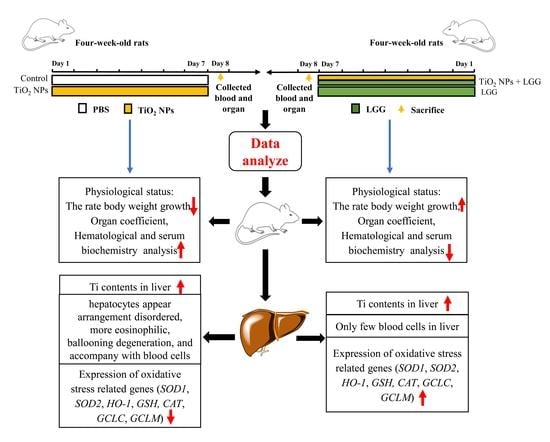
Protective Effect of Lactobacillus rhamnosus GG on TiO2 Nanoparticles-Induced Oxidative Stress Damage in the Liver of Young Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of TiO2 NPs
2.2. Probiotics Preparation
2.3. Animals Administration
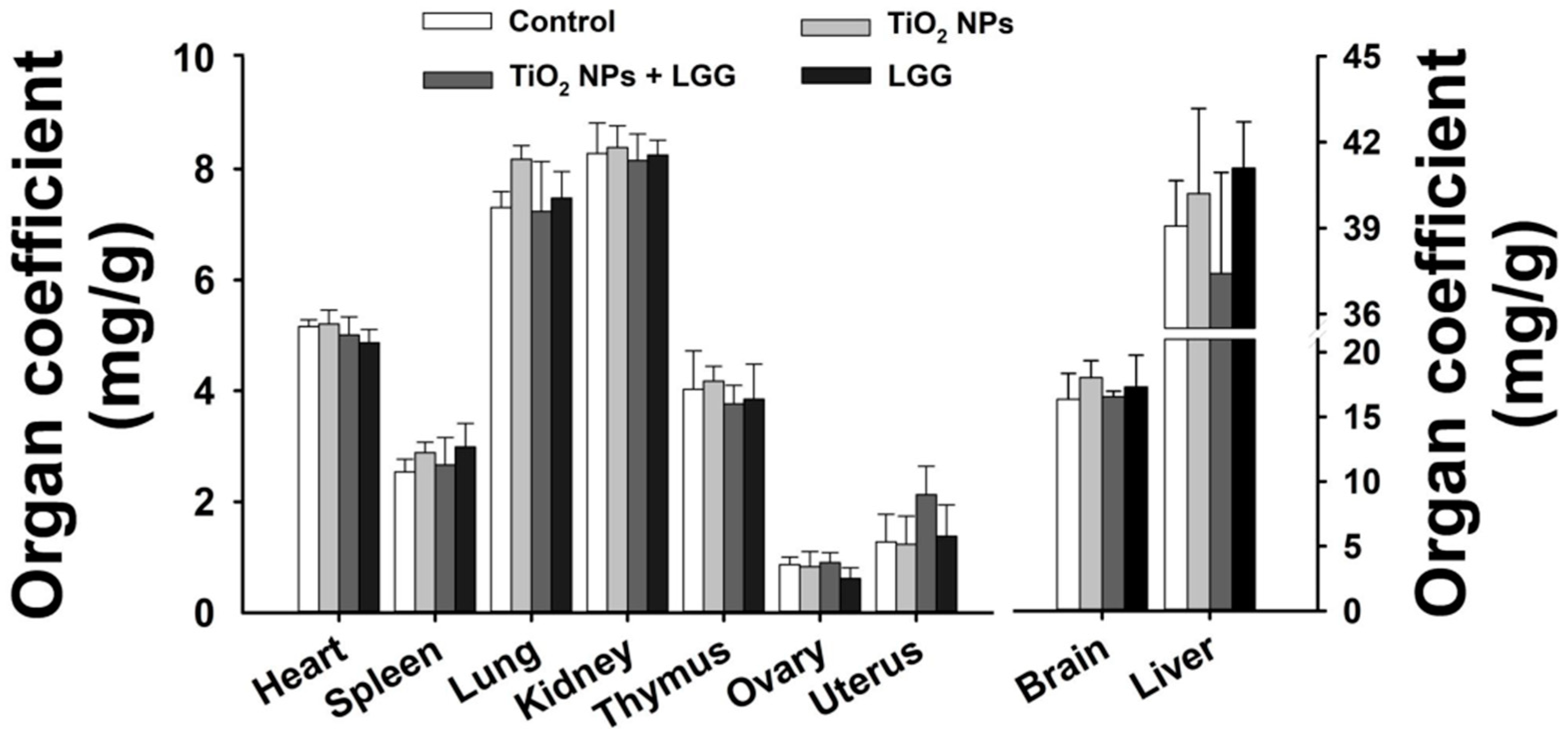
2.4. Organ Coefficient
2.5. Analysis of Hematology
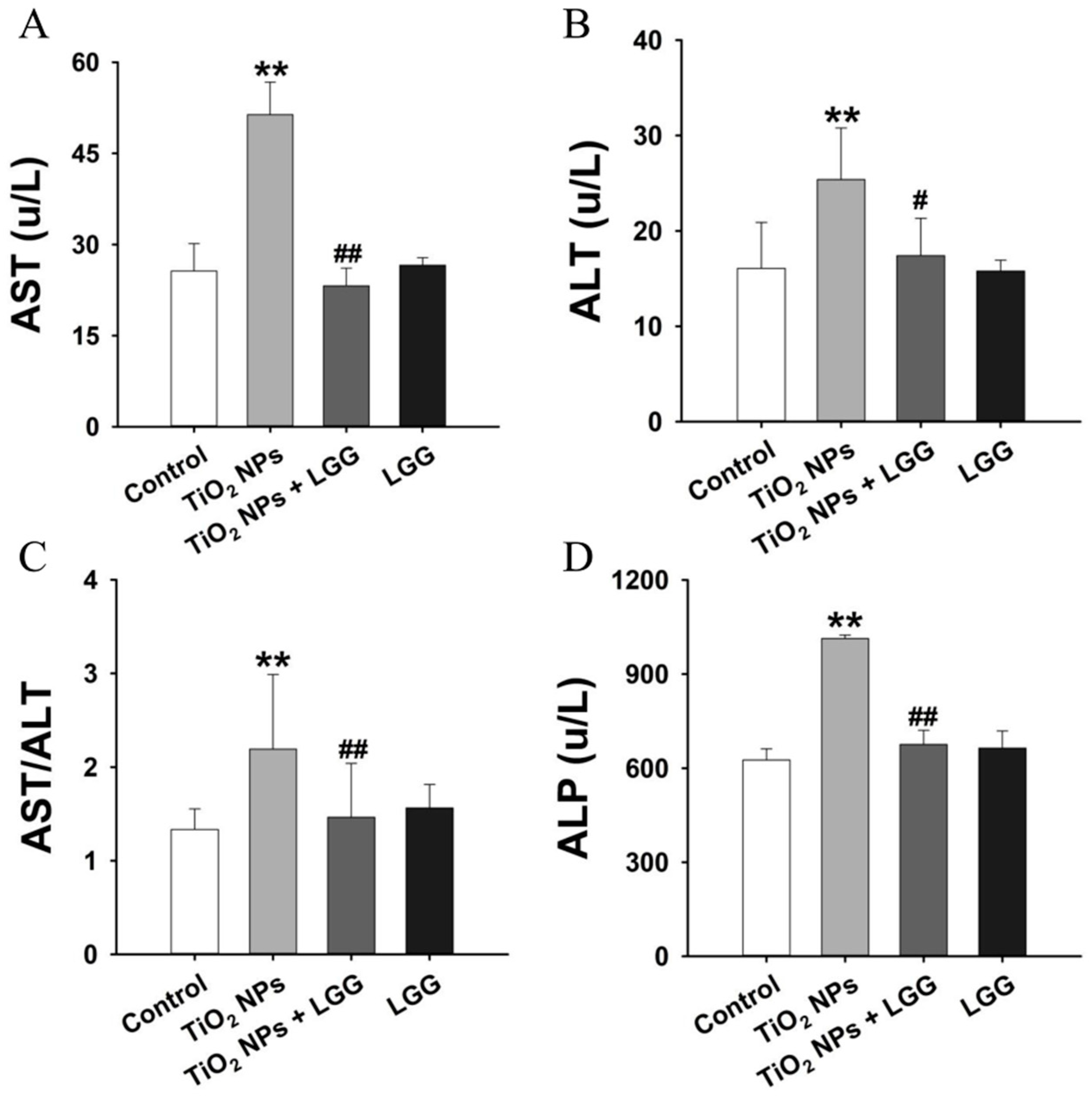
2.6. Evaluation of Serum Biochemistry
2.7. Analysis Contents of Ti
2.8. Histopathological Examination of Liver
2.9. Analysis of Gene Expression
2.10. Statistical Analysis
3. Results
3.1. Characterization of TiO2 NPs
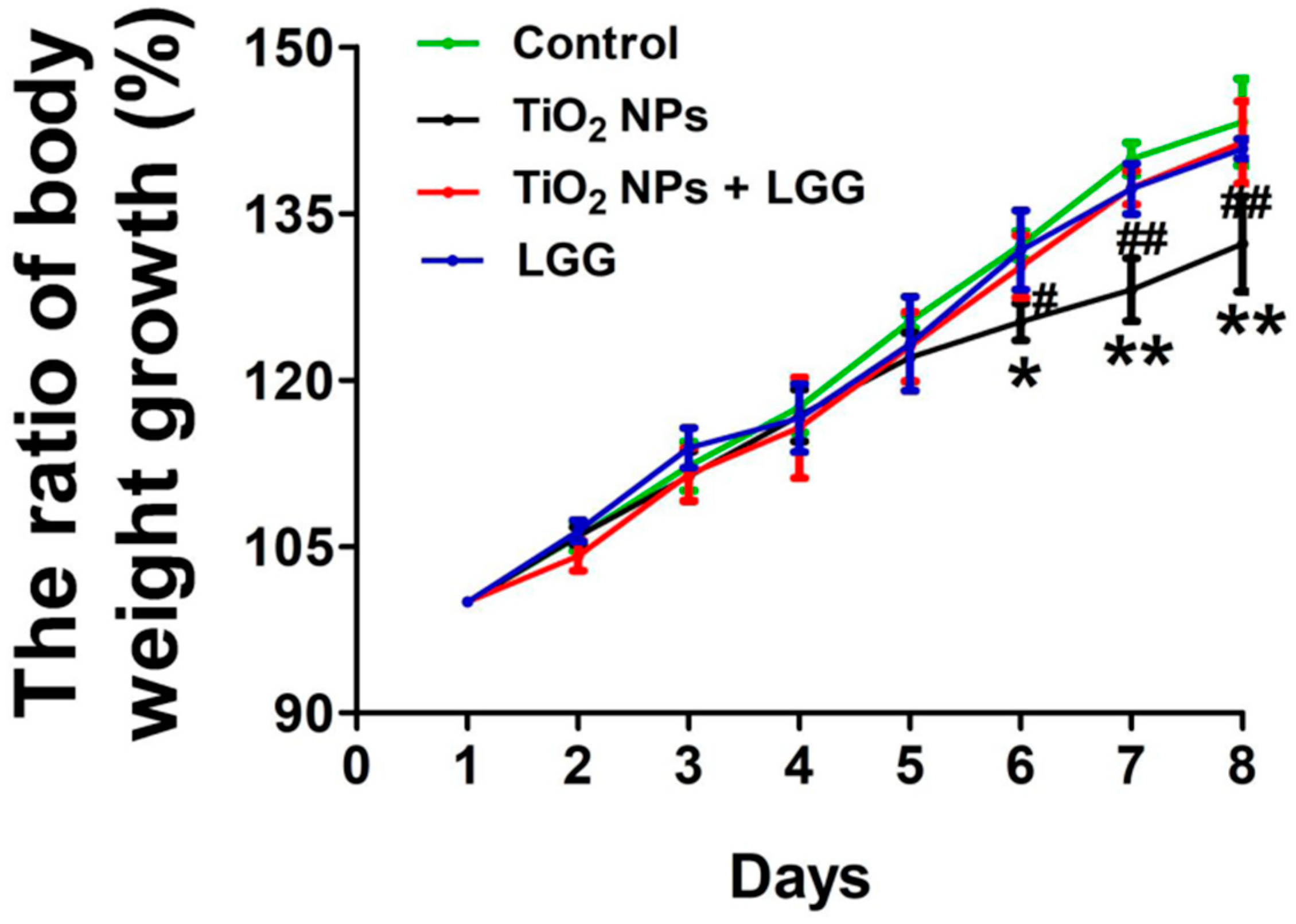
3.2. Body Weight Changes in Young Rats
3.3. Organ Coefficient
3.4. Hematology
3.5. Serum Biochemistry
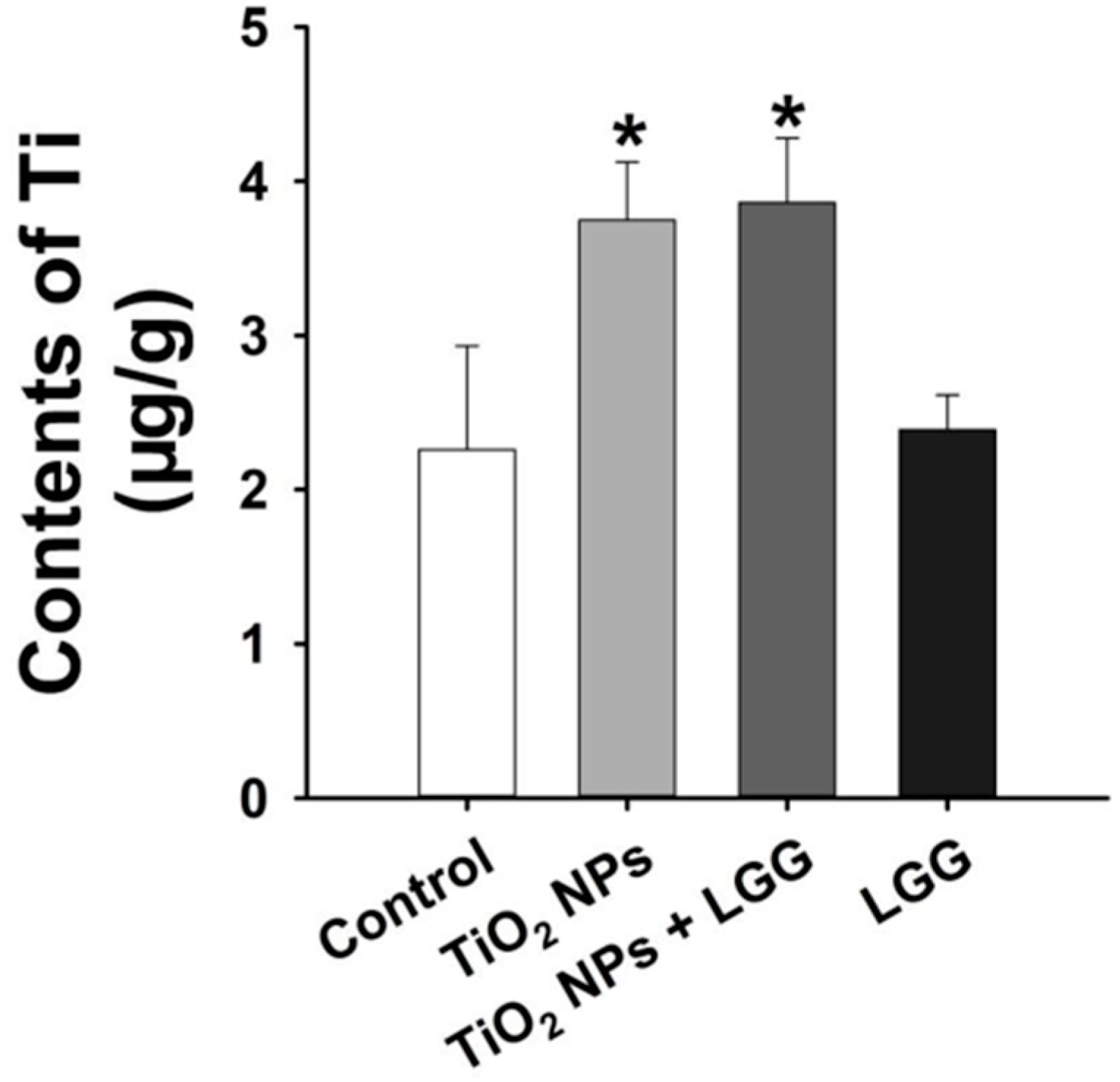
3.6. Ti Contents in the Liver
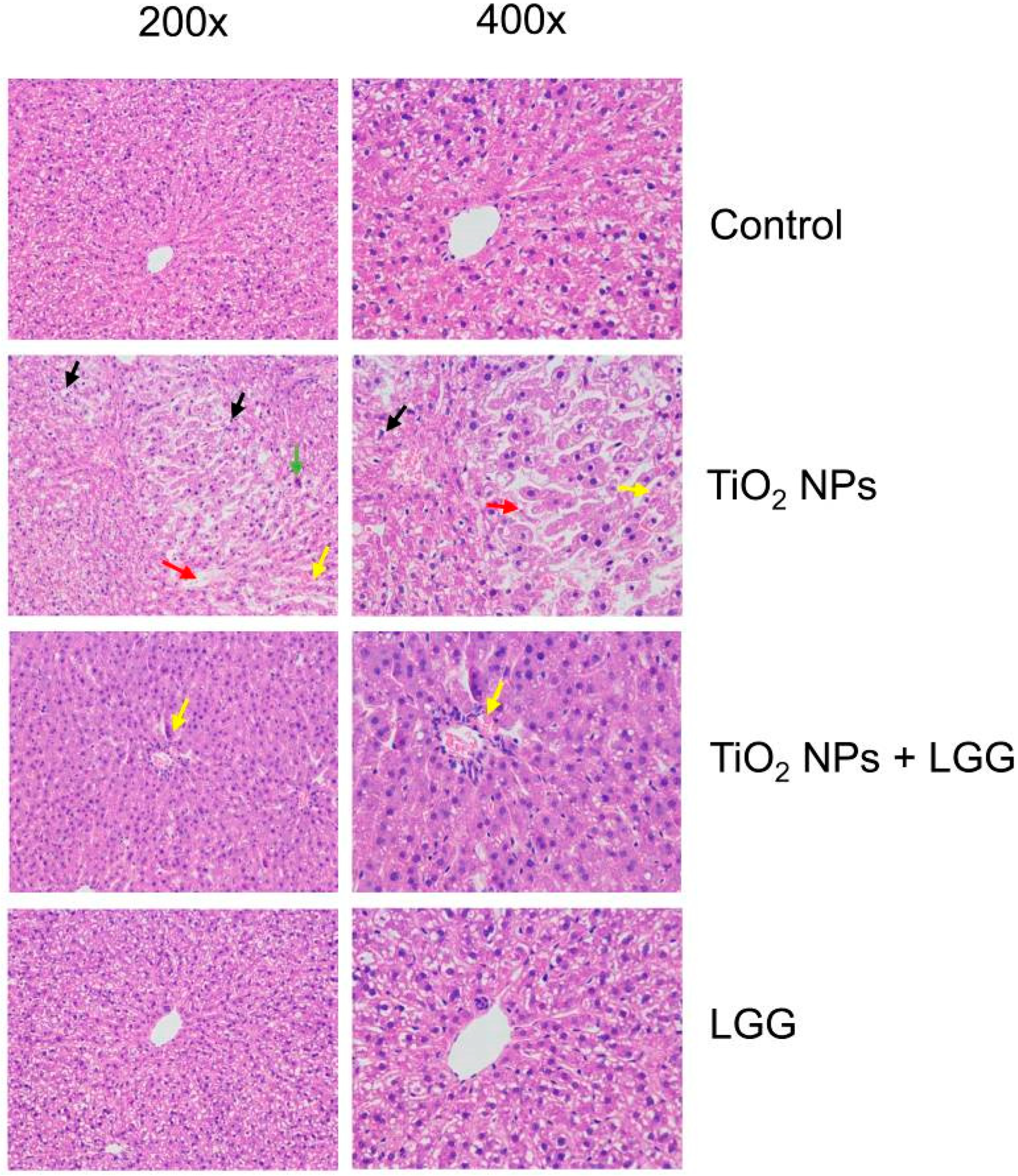
3.7. Histopathological Evaluation
3.8. Levels of Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Peters, R.J.; Bouwmeester, H.; Gottardo, S.; Amenta, V.; Arena, M.; Brandhoff, P.; Marvin, H.J.; Mech, A.; Moniz, F.B.; Pesudo, L.Q.; et al. Nanomaterials for products and application in agriculture, feed and food. Trends Food Sci. Technol. 2016, 54, 155–164. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymer 2020, 12, 1397. [Google Scholar] [CrossRef]
- Chandra, H.; Singh, C.; Kumari, P.; Yadav, S.; Mishra, A.P.; Laishevtcev, A.; Brisc, C.; Brisc, M.C.; Munteanu, M.A.; Bungau, S. Promising Roles of Alternative Medicine and Plant-Based Nanotechnology as Remedies for Urinary Tract Infections. Molecules 2020, 25, 5593. [Google Scholar] [CrossRef]
- Santos, B.T.; Pérez, C.F.; Bourzac, J.F.I.; Nápoles, Y.M.; González, W.R.; Bourg, V.; Torralba, D.; Pérez, V.; Mouriño, A.; Ayala, J.; et al. Remote induction of cellular immune response in mice by anti-meningococcal nanocochleates-nanoproteoliposomes. Sci. Total Environ. 2019, 668, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Chen, J.; Wang, M.; Sheng, X.; Chen, X.; Feng, X.; Mao, S.S. Titanium dioxide nanostructures for photoelectrochemical applications. Prog. Mater. Sci. 2018, 98, 299–385. [Google Scholar] [CrossRef]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent Progress on Titanium Dioxide Nanomaterials for Photocatalytic Applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef] [PubMed]
- Yemmireddy, V.K.; Hung, Y.C. Selection of photocatalytic bactericidal titanium dioxide (TiO2) nanopar-ticles for food safety applications. LWT Food Sci. Technol. 2015, 61, 1–6. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Lee, B.W.; Okada, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; et al. Comparison of pulmonary inflammatory responses following intratracheal instillation and inhalation of nanopar-ticles. Nanotoxicology 2016, 10, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, Y.; Chen, Z.; Xu, H.; Zhou, J.; Tang, S.; Xu, Z.; Kong, F.; Li, X.; Zhang, Y.; et al. Cardiopulmonary effects induced by occupational exposure to titanium dioxide nanoparticles. Nanotoxicology 2018, 12, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, S.; Zhou, L.; Sun, L. The Potential Liver, Brain, and Embryo Toxicity of Titanium Dioxide Nanoparticles on Mice. Nanoscale Res. Lett. 2017, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yin, S.; Tang, M.; Chen, J.; Yang, Z.; Zhang, W.; Chen, L.; Yang, B.; Li, Z.; Zha, Y.; et al. Effects of Developmental Exposure to TiO2 Nanoparticles on Synaptic Plasticity in Hippocampal Dentate Gyrus Area: An In Vivo Study in Anesthetized Rats. Biol. Trace Elem. Res. 2011, 143, 1616–1628. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Pu, Y.; Yin, L.; Li, Y.; Zhang, X.; Liang, G.; Li, X.; Zhang, J. Small-sized titanium dioxide nanoparticles mediate immune toxicity in rat pulmonary alveolar macrophages in vivo. J. Nanosci. Nanotechnol. 2010, 10, 5161–5169. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, Y.-Q. Murine liver damage caused by exposure to nano-titanium dioxide. Nanotechnol. 2016, 27, 112001. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.-J.; Ba, T.; Pu, J.; Cui, X.-X.; Jia, G. Effects of TiO2; nanoparticles on antioxidant function and element content of liver and kidney tissues in young and adult rats. Beijing Da Xue Xue Bao Yi Xue Ban = J. Peking Univ. Health Sci. 2014, 46, 395–399. [Google Scholar]
- Wang, Y.; Chen, Z.; Ba, T.; Pu, J.; Chen, T.; Song, Y.; Gu, Y.; Qian, Q.; Xu, Y.; Xiang, K.; et al. Susceptibility of Young and Adult Rats to the Oral Toxicity of Titanium Dioxide Nanoparticles. Small 2013, 9, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, B.; Li, L.; Guo, Q.; Yang, D.; Wei, X.; Fan, X.; Liu, J.; Wu, Q.; Oh, Y.; et al. The toxic effects of titanium dioxide nanoparticles on plasma glucose metabolism are more severe in developing mice than in adult mice. Environ. Toxicol. 2019, 35, 443–456. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Assadian, E.; Dezhampanah, H.; Seydi, E.; Pourahmad, J. Toxicity of Fe2O3 nanoparticles on human blood lymphocytes. J. Biochem. Mol. Toxicol. 2019, 33, e22303. [Google Scholar] [CrossRef]
- Saliani, M.; Jalal, R.; Goharshadi, E.K. Mechanism of oxidative stress involved in the toxicity of ZnO nanoparticles against eukaryotic cells. Nanomed. J. 2016, 3, 1–14. [Google Scholar]
- Shukla, R.K.; Kumar, A.; Vallabani, N.V.S.; Pandey, A.K.; Dhawan, A. Titanium dioxide nanoparticle-induced oxidative stress triggers DNA damage and hepatic injury in mice. Nanomedicine 2014, 9, 1423–1434. [Google Scholar] [CrossRef]
- Azim, S.A.A.; Darwish, H.A.; Rizk, M.Z.; Ali, S.A.; Kadry, M.O. Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: Possible role of some antioxi-dants-ScienceDirect. Exp. Toxicol. Pathol. 2015, 67, 305–314. [Google Scholar] [CrossRef]
- Shen, Q.; Shang, N.; Li, P. In Vitro and In Vivo Antioxidant Activity of Bifidobacterium animalis 01 Isolated from Centenarians. Curr. Microbiol. 2010, 62, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wang, Z.; Zhang, Y.; Zhang, J.; Wang, L.; Dong, X.; Su, F.; Yao, G.; Wang, S.; Zhang, H. Effect ofLactobacillus plantarumP-8 on lipid metabolism in hyperlipidemic rat model. Eur. J. Lipid Sci. Technol. 2012, 114, 1230–1236. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Huys, G.; Daube, G. Probiotics: An update. J. de Pediatr. 2015, 91, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, Y.; Chen, L.; Lv, S.; Liu, S.; Nie, P.; Aguilar, Z.P.; Xu, H. Restraining the TiO2 nanoparticles-induced intestinal inflammation mediated by gut microbiota in juvenile rats via ingestion of Lactobacillus rhamnosus GG. Ecotoxicol. Environ. Saf. 2020, 206, 111393. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.; Echeverría, G.; Viñas, I.; López, M.; Abadias, M. Biopreservation of fresh-cut pear using Lactobacillus rhamnosus GG and effect on quality and volatile compounds. LWT 2018, 87, 581–588. [Google Scholar] [CrossRef]
- Jia, Z.; Pang, X.; Lv, J. Reduced-Fat Response of Lactobacillus casei subsp. casei SY13 on a Time and Dose-Dependent Model. Front. Microbiol. 2018, 9, 3200. [Google Scholar] [CrossRef] [PubMed]
- Casotti, V.; D’Antiga, L. Basic principles of liver physiology. In Pediatric Hepatology and Liver Transplantation; Springer: Berlin, Germany, 2019; pp. 21–39. [Google Scholar]
- Mossa, A.T.H.; Refaie, A.A.; Ramadan, A.; Bouajila, J. Amelioration of Prallethrin-Induced Oxidative Stress and Hepatotoxicity in Rat by the Administration of Ori-ganum majorana Essential Oil. BioMed Res. Int. 2013, 2013, 859085. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shi, H.; Ruth, M.; Yu, H.; Lazar, L.; Zou, B.; Yang, C.; Wu, A.; Zhao, J. Acute Toxicity of Intravenously Administered Titanium Dioxide Nanoparticles in Mice. PLoS ONE 2013, 8, e70618. [Google Scholar] [CrossRef] [PubMed]
- Ashar, W.M.P.; Muthu, M.H.S. Fenvalerate induced hepatotoxicity and its amelioration by quercetin. Int. J. Pharm. Res. 2012, 4, 1391–1400. [Google Scholar]
- Esmaeillou, M.; Moharamnejad, M.; Hsankhani, R.; Tehrani, A.A.; Maadi, H. Toxicity of ZnO nanoparticles in healthy adult mice. Environ. Toxicol. Pharmacol. 2013, 35, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-X.; Lv, Y.; Xu, A.-M.; Wang, H.-Z. The prognostic significance of serum gamma-glutamyltransferase levels and AST/ALT in primary hepatic carcinoma. BMC Cancer 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Gratz, S.W.; Mykkanen, H.; El-Nezami, H.S. Probiotics and gut health: A special focus on liver diseases. Word J. Gastroenterol. 2010, 16, 403–410. [Google Scholar] [CrossRef]
- Bouhafs, L.; Moudilou, E.N.; Exbrayat, J.M.; Lahouel, M.; Idoui, T. Protective effects of probioticLactobacillus plantarumBJ0021 on liver and kidney oxidative stress and apoptosis induced by endosulfan in pregnant rats. Ren. Fail. 2015, 37, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, K.M.; El-Amir, Y.O. Protective effects of thymoquinone and avenanthramides on titanium dioxide nanoparticles induced toxicity in Sprague-Dawley rats. Pathol. Res. Pract. 2017, 213, 13–22. [Google Scholar] [CrossRef]
- Suker, D.K.; Jasim, F.A. Liver histopathological alteration after repeated intra-tracheal instillation of titanium dioxide in male rats. Gastroenterol. Hepatol. Bed Bench 2018, 11, 159–168. [Google Scholar]
- Yao, L.; Chen, L.; Chen, B.; Tang, Y.; Zhao, Y.; Liu, S.; Xu, H. Toxic effects of TiO2 NPs in the blood-milk barrier of the maternal dams and growth of offspring. Ecotoxicol. Environ. Saf. 2021, 208, 111762. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Kirpich, I.; Ma, Z.; Wang, C.; Zhang, M.; Suttles, J.; McClain, C.; Feng, W. Lactobacillus rhamnosus GG reduces hepatic TNFα production and inflammation in chronic alcohol-induced liver injury. J. Nutr. Biochem. 2013, 24, 1609–1615. [Google Scholar] [CrossRef]
- Di Zhou, S.H.; Yan, T.; Long, C.; Xu, J.; Zheng, P.; Chen, Z.; Jia, G. Toxicity of titanium dioxide nanoparticles induced by reactive oxygen species. React. Oxyg. Species 2019, 8, 267–275. [Google Scholar]
- Hu, H.; Fan, X.; Yin, Y.; Guo, Q.; Yang, D.; Wei, X.; Zhang, B.; Liu, J.; Wu, Q.; Oh, Y.; et al. Mechanisms of titanium dioxide nanoparticle-induced oxidative stress and modulation of plasma glucose in mice. Environ. Toxicol. 2019, 34, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.; Wu, C.-H.; Aronstam, R.S. Toxicity of Transition Metal Oxide Nanoparticles: Recent Insights from in vitro Studies. Materials 2010, 3, 4842–4859. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.-L.; Jenck, F.; Martin, J.R.; Perrin, S.; Haefely, W.E. Effects of repeated mild stress and two antidepressant treatments on the behavioral response to 5HT1C receptor activation in rats. Psychopharmacology 1993, 110, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Alam, J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Tehrani, H.S.; Moosavi-Movahedi, A.A. Biology, Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef]
- Korge, P.; Calmettes, G.; Weiss, J.N. Increased reactive oxygen species production during reductive stress: The roles of mitochondrial glutathione and thioredoxin reductases. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 514–525. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Franklin, C.C.; Backos, D.S.; Mohar, I.; White, C.C.; Forman, H.J.; Kavanagh, T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Asp. Med. 2009, 30, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Ayer, A.; Zarjou, A.; Agarwal, A.; Stocker, R. Heme Oxygenases in Cardiovascular Health and Disease. Physiol. Rev. 2016, 96, 1449–1508. [Google Scholar] [CrossRef]
- Weldy, C.S.; Luttrell, I.P.; White, C.C.; Morgan-Stevenson, V.; Bammler, T.K.; Beyer, R.P.; Afsharinejad, Z.; Kim, F.; Chitaley, K.; Kavanagh, T.J. Glutathione (GSH) and the GSH synthesis gene Gclm modulate vascular reactivity in mice. Free. Radic. Biol. Med. 2012, 53, 1264–1278. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Miller, M.L.; Shen, D.; Shertzer, H.G.; Stringer, K.F.; Wang, B.; Schneider, S.N.; Nebert, D.W.; Dalton, T.P. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology 2007, 45, 1118–1128. [Google Scholar] [CrossRef]
- Goyal, N.; Rishi, P.; Shukla, G. Lactobacillus rhamnosus GG antagonizes Giardia intestinalis induced oxidative stress and intestinal disaccharidases: An experimental study. World J. Microbiol. Biotechnol. 2013, 29, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, X.-L.; Le, G.-W.; Shi, Y.-H. Inhibition of Fe-induced colon oxidative stress by lactobacilli in mice. World J. Microbiol. Biotechnol. 2012, 29, 209–216. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer | Sequence (5′-3′) |
|---|---|---|
| SOD1 | Forward | TTTTGCTCTCCCAGGCCG |
| Reverse | ACCGCCATGTTTCTTAGAGTG | |
| SOD2 | Forward | ACTTGAAACGTGTAACTAGGC |
| Reverse | CTTTCATACAATACACAGTCGG | |
| HO-1 | Forward | TTTTCACCTTCCCAGCAT |
| Reverse | TTAGCCTCTTCTGTCACCCT | |
| CAT | Forward | ATAGCCAGAAGAGAAACCCACA |
| Reverse | CCTCTCCATTCGCATTAACCAG | |
| GSH | Forward | ATCCCACTGCGCTCATGACC |
| Reverse | AGCCAGCCATCACCAAGCC | |
| GCLC | Forward | GAGCGAGATGCCGTCTTACA |
| Reverse | TTGCTACACCCATCCACCAC | |
| GCLM | Forward | TGTTTGACCAAGTGCCCAT |
| Reverse | ATCTAAAATGCCTTCGGTGT | |
| GAPDH | Forward | TCCCTCAAGATTGTCAGCAA |
| Reverse | AGATCCACAACGGATACATT |
| Indexes | Control | TiO2 NPs | TiO2 NPs + LGG | LGG |
|---|---|---|---|---|
| WBC (109/L) | 5.57 ± 2.39 | 9.7 ± 1.5 * | 6.17 ± 1.05 # | 6.43 ± 2.01 |
| Lymph (109/L) | 4.8 ± 2.11 | 6.17 ± 2.67 | 5.3 ± 1.19 | 4.85 ± 1.75 |
| Mon (109/L) | 0.1 ± 0.08 | 0.18 ± 0.15 | 0.12 ± 0.02 | 0.13 ± 0.05 |
| NEUT (109/L) | 0.55 ± 0.15 | 1.07 ± 0.68 * | 0.6 ± 0.1 # | 0.7 ± 0.22 |
| RBC (1012/L) | 2.33 ± 1.08 | 2.44 ± 0.77 | 2.12 ± 0.26 | 2.26 ± 0.15 |
| HGB (g/L) | 85.67 ± 22.51 | 94.5 ± 17.64 | 81 ± 15.9 | 92 ± 13.93 |
| PLT (1012/L) | 930.33 ± 340.19 | 687.67 ± 121.5 | 1072.5 ± 25.5 | 896.5 ± 85.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, P.; Wang, M.; Zhao, Y.; Liu, S.; Chen, L.; Xu, H. Protective Effect of Lactobacillus rhamnosus GG on TiO2 Nanoparticles-Induced Oxidative Stress Damage in the Liver of Young Rats. Nanomaterials 2021, 11, 803. https://doi.org/10.3390/nano11030803
Nie P, Wang M, Zhao Y, Liu S, Chen L, Xu H. Protective Effect of Lactobacillus rhamnosus GG on TiO2 Nanoparticles-Induced Oxidative Stress Damage in the Liver of Young Rats. Nanomaterials. 2021; 11(3):803. https://doi.org/10.3390/nano11030803
Chicago/Turabian StyleNie, Penghui, Mengqi Wang, Yu Zhao, Shanji Liu, Ling Chen, and Hengyi Xu. 2021. "Protective Effect of Lactobacillus rhamnosus GG on TiO2 Nanoparticles-Induced Oxidative Stress Damage in the Liver of Young Rats" Nanomaterials 11, no. 3: 803. https://doi.org/10.3390/nano11030803
APA StyleNie, P., Wang, M., Zhao, Y., Liu, S., Chen, L., & Xu, H. (2021). Protective Effect of Lactobacillus rhamnosus GG on TiO2 Nanoparticles-Induced Oxidative Stress Damage in the Liver of Young Rats. Nanomaterials, 11(3), 803. https://doi.org/10.3390/nano11030803