Investigation of the Immune Modulatory Potential of Zinc Oxide Nanoparticles in Human Lymphocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Zinc Oxide Nanoparticles
2.2. Preparation of Human Lymphocytes
2.3. Annexin-V/Propidiumiodide Fluorescence-Activated Cell Sorting (FACS)
2.4. Flow Cytometry Analysis
2.5. Statistics
3. Results
3.1. Nanoparticle Characterization
3.2. Annexin-V/Propidiumiodide FACS
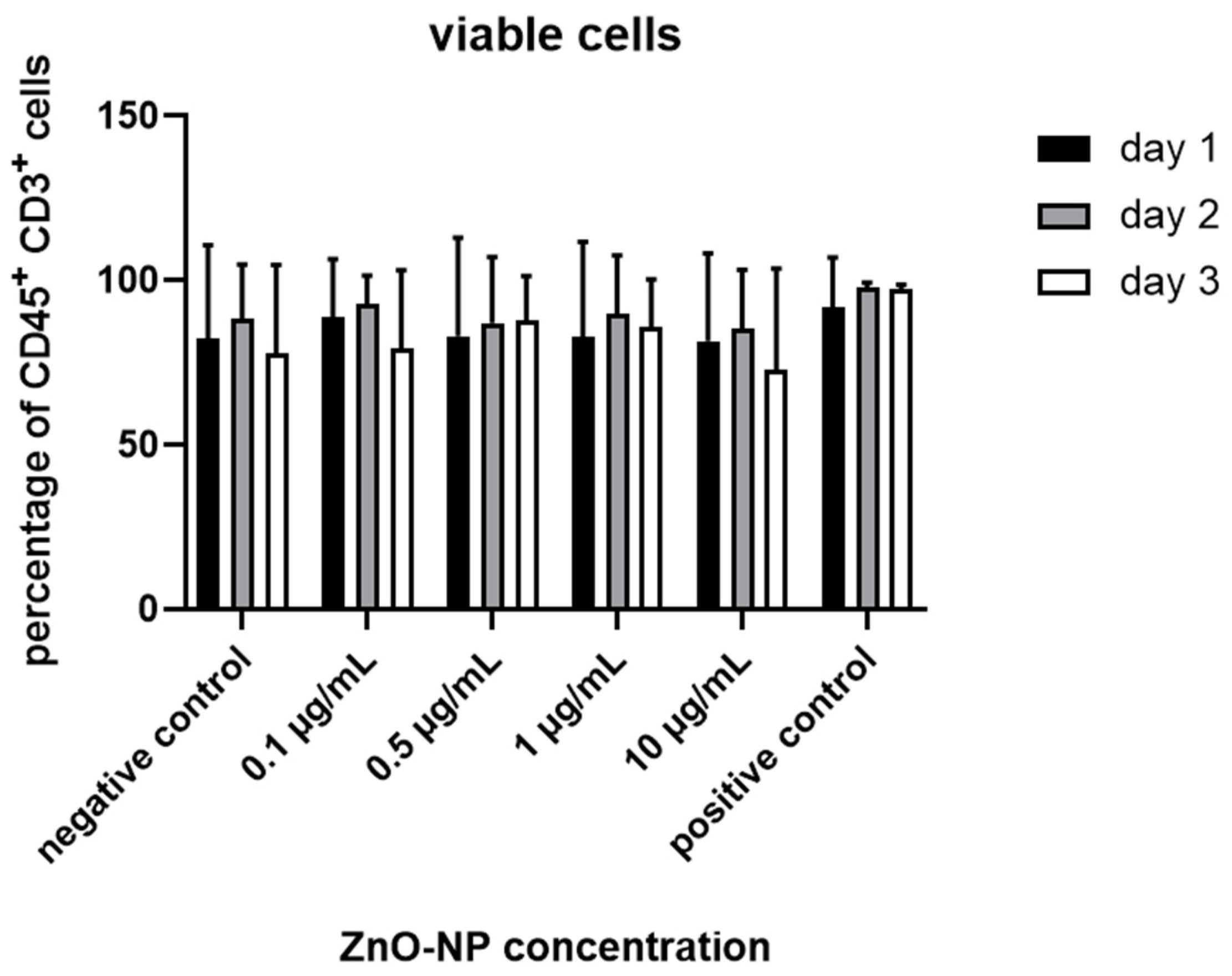
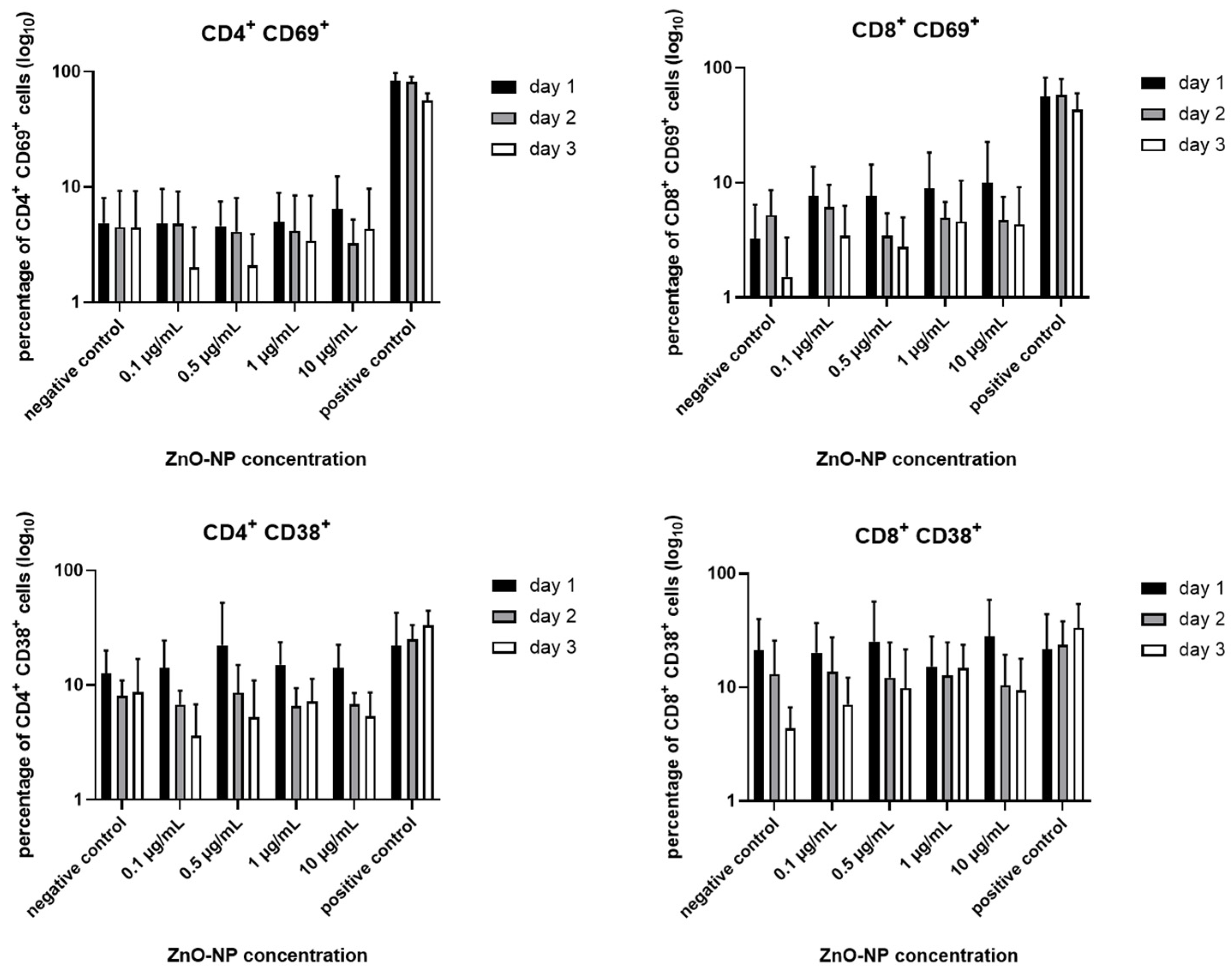
3.3. Cell Viability, T Cell Subtypes and T Cell Activation
3.4. CD4+ T Cell Differentiation and HLA-DR Expression
3.5. Proportion of Terminally Differentiated CD4+ and CD8+ T Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Monteiro-Riviere, N.A.; Wiench, K.; Landsiedel, R.; Schulte, S.; Inman, A.O.; Riviere, J.E. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in uvb sunburned skin: An in vitro and in vivo study. Toxicol. Sci. 2011, 123, 264–280. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic nanoparticles in mr imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Rabbani, G.; Baig, M.H.; Lim, J.H.; Khan, M.E.; Lee, E.J.; Ashraf, G.M.; Choi, I. Nanoparticle-based drugs: A potential armamentarium of effective anti-cancer therapies. Curr. Drug Metab. 2018, 19, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, M.; Nasrollahzadeh, M.; Jaleh, B.; Jamalipour Soufi, G.; Iravani, S. Carbon-based nanomaterials for targeted cancer nanotherapy: Recent trends and future prospects. J. Drug Target. 2021, 1–78. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.D.F.; Coimbra, J.S.D.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Lademann, J.; Ribaud, C.; Roberts, M.S. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit. Rev. Toxicol. 2007, 37, 251–277. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Hipler, U.C.; Boldt, S.; Strehle, J.; Wollina, U. Skin-protective effects of a zinc oxide-functionalized textile and its relevance for atopic dermatitis. Clin. Cosmet. Investig. Derm. 2013, 6, 115–121. [Google Scholar]
- Pairoj, S.; Damrongsak, P.; Damrongsak, B.; Jinawath, N.; Kaewkhaw, R.; Ruttanasirawit, C.; Leelawattananon, T.; Locharoenrat, K. Antitumor activities of carboplatin-doxorubicin-zno complexes in different human cancer cell lines (breast, cervix uteri, colon, liver and oral) under uv exposition. Artif. Cells Nanomed. Biotechnol. 2021, 49, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Karg, E.; Roth, C.; Schulz, H.; Ziesenis, A.; Heinzmann, U.; Schramel, P.; Heyder, J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ. Health Perspect. 2001, 109 (Suppl. 4), 547–551. [Google Scholar]
- Alarifi, S.; Ali, D.; Alkahtani, S.; Verma, A.; Ahamed, M.; Ahmed, M.; Alhadlaq, H.A. Induction of oxidative stress, DNA damage, and apoptosis in a malignant human skin melanoma cell line after exposure to zinc oxide nanoparticles. Int. J. Nanomed. 2013, 8, 983–993. [Google Scholar]
- Cardozo, T.R.; De Carli, R.F.; Seeber, A.; Flores, W.H.; da Rosa, J.A.N.; Kotzal, Q.S.G.; Lehmann, M.; da Silva, F.R.; Dihl, R.R. Genotoxicity of zinc oxide nanoparticles: An in vivo and in silico study. Toxicol. Res. 2019, 8, 277–286. [Google Scholar] [CrossRef]
- El Yamani, N.; Collins, A.R.; Runden-Pran, E.; Fjellsbo, L.M.; Shaposhnikov, S.; Zienolddiny, S.; Dusinska, M. In vitro genotoxicity testing of four reference metal nanomaterials, titanium dioxide, zinc oxide, cerium oxide and silver: Towards reliable hazard assessment. Mutagenesis 2017, 32, 117–126. [Google Scholar] [CrossRef]
- Moratin, H.; Scherzad, A.; Gehrke, T.; Ickrath, P.; Radeloff, K.; Kleinsasser, N.; Hackenberg, S. Toxicological characterization of zno nanoparticles in malignant and non-malignant cells. Environ. Mol. Mutagen. 2017, 59, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Ickrath, P.; Kleinsasser, N.; Ding, X.; Ginzkey, C.; Beyersdorf, N.; Hagen, R.; Kerkau, T.; Hackenberg, S. Characterization of t-cell subpopulations in patients with chronic rhinosinusitis with nasal polyposis. Allergy Rhinol. 2017, 8, 139–147. [Google Scholar] [CrossRef]
- Ickrath, P.; Scherzad, A.; Kleinsasser, N.; Ginzkey, C.; Hagen, R.; Hackenberg, S. Influence of nasal polyp tissue on the differentiation and activation of t lymphocytes in a co-culture system. Biomed. Rep. 2019, 10, 119–126. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoet, P.H.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.F.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef]
- Jani, P.U.; Mccarthy, D.E.; Florence, A.T. Titanium-dioxide (rutile) particle uptake from the rat gi tract and translocation to systemic organs after oral-administration. Int. J. Pharm. 1994, 105, 157–168. [Google Scholar] [CrossRef]
- Bakand, S.; Hayes, A. Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef]
- Pagano, S.; Coniglio, M.; Valenti, C.; Negri, P.; Lombardo, G.; Costanzi, E.; Cianetti, S.; Montaseri, A.; Marinucci, L. Biological effects of resin monomers on oral cell populations: Descriptive analysis of literature. Eur. J. Paediatr. Dent. 2019, 20, 224–232. [Google Scholar]
- Kim, J.; Gambhir, V.; Alatery, A.; Basta, S. Delivery of exogenous antigens to induce cytotoxic cd8+ t lymphocyte responses. J. Biomed. Biotechnol. 2010, 2010, 218752. [Google Scholar] [CrossRef][Green Version]
- Bhat, P.; Leggatt, G.; Waterhouse, N.; Frazer, I.H. Interferon-gamma derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 2017, 8, e2836. [Google Scholar] [CrossRef] [PubMed]
- Frankel, S.R.; Baeuerle, P.A. Targeting t cells to tumor cells using bispecific antibodies. Curr. Opin. Chem. Biol. 2013, 17, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Lindau, D.; Gielen, P.; Kroesen, M.; Wesseling, P.; Adema, G.J. The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory t cells and natural killer t cells. Immunology 2013, 138, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Qian, R.; Liu, T.; Wu, T.; Wang, T. Nanoparticulate carriers used as vaccine adjuvant delivery systems. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 449–484. [Google Scholar] [CrossRef] [PubMed]
- Greulich, C.; Diendorf, J.; Gessmann, J.; Simon, T.; Habijan, T.; Eggeler, G.; Schildhauer, T.A.; Epple, M.; Koller, M. Cell type-specific responses of peripheral blood mononuclear cells to silver nanoparticles. Acta Biomater. 2011, 7, 3505–3514. [Google Scholar] [CrossRef]
- Hanley, C.; Thurber, A.; Hanna, C.; Punnoose, A.; Zhang, J.; Wingett, D.G. The influences of cell type and zno nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale Res. Lett. 2009, 4, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Surendran, S.P.; Moon, M.J.; Park, R.; Jeong, Y.Y. Bioactive nanoparticles for cancer immunotherapy. Int. J. Mol. Sci. 2018, 19, 3877. [Google Scholar] [CrossRef]
- Hosseini, M.; Haji-Fatahaliha, M.; Jadidi-Niaragh, F.; Majidi, J.; Yousefi, M. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Getts, D.R.; Shea, L.D.; Miller, S.D.; King, N.J. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015, 36, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B. Hide and seek: Nanomaterial interactions with the immune system. Front. Immunol. 2019, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tongchusak, S.; Mizukami, Y.; Kang, Y.J.; Ioji, T.; Touma, M.; Reinhold, B.; Keskin, D.B.; Reinherz, E.L.; Sasada, T. Induction of anti-tumor cytotoxic t cell responses through plga-nanoparticle mediated antigen delivery. Biomaterials 2011, 32, 3666–3678. [Google Scholar] [CrossRef] [PubMed]
- Zupancic, E.; Curato, C.; Paisana, M.; Rodrigues, C.; Porat, Z.; Viana, A.S.; Afonso, C.A.M.; Pinto, J.; Gaspar, R.; Moreira, J.N.; et al. Rational design of nanoparticles towards targeting antigen-presenting cells and improved t cell priming. J. Control. Release 2017, 258, 182–195. [Google Scholar] [CrossRef]
- Cruz, L.J.; Rosalia, R.A.; Kleinovink, J.W.; Rueda, F.; Lowik, C.W.; Ossendorp, F. Targeting nanoparticles to cd40, dec-205 or cd11c molecules on dendritic cells for efficient cd8(+) t cell response: A comparative study. J. Control. Release 2014, 192, 209–218. [Google Scholar] [CrossRef]
- Getts, D.R.; Martin, A.J.; McCarthy, D.P.; Terry, R.L.; Hunter, Z.N.; Yap, W.T.; Getts, M.T.; Pleiss, M.; Luo, X.; King, N.J.; et al. Microparticles bearing encephalitogenic peptides induce t-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 2012, 30, 1217–1224. [Google Scholar] [CrossRef]
- Wang, C.; Sun, W.; Ye, Y.; Bomba, H.N.; Gu, Z. Bioengineering of artificial antigen presenting cells and lymphoid organs. Theranostics 2017, 7, 3504–3516. [Google Scholar] [CrossRef]
- Meunier, E.; Coste, A.; Olagnier, D.; Authier, H.; Lefevre, L.; Dardenne, C.; Bernad, J.; Beraud, M.; Flahaut, E.; Pipy, B. Double-walled carbon nanotubes trigger il-1beta release in human monocytes through nlrp3 inflammasome activation. Nanomedicine 2012, 8, 987–995. [Google Scholar] [CrossRef]
- Potter, T.M.; Neun, B.W.; Rodriguez, J.C.; Ilinskaya, A.N.; Dobrovolskaia, M.A. Analysis of pro-inflammatory cytokine and type ii interferon induction by nanoparticles. Methods Mol. Biol. 2018, 1682, 173–187. [Google Scholar]
- Mitchell, L.A.; Lauer, F.T.; Burchiel, S.W.; McDonald, J.D. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat. Nanotechnol. 2009, 4, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Degheidy, H.; Casey, B.J.; Goering, P.L. Flow cytometry evaluation of in vitro cellular necrosis and apoptosis induced by silver nanoparticles. Food Chem. Toxicol. 2015, 85, 45–51. [Google Scholar] [CrossRef] [PubMed]
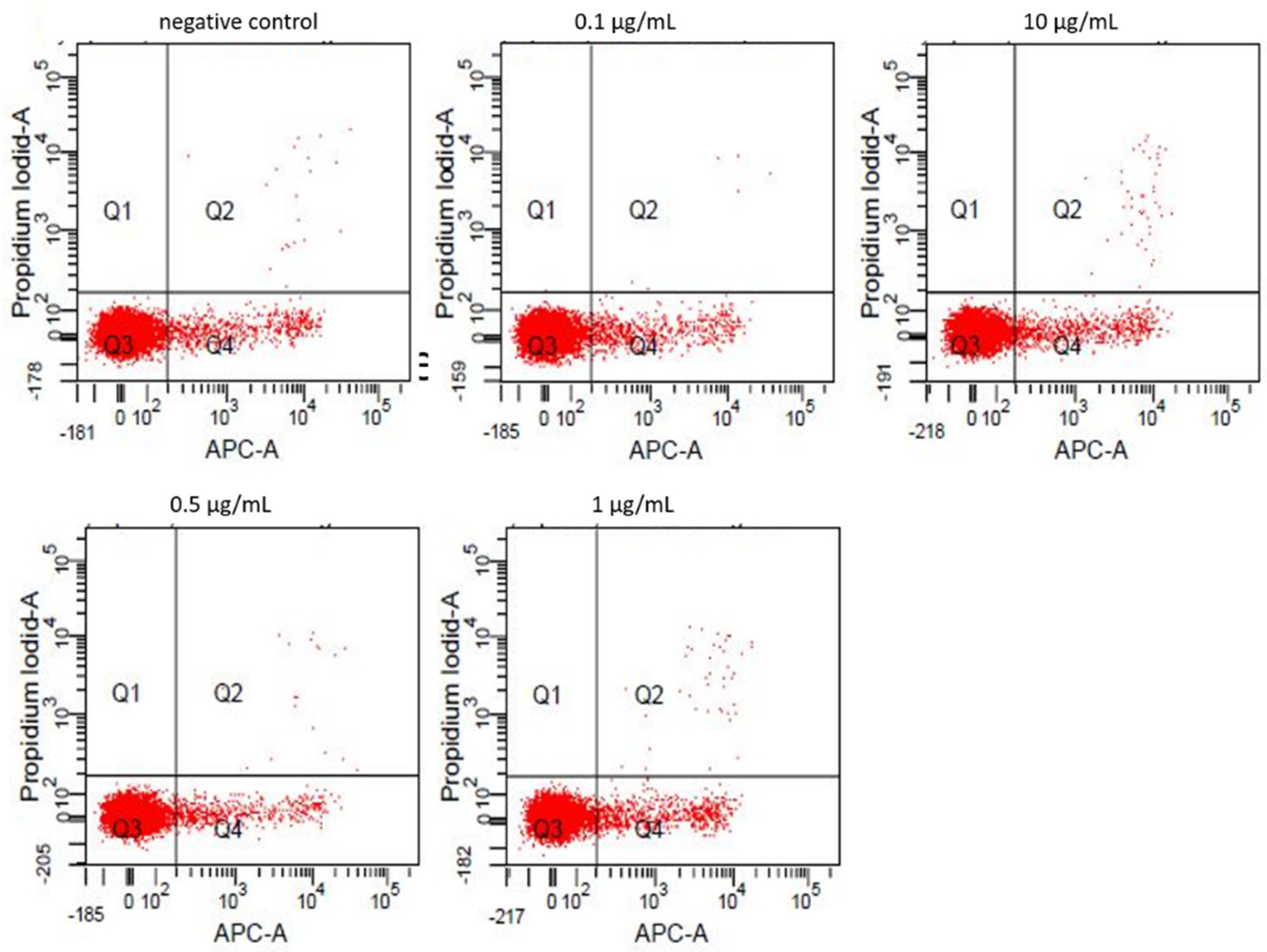




| Viable (%) | Apoptotic (%) | Necrotic (%) | |
|---|---|---|---|
| negative control | 94.2 | 5.6 | 0.2 |
| 0.1 µg/mL | 94.2 | 5.7 | 0.1 |
| 0.5 µg/mL | 95.0 | 4.09 | 0.2 |
| 1 µg/mL | 92.7 | 6.7 | 0.4 |
| 10 µg/mL | 93.7 | 5.9 | 0.4 |
| 25 µg/mL | 78.4 | 16.2 | 5.1 |
| 50 µg/mL | 68.6 | 20.8 | 10.3 |
| 75 µg/mL | 69.1 | 20.6 | 10.0 |
| 100 µg/mL | 63.8 | 21.6 | 13.9 |
| Day 1 | Naïve (%) | Memory (%) | rTreg (%) | aTreg (%) | FoxP3low (%) |
|---|---|---|---|---|---|
| negative control | 51.93 ± 21.41 | 42.28 ± 0.68 | 2.20 ± 1.24 | 1.12 ± 1.10 | 2.76 ± 1.20 |
| 0.1 µg/mL | 49.56 ± 22.93 | 45.68 ± 23.78 | 1.72 ± 1.73 | 1.06 ± 1.21 | 2.21 ± 1.33 |
| 0.5 µg/mL | 56.07 ± 19.56 | 39.21 ± 19.88 | 2.07± 2.27 | 0.89 ± 0.76 | 2.17 ± 1.38 |
| 1 µg/mL | 50.58 ± 21.90 | 44.60 ± 23.11 | 1.72 ± 1.42 | 0.86 ± 0.76 | 2.38 ± 1.52 |
| 10 µg/mL | 54.30 ± 19.07 | 41.66 ± 19.12 | 1.44 ± 1.40 | 0.47 ± 0.43 | 2.48 ± 1.52 |
| positive control | 57.97 ± 15.45 | 26.50 ± 15.15 | 4.95 ± 2.39 | 3.88 ± 2.12 | 4.73 ± 3.30 |
| Day 2 | Naïve (%) | Memory (%) | rTreg (%) | aTreg (%) | FoxP3low (%) |
|---|---|---|---|---|---|
| negative control | 52.71 ± 19.87 | 42.28 ± 20.07 | 1.60 ± 0.95 | 0.88 ± 1.00 | 2.68 ± 1.31 |
| 0.1 µg/mL | 46.84 ± 22.93 | 48.40 ± 23.16 | 1.59 ± 1.46 | 0.89 ± 0.82 | 2.63 ± 1.14 |
| 0.5 µg/mL | 49.82 ± 19.50 | 46.19 ± 20.48 | 1.43 ± 1.62 | 0.79 ± 0.77 | 2.17 ± 1.30 |
| 1 µg/mL | 47.96 ± 21.09 | 48.44 ± 21.62 | 1.18 ± 0.90 | 0.72 ± 0.69 | 2.28 ± 1.36 |
| 10 µg/mL | 49.44 ± 22.72 | 46.45 ± 22.36 | 1.54 ± 1.11 | 0.48 ± 0.52 | 2.44 ± 1.20 |
| positive control | 53.47 ± 12.47 | 19.00 ± 9.025 | 9.04 ± 3.16 | 7.11 ± 5.40 | 4.50 ± 3.22 |
| Day 3 | Naïve (%) | Memory (%) | rTreg (%) | aTreg (%) | FoxP3low (%) |
|---|---|---|---|---|---|
| negative control | 48.93 ± 10.28 | 47.18 ± 10.00 | 1.11 ± 0.68 | 0.69 ± 0.63 | 2.61 ± 0.94 |
| 0.1 µg/mL | 42.10 ± 13.62 | 54.66 ± 14.12 | 0.99 ± 0.98 | 0.54 ± 0.52 | 2.09 ± 0.51 |
| 0.5 µg/mL | 38.94 ± 20.41 | 57.15 ± 20.60 | 1.14 ± 1.14 | 0.69 ± 0.60 | 2.43 ± 0.51 |
| 1 µg/mL | 47.05 ± 15.36 | 50.08 ± 16.43 | 1.25 ± 0.92 | 0.47 ± 0.61 | 1.66 ± 0.76 |
| 10 µg/mL | 49.53 ± 12.57 | 46.56 ± 11.58 | 2.29 ± 3.67 | 0.35 ± 0.36 | 1.72 ± 1.08 |
| positive control | 43.35 ± 12.94 | 20.69 ± 7.53 | 10.74 ± 8.74 | 9.55 ± 3.89 | 6.09 ± 3.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moratin, H.; Ickrath, P.; Scherzad, A.; Meyer, T.J.; Naczenski, S.; Hagen, R.; Hackenberg, S. Investigation of the Immune Modulatory Potential of Zinc Oxide Nanoparticles in Human Lymphocytes. Nanomaterials 2021, 11, 629. https://doi.org/10.3390/nano11030629
Moratin H, Ickrath P, Scherzad A, Meyer TJ, Naczenski S, Hagen R, Hackenberg S. Investigation of the Immune Modulatory Potential of Zinc Oxide Nanoparticles in Human Lymphocytes. Nanomaterials. 2021; 11(3):629. https://doi.org/10.3390/nano11030629
Chicago/Turabian StyleMoratin, Helena, Pascal Ickrath, Agmal Scherzad, Till Jasper Meyer, Sebastian Naczenski, Rudolf Hagen, and Stephan Hackenberg. 2021. "Investigation of the Immune Modulatory Potential of Zinc Oxide Nanoparticles in Human Lymphocytes" Nanomaterials 11, no. 3: 629. https://doi.org/10.3390/nano11030629
APA StyleMoratin, H., Ickrath, P., Scherzad, A., Meyer, T. J., Naczenski, S., Hagen, R., & Hackenberg, S. (2021). Investigation of the Immune Modulatory Potential of Zinc Oxide Nanoparticles in Human Lymphocytes. Nanomaterials, 11(3), 629. https://doi.org/10.3390/nano11030629






