylate reaction. RSC Adv. 2015, 5, 18997–19001. [Google Scholar] [CrossRef]
- Zeng, H.; Wasylczyk, P.; Cerretti, G.; Martella, D.; Parmeggiani, C.; Wiersma, D.S. Alignment engineering in liquid crystalline elastomers: Free-form microstructures with multiple functionalities. Appl. Phys. Lett. 2015, 106, 111902. [Google Scholar] [CrossRef]
- Wang, Z.; He, Q.; Wang, Y.; Cai, S. Programmable actuation of liquid crystal elastomers: Via “living” exchange reaction. Soft Matter 2019, 15, 2811–2816. [Google Scholar] [CrossRef] [PubMed]
- Jampani, V.S.R.; Volpe, R.H.; De Sousa, K.R.; Machado, J.F.; Yakacki, C.M.; Lagerwall, J.P.F. Liquid crystal elastomer shell actuators with negative order parameter. Sci. Adv. 2019, 5, eaaw2476. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.P.; Vollrath, F. Biological liquid crystal elastomers. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Woodhead-Galloway, J.; Knight, D.P. Some observations on the fine structure of elastoidin. Proc. R. Soc. Lond. B 1977, 195, 355–364. [Google Scholar] [PubMed]
- Hukins, D.W.; Woodhead-Galloway, J.; Knight, D.P. Molecular tilting in dried elastoiding and its implications for the structures of other collagen fibrils. Biochem. Biophys. Res. Commun. 1976, 73, 1049–1055. [Google Scholar] [CrossRef]
- Hukins, D.W.L.; Woodhead-Galloway, J. Collagen Fibrils as Examples of Smectic a Biological Fibres. Mol. Cryst. Liq. Cryst. 1977, 41, 33–39. [Google Scholar] [CrossRef]
- Martin, R.; Farjanel, J.; Eichenberger, D.; Colige, A.; Kessler, E.; Hulmes, D.J.S.; Giraud-Guille, M.M. Liquid crystalline ordering of procollagen as a determinant of three-dimensional extracellular matrix architecture. J. Mol. Biol. 2000, 301, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Misof, K.; Rapp, G.; Fratzl, P. A new molecular model for collagen elasticity based on synchrotron x- ray scattering evidence. Biophys. J. 1997, 72, 1376–1381. [Google Scholar] [CrossRef]
- Eisoldt, L.; Smith, A.; Scheibel, T. Decoding the secrets of spider silk. Mater. Today 2011, 14, 80–86. [Google Scholar] [CrossRef]
- Vehoff, T.; Glišović, A.; Schollmeyer, H.; Zippelius, A.; Salditt, T. Mechanical properties of spider dragline silk: Humidity, hysteresis, and relaxation. Biophys. J. 2007, 93, 4425–4432. [Google Scholar] [CrossRef]
- Vollrath, F.; Knight, D.P. Liquid crystal spinning of spider silk. Nature 2002, 410, 541–548. [Google Scholar]
- Römer, L.; Scheibel, T. The elaborate structure of spider silk: Structure and function of a natural high performance fiber. Prion 2008, 2, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Grubb, D.T.; Jelinsk, L.W. Small-angle X-ray scattering of spider dragline silk. Macromolecules 1997, 30, 8254–8261. [Google Scholar] [CrossRef]
- Lazaris, A.; Arcidiacono, S.; Huang, Y.; Zhou, J.F.; Duguay, F.; Chretien, N.; Welsh, E.A.; Soares, J.W.; Karatzas, C.N. Spider silk fibers spun from soluble recombinant silk produced in mammalian cells. Science 2002, 295, 472–476. [Google Scholar] [CrossRef]
- Rey, A.D.; Herrera-Valencia, E.E. Invited review: Liquid crystal models of biological materials and silk spinning. Biopolymers 2012, 97, 374–396. [Google Scholar] [CrossRef] [PubMed]
- Afzal, Z.R.; Prabhakar, P.; Prabhakar, P. Optimal tool path planning for 3D printing with spatio-temporal and thermal constraints. In Proceedings of the 2019 6th Indian Control Conference (ICC), Hyderabad, India, 18–20 December 2019; pp. 176–181. [Google Scholar]
- Gu, G.X.; Su, I.; Sharma, S.; Voros, J.L.; Qin, Z.; Buehler, M.J. Three-dimensional-printing of bio-inspired composites. J. Biomech. Eng. 2016, 138, 1–16. [Google Scholar]
- Lefèvre, T.; Auger, M. Spider silk inspired materials and sustainability: Perspective. Mater. Technol. 2016, 31, 384–399. [Google Scholar] [CrossRef]
- Shang, L.; Yu, Y.; Liu, Y.; Chen, Z.; Kong, T.; Zhao, Y. Spinning and Applications of Bioinspired Fiber Systems. ACS Nano 2019, 13, 2749–2772. [Google Scholar] [CrossRef]
- Ling, S.; Qin, Z.; Li, C.; Huang, W.; Kaplan, D.L.; Buehler, M.J. Polymorphic regenerated silk fibers assembled through bioinspired spinning. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bowen, C.H.; Dai, B.; Sargent, C.J.; Bai, W.; Ladiwala, P.; Feng, H.; Huang, W.; Kaplan, D.L.; Galazka, J.M.; Zhang, F. Recombinant Spidroins Fully Replicate Primary Mechanical Properties of Natural Spider Silk. Biomacromolecules 2018, 19, 3853–3860. [Google Scholar] [CrossRef] [PubMed]
- Jansson, R.; Lau, C.H.; Ishida, T.; Ramström, M.; Sandgren, M.; Hedhammar, M. Functionalized silk assembled from a recombinant spider silk fusion protein (Z-4RepCT) produced in the methylotrophic yeast Pichia pastoris. Biotechnol. J. 2016, 11, 687–699. [Google Scholar] [CrossRef]
- Menassa, R.; Zhu, H.; Karatzas, C.N.; Lazaris, A.; Richman, A.; Brandle, J. Spider dragline silk proteins in transgenic tobacco leaves: Accumulation and field production. Plant Biotechnol. J. 2004, 2, 431–438. [Google Scholar] [CrossRef]
- Grip, S.; Rising, A.; Nimmervoll, H.; Storckenfeldt, E.; Mcqueen-Mason, S.J.; Pouchkina-Stantcheva, N.; Vollrath, F.; Engström, W.; Fernandez-Arias, A. Transient expression of a major ampullate spidroin 1 gene fragment from Euprosthenops sp. in mammalian cells. Cancer Genom. Proteom. 2006, 3, 83–88. [Google Scholar]
- Service, R. Mammalian Cells Spin A Spidery New Yarn. Sciencemag 2002, 295, 64–65. [Google Scholar]
- Teague, B.P.; Guye, P.; Weiss, R. Synthetic morphogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, 1–15. [Google Scholar] [CrossRef]
- Aharoni, H.; Xia, Y.; Zhang, X.; Kamien, R.D.; Yang, S. Universal inverse design of surfaces with thin nematic elastomer sheets. Proc. Natl. Acad. Sci. USA 2018, 115, 7206–7211. [Google Scholar] [CrossRef]
- Lavrentovich, O.D. Prepatterned liquid crystal elastomers as a step toward artificial morphogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, 7171–7173. [Google Scholar] [CrossRef]
- De Haan, L.T.; Gimenez-Pinto, V.; Konya, A.; Nguyen, T.S.; Verjans, J.M.N.; Sánchez-Somolinos, C.; Selinger, J.V.; Selinger, R.L.B.; Broer, D.J.; Schenning, A.P.H.J. Accordion-like actuators of multiple 3D patterned liquid crystal polymer films. Adv. Funct. Mater. 2014, 24, 1251–1258. [Google Scholar] [CrossRef]
- Guin, T.; Settle, M.J.; Kowalski, B.A.; Auguste, A.D.; Beblo, R.V.; Reich, G.W.; White, T.J. Layered liquid crystal elastomer actuators. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, B.A.; Mostajeran, C.; Godman, N.P.; Warner, M.; White, T.J. Curvature by design and on demand in liquid crystal elastomers. Phys. Rev. E 2018, 97, 1–5. [Google Scholar] [CrossRef] [PubMed]
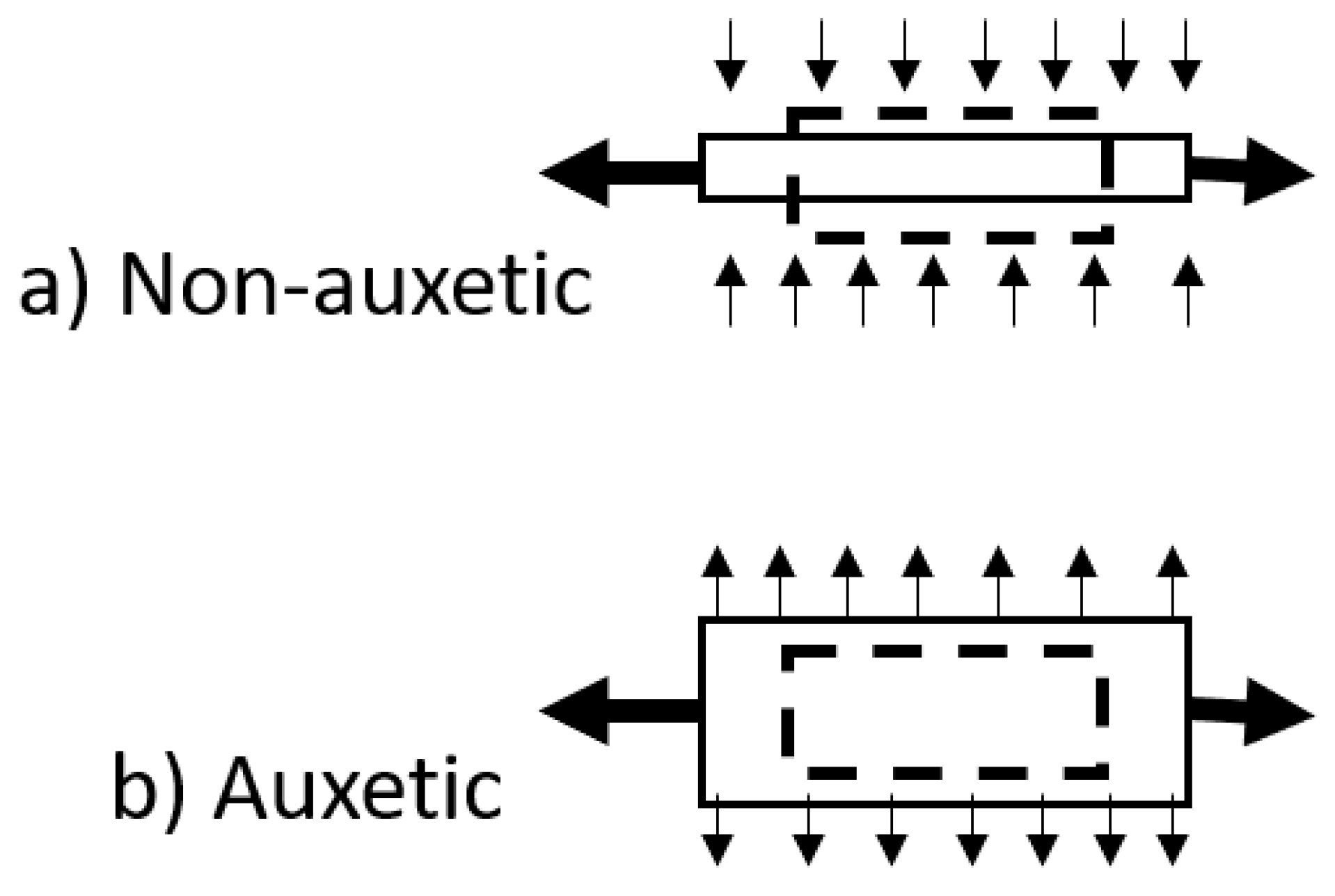
- Huang, C.; Chen, L. Negative Poisson’s Ratio in Modern Functional Materials. Adv. Mater. 2016, 28, 8079–8096. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Li, T.; Cao, S.; Wang, L. Hoberman-sphere-inspired lattice metamaterials with tunable negative thermal expansion. Compos. Struct. 2018, 189, 586–597. [Google Scholar] [CrossRef]
- Novak, N.; Vesenjak, M.; Ren, Z. Auxetic Cellular Materials—A Review. Stroj. Vestn. J. Mech. Eng. 2016, 62, 485–493. [Google Scholar] [CrossRef]
- Baughman, R.H.; Shacklette, J.M.; Zakhidov, A.A.; Stafstro, S. Negative Poisson′s ratios as a common feature of cubic metals. Nature 1998, 392, 362–365. [Google Scholar] [CrossRef]
- Grima, J.N.; Gatt, R.; Zammit, V.; Williams, J.J.; Evans, K.E.; Alderson, A.; Walton, R.I. Natrolite: A zeolite with negative Poisson′s ratios. J. Appl. Phys. 2007, 101, 101–104. [Google Scholar] [CrossRef]
- Yeganeh-Haeri, A.; Weidner, D.J.; Parise, J.B. Elasticity of α-cristobalite: A silicon dioxide with a negative poisson′s ratio. Science 1992, 257, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.; Vincent, J.F.; Hillerton, J.E. Poisson′s ratio in skin. Biomed. Mater. Eng. 1991, 1, 19–23. [Google Scholar]
- Veronda, D.R.; Westmann, R.A. Mechanical characterization of skin-Finite deformations. J. Biomech. 1970, 3, 111–124. [Google Scholar] [CrossRef]
- Williams, J.L.; Lewis, J.L. Properties and an anisotropic model of cancellous bone from the proximal tibial epiphysis. J. Biomech. Eng. 1982, 104, 50–56. [Google Scholar] [CrossRef]
- Gatt, R.; Vella Wood, M.; Gatt, A.; Zarb, F.; Formosa, C.; Azzopardi, K.M.; Casha, A.; Agius, T.P.; Schembri-Wismayer, P.; Attard, L.; et al. Negative Poisson’s ratios in tendons: An unexpected mechanical response. Acta Biomater. 2015, 24, 201–208. [Google Scholar] [CrossRef]
- Mardling, P.; Alderson, A.; Jordan-Mahy, N.; le Maitre, C.L. The use of auxetic materials in tissue engineering. Biomater. Sci. 2020, 8, 2074–2083. [Google Scholar] [CrossRef]
- Alderson, K.L.; Pickles, A.P.; Neale, P.J.; Evans, K.E. Auxetic polyethylene: The effect of a negative poisson′s ratio on hardness. Acta Metall. Mater. 1994, 42, 2261–2266. [Google Scholar] [CrossRef]
- Verma, P.; Shofner, M.L.; Griffin, A.C. Deconstructing the auxetic behavior of paper. Phys. Status Solidi Basic Res. 2014, 251, 289–296. [Google Scholar] [CrossRef]
- Domaschke, S.; Morel, A.; Fortunato, G.; Ehret, A.E. Random auxetics from buckling fibre networks. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Higuchi, J.; Fortunato, G.; Wozniak, B.; Chodara, A.; Lojkowski, W. Biological Barrier Membrane. WO 2020/085927 A1, 30 April 2020. [Google Scholar]
- Kim, H.W.; Kim, T.Y.; Park, H.K.; You, I.; Kwak, J.; Kim, J.C.; Hwang, H.; Kim, H.S.; Jeong, U. Hygroscopic Auxetic On-Skin Sensors for Easy-to-Handle Repeated Daily Use. ACS Appl. Mater. Interfaces 2018, 10, 40141–40148. [Google Scholar] [CrossRef]
- Umakiran, T.; Kumar, A. Auxetic Textile and its Applications. Man Made Text. India 2009, 52, 265. [Google Scholar]
- Evans, K.E.; Alderson, K.L. Auxetic materials: The positive side of being negative. Eng. Sci. Educ. J. 2000, 9, 148–154. [Google Scholar] [CrossRef]
- Mcmullan, P.J.; Kumar, S.; Griffin, A.C. NTC Project: M04-GT21 Textile Fibres Engineered From Molecular Auxetic Polymers National Textile Center Annual Report; National Textile Center: Philadelphia, PA, USA, 2005; pp. 1–10. [Google Scholar]
- Küpfer, J.; Finkelnann, H. Nematic liquid single crystal elastomers. Makromol. Chem. Rapid Commun. 1991, 12, 717–726. [Google Scholar] [CrossRef]
- Li, M.H.; Keller, P.; Antonietti, M.; Lacey, D.; Meyer, R.B. Artificial muscles based on liquid crystal elastomers. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 2763–2777. [Google Scholar] [CrossRef]
- Ambrogi, V.; Giamberini, M.; Cerruti, P.; Pucci, P.; Menna, N.; Mascolo, R.; Carfagna, C. Liquid crystalline elastomers based on diglycidyl terminated rigid monomers and aliphatic acids. Part 1. Synthesis and characterization. Polymer 2005, 46, 2105–2121. [Google Scholar] [CrossRef]
- Boothby, J.M.; Kim, H.; Ware, T.H. Shape changes in chemoresponsive liquid crystal elastomers. Sens. Actuators B Chem. 2017, 240, 511–518. [Google Scholar] [CrossRef]
- Michal, B.T.; McKenzie, B.M.; Felder, S.E.; Rowan, S.J. Metallo-, thermo-, and photoresponsive shape memory and actuating liquid crystalline elastomers. Macromolecules 2015, 48, 3239–3246. [Google Scholar] [CrossRef]
- Ferrantini, C.; Pioner, J.M.; Martella, D.; Coppini, R.; Piroddi, N.; Paoli, P.; Calamai, M.; Pavone, F.S.; Wiersma, D.S.; Tesi, C.; et al. Development of Light-Responsive Liquid Crystalline Elastomers to Assist Cardiac Contraction. Circ. Res. 2019, 124, e44–e54. [Google Scholar] [CrossRef]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases 2012, 7, 1–27. [Google Scholar] [CrossRef]
- Bera, T.; Malcuit, C.; Clements, R.J.; Hegmann, E. Role of surfactant during microemulsion photopolymerization for the creation of three-dimensional liquid crystal elastomer microsphere spatial cell scaffolds. Front. Mater. 2016, 3, 1–8. [Google Scholar] [CrossRef][Green Version]
- DiRienzo, A.L.; Yakacki, C.M.; Frensemeier, M.; Schneider, A.S.; Safranski, D.L.; Hoyt, A.J.; Frick, C.P. Porous poly(para-phenylene) scaffolds for load-bearing orthopedic applications. J. Mech. Behav. Biomed. Mater. 2014, 30, 347–357. [Google Scholar] [CrossRef]
- Naciri, J.; Srinivasan, A.; Jeon, H.; Nikolov, N.; Keller, P.; Ratna, B.R. Nematic elastomer fiber actuator. Macromolecules 2003, 36, 8499–8505. [Google Scholar] [CrossRef]
- Sharma, A.; Neshat, A.; Mahnen, C.J.; Nielsen, A.D.; Snyder, J.; Stankovich, T.L.; Daum, B.G.; Laspina, E.M.; Beltrano, G.; Gao, Y.; et al. Biocompatible, biodegradable and porous liquid crystal elastomer scaffolds for spatial cell cultures. Macromol. Biosci. 2015, 15, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Turiv, T.; Krieger, J.; Babakhanova, G.; Yu, H.; Shiyanovskii, S.V.; Wei, Q.H.; Kim, M.H.; Lavrentovich, O.D. Topology control of human cells monolayer by liquid crystal elastomer. arXiv 2020, arXiv:2003.12648. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.; Jull, E.I.L.; Mandle, R.J.; Raistrick, T.; Hine, P.J.; Gleeson, H.F. Liquid Crystal Elastomers for Biological Applications. Nanomaterials 2021, 11, 813. https://doi.org/10.3390/nano11030813
Hussain M, Jull EIL, Mandle RJ, Raistrick T, Hine PJ, Gleeson HF. Liquid Crystal Elastomers for Biological Applications. Nanomaterials. 2021; 11(3):813. https://doi.org/10.3390/nano11030813
Chicago/Turabian StyleHussain, Mariam, Ethan I. L. Jull, Richard J. Mandle, Thomas Raistrick, Peter J. Hine, and Helen F. Gleeson. 2021. "Liquid Crystal Elastomers for Biological Applications" Nanomaterials 11, no. 3: 813. https://doi.org/10.3390/nano11030813
APA StyleHussain, M., Jull, E. I. L., Mandle, R. J., Raistrick, T., Hine, P. J., & Gleeson, H. F. (2021). Liquid Crystal Elastomers for Biological Applications. Nanomaterials, 11(3), 813. https://doi.org/10.3390/nano11030813







