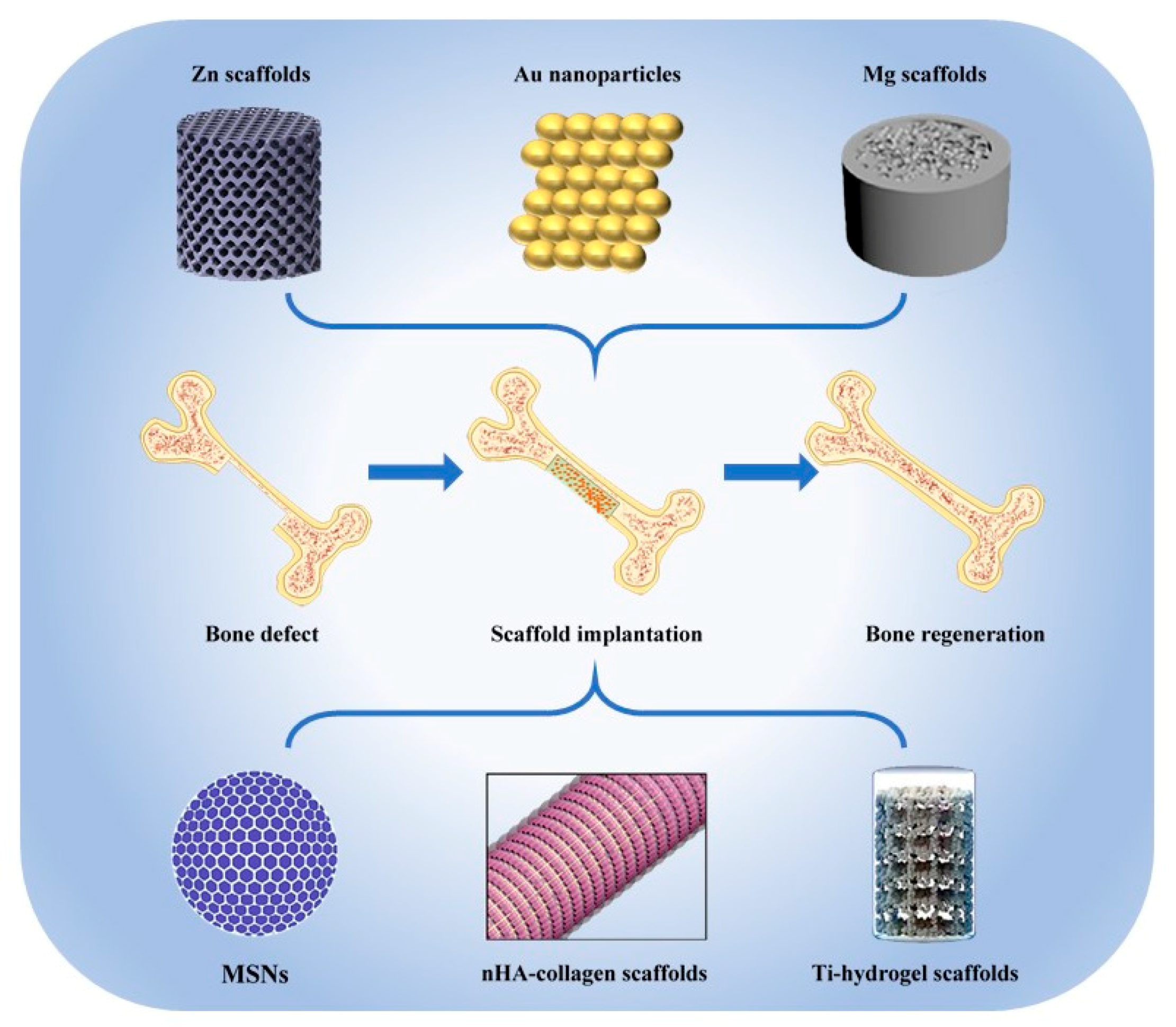
Novel Inorganic Nanomaterial-Based Therapy for Bone Tissue Regeneration
Abstract
1. Introduction
2. Nano Hydroxyapatites
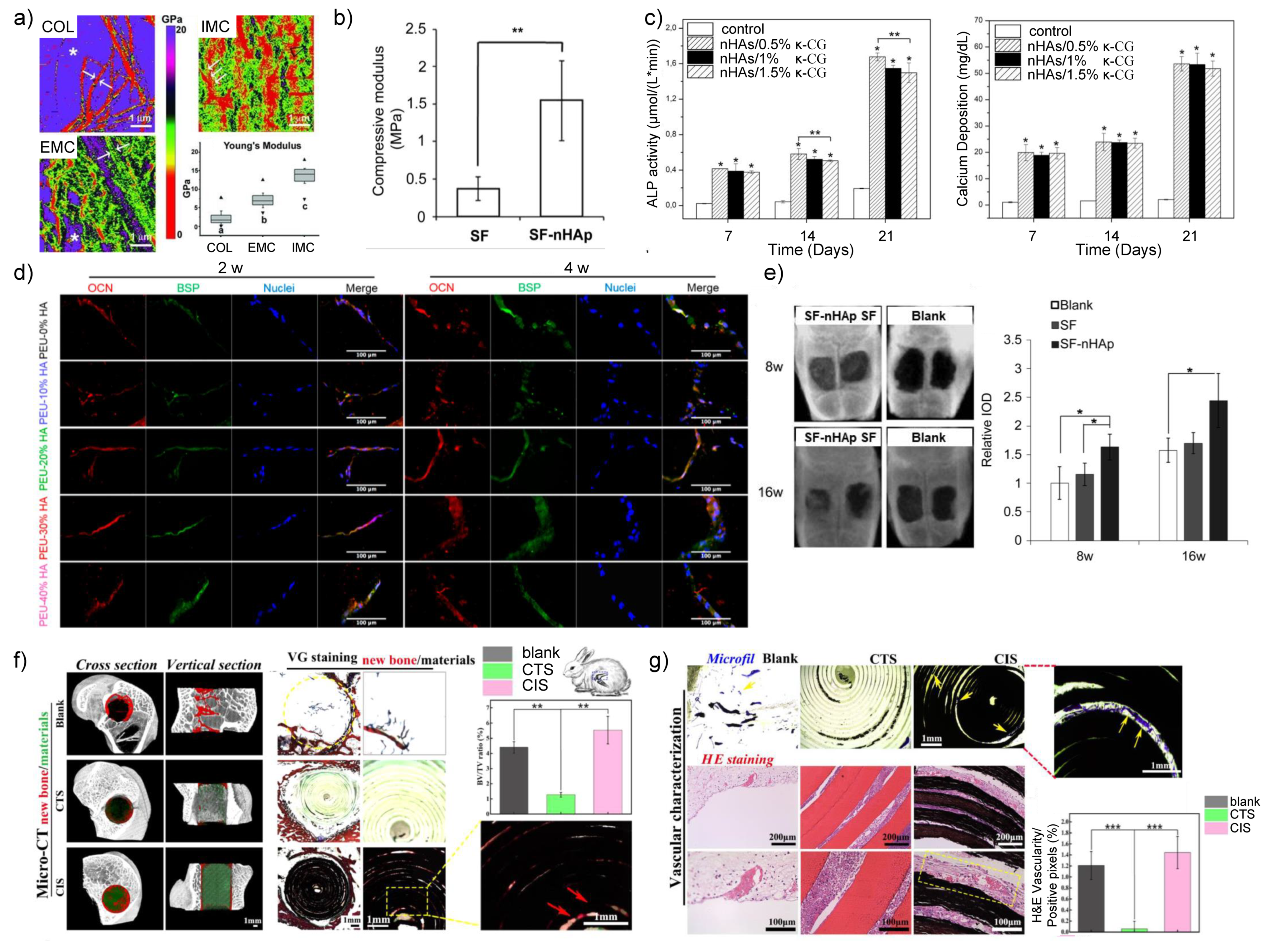
2.1. nHA/Polymer Composites
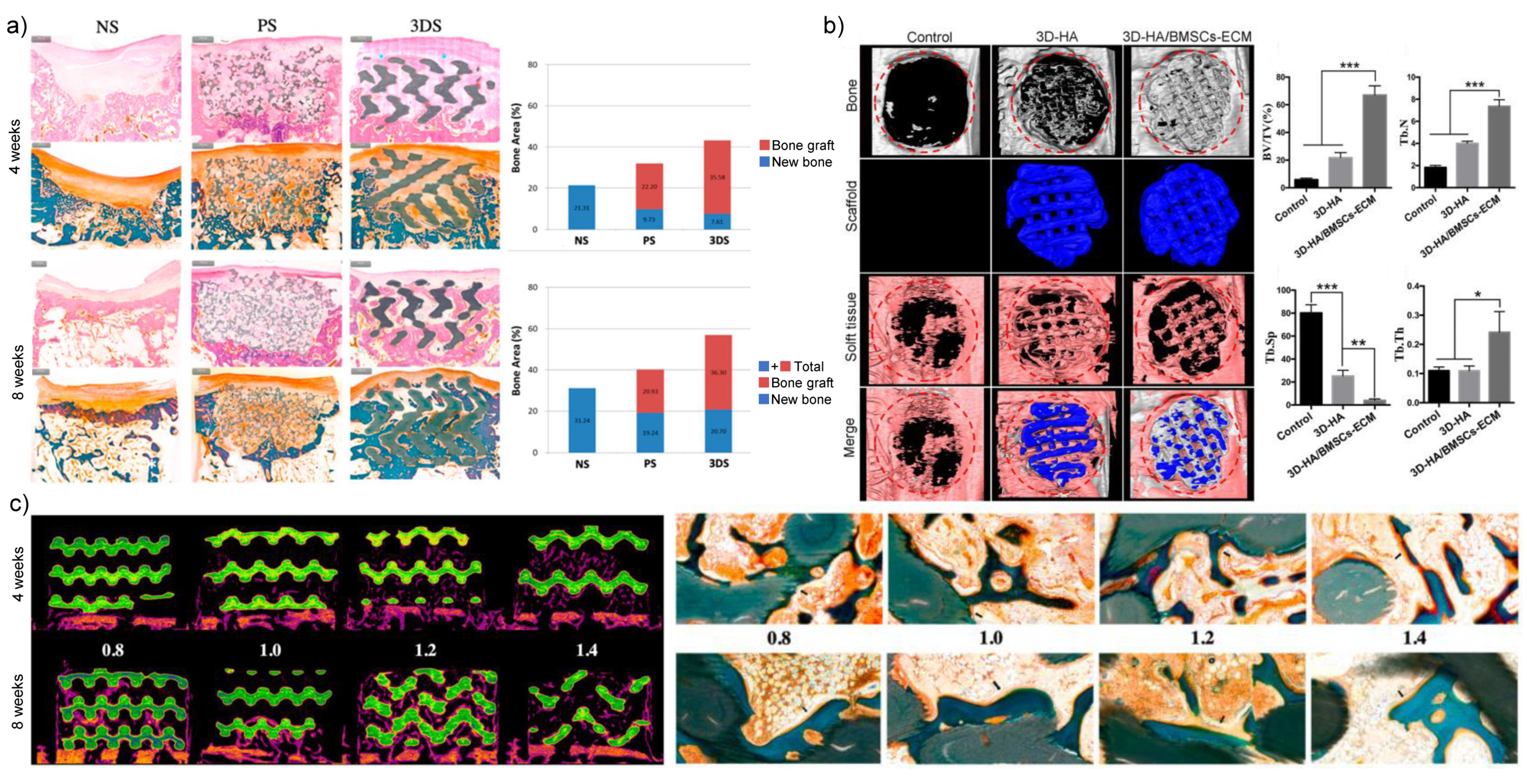
2.2. 3D Printed nHA-Based Inorganic Nanomaterials
3. Nano Silica
3.1. Drug Delivery Carrier
3.2. Modification of MSN-Based Scaffolds
4. Metallic Nanomaterials
4.1. Ti-Based Nanomaterials
4.1.1. Nanoscale Surface Modification of Ti-Based Biomaterials
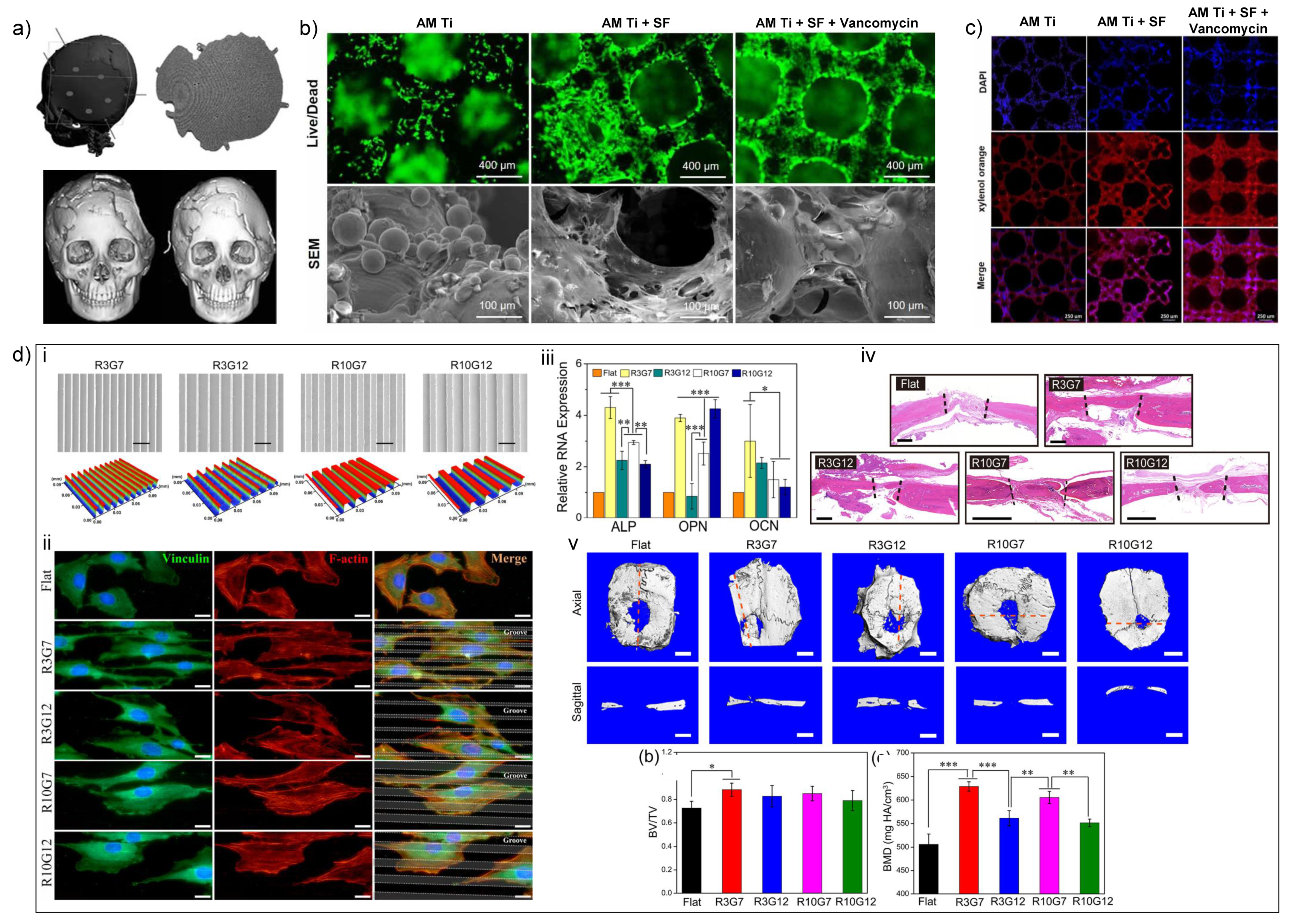
4.1.2. Additive Manufacturing of Ti-Based Biomaterials
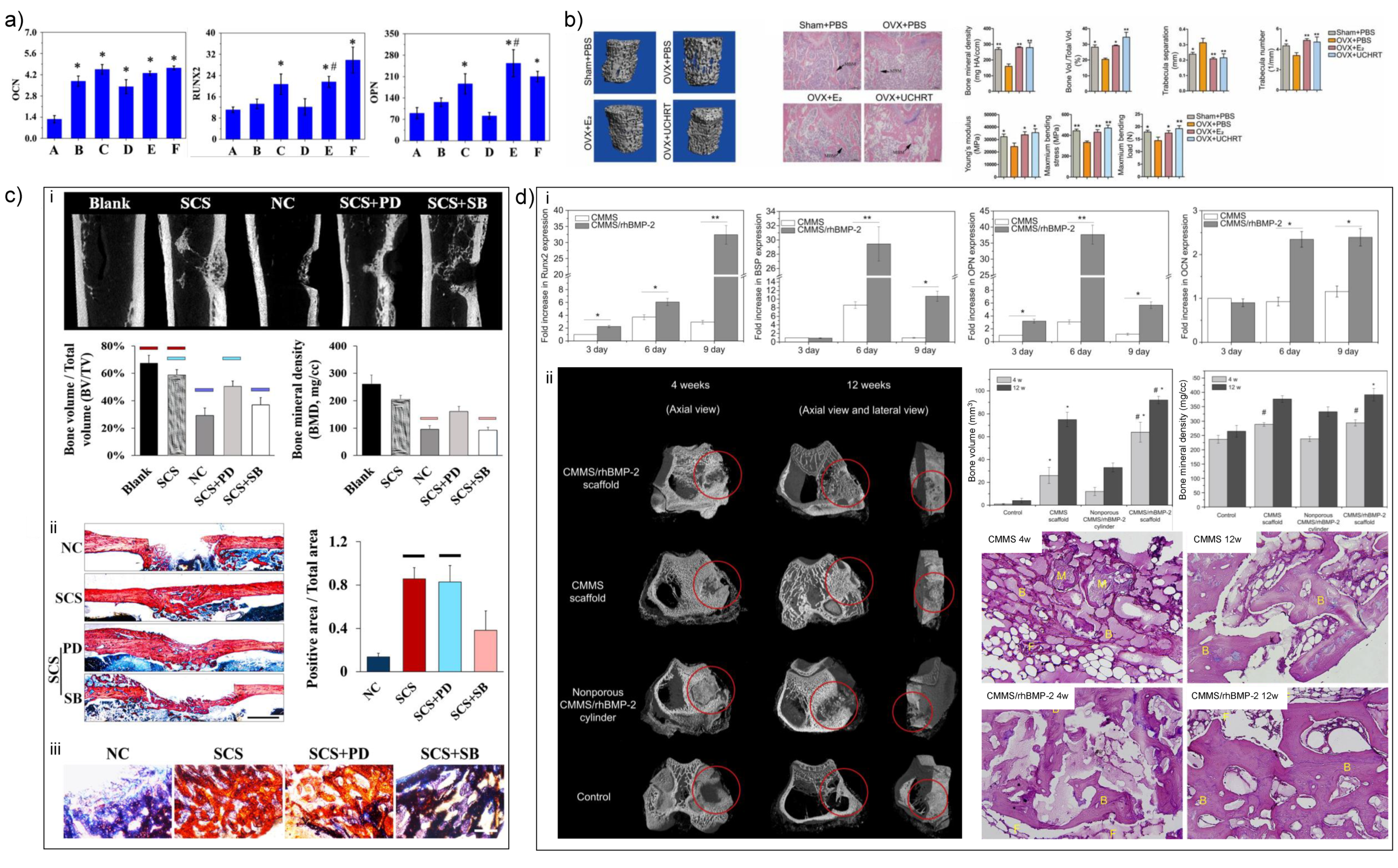
4.2. Mg-Based Biomaterials
4.2.1. Nanoscale Surface Modification of Mg-Based Biomaterials
4.2.2. Additive Manufacturing of Mg-Based Biomaterials
4.3. Zn-Based Biomaterials
4.4. Au-Based Biomaterials
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wegst, U.G.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Griffin, K.S.; Davis, K.M.; McKinley, T.O.; Anglen, J.O.; Chu, T.-M.G.; Boerckel, J.D.; Kacena, M.A. Evolution of Bone Grafting: Bone Grafts and Tissue Engineering Strategies for Vascularized Bone Regeneration. Clin. Rev. Bone Miner. Metab. 2015, 13, 232–244. [Google Scholar] [CrossRef]
- Burk, T.; Del Valle, J.; Finn, R.A.; Phillips, C. Maximum Quantity of Bone Available for Harvest From the Anterior Iliac Crest, Posterior Iliac Crest, and Proximal Tibia Using a Standardized Surgical Approach: A Cadaveric Study. J. Oral Maxillofac. Surg. 2016, 74, 2532–2548. [Google Scholar] [CrossRef]
- Cross, L.M.; Thakur, A.; Jalili, N.A.; Detamore, M.; Gaharwar, A.K. Nanoengineered biomaterials for repair and regeneration of orthopedic tissue interfaces. Acta Biomater. 2016, 42, 2–17. [Google Scholar] [CrossRef]
- Morse, D.E. Silicon biotechnology: Harnessing biological silica production to construct new materials. Trends Biotechnol. 1999, 17, 230–232. [Google Scholar] [CrossRef]
- Lee, J.W.; Han, H.S.; Han, K.J.; Park, J.; Jeon, H.; Ok, M.R.; Seok, H.K.; Ahn, J.P.; Lee, K.E.; Lee, D.H. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. USA 2016, 716. [Google Scholar] [CrossRef] [PubMed]
- Ljungblad, U. Statistical process control applied to additive manufacturing enables series production of orthopedic implants. In Proceedings of the 21st International DAAAM Symposium, Zadar, Croatia, 20–23 October 2010; DAAAM International Vienna: Vienna, Austria, 2010. [Google Scholar]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mat. Sci Eng. C Mater. 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Yu, M.; Wang, Y.; Jin, S.; Li, Z.; Cui, S.; He, D.; Zhang, T.; Wang, T.; et al. Thermodynamically Controlled Self-Assembly of Hierarchically Staggered Architecture as an Osteoinductive Alternative to Bone Autografts. Adv. Funct. Mater. 2019, 29, 1806445. [Google Scholar] [CrossRef]
- Li, Y.; Pavanram, P.; Zhou, J.; Lietaert, K.; Taheri, P.; Li, W.; San, H.; Leeflang, M.A.; Mol, J.M.C.; Jahr, H.; et al. Additively manufactured biodegradable porous zinc. Acta Biomater. 2020, 101, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Lee, H.; Jang, T.S.; Seong, Y.J.; Kim, H.E.; Koh, Y.H.; Song, J.; Jung, H.D. Biomimetic porous Mg with tunable mechanical properties and biodegradation rates for bone regeneration. Acta Biomater. 2019, 84, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhou, Y.; Shao, J.; Chen, Z.; Song, B.; Chang, J.; Wu, C.; Xiao, Y. Stimulation of osteogenesis and angiogenesis of hBMSCs by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta Biomater. 2015, 21, 178–189. [Google Scholar] [CrossRef]
- Qiao, S.; Wu, D.; Li, Z.; Zhu, Y.; Zhan, F.; Lai, H.; Gu, Y. The combination of multi-functional ingredients-loaded hydrogels and three-dimensional printed porous titanium alloys for infective bone defect treatment. J. Tissue Eng. 2020, 11, 2041731420965797. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Kou, X.I.; Wang, X.; Zhou, Y. Hierarchical Intrafibrillar Nanocarbonated Apatite Assembly Improves the Nanomechanics and Cytocompatibility of Mineralized Collagen. Adv. Funct. Mater. 2012, 23, 1404–1411. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, S.; Cui, S.J.; Kou, X.X.; Wang, X.D.; Liu, X.M.; Sun, Y.; Wang, G.N.; Liu, Y.; Zhou, Y.H. The surface chemistry of nanoscale mineralized collagen regulates periodontal ligament stem cell fate. ACS Appl. Mater. Interfaces 2016, 15958. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.; Luo, D.; Xue, Z.; Yang, X.; Gu, L.; Zhou, Y.; Wang, T. Hierarchically Staggered Nanostructure of Mineralized Collagen as a Bone-Grafting Scaffold. Adv. Mater. 2016, 28, 8740–8748. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B. Preparation and characterization of composites based on the blends of collagen, chitosan and hyaluronic acid with nano-hydroxyapatite. Int. J. Biol. Macromol. 2017, 102, 658–666. [Google Scholar] [CrossRef]
- Nazeer, M.A.; Yilgor, E.; Yilgor, I. Intercalated chitosan/hydroxyapatite nanocomposites: Promising materials for bone tissue engineering applications. Carbohydr. Polym. 2017, 175, 38–46. [Google Scholar] [CrossRef]
- Uswatta, S.P.; Okeke, I.U.; Jayasuriya, A.C. Injectable porous nano-hydroxyapatite/chitosan/tripolyphosphate scaffolds with improved compressive strength for bone regeneration. Mater. Sci Eng. C 2016, 69, 505–512. [Google Scholar] [CrossRef]
- Liu, H.; Xu, G.W.; Wang, Y.F.; Zhao, H.S.; Xiong, S.; Wu, Y.; Heng, B.C.; An, C.R.; Zhu, G.H.; Xie, D.H. Composite scaffolds of nano-hydroxyapatite and silk fibroin enhance mesenchymal stem cell-based bone regeneration via the interleukin 1 alpha autocrine/paracrine signaling loop. Biomaterials 2015, 49, 103–112. [Google Scholar] [CrossRef]
- Moeini, S.; Mohammadi, M.R.; Simchi, A. In-situ solvothermal processing of polycaprolactone/hydroxyapatite nanocomposites with enhanced mechanical and biological performance for bone tissue engineering. Bioact. Mater. 2017, 2, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, Y.; Li, S.; Seifert, G.V.; Becker, M.L. Three-Dimensional Printing of Nano Hydroxyapatite/Poly(ester urea) Composite Scaffolds with Enhanced Bioactivity. Biomacromolecules 2017, 18, 4171–4183. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, J.; Fortunato, G.; Wozniak, B.; Chodara, A.; Domaschke, S.; Meczynska-Wielgosz, S.; Kruszewski, M.; Dommann, A.; Lojkowski, W. Polymer Membranes Sonocoated and Electrosprayed with Nano-Hydroxyapatite for Periodontal Tissues Regeneration. Nanomaterials 2019, 9, 1625. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Umar, A.; Sindhu, A.; Mohammed, H.; Al-Hadeethi, Y.; Guo, Z. Fabrication and in-vitro biocompatibility of freeze-dried CTS-nHA and CTS-nBG scaffolds for bone regeneration applications. Int. J. Biol. Macromol. 2020, 149, 1–10. [Google Scholar] [CrossRef]
- Bohner, M.; Miron, R.J. A proposed mechanism for material-induced heterotopic ossification. Mater. Today 2019. [Google Scholar] [CrossRef]
- Gonzalez Ocampo, J.I.; Machado de Paula, M.M.; Bassous, N.J.; Lobo, A.O.; Ossa Orozco, C.P.; Webster, T.J. Osteoblast responses to injectable bone substitutes of kappa-carrageenan and nano hydroxyapatite. Acta Biomater. 2019, 83, 425–434. [Google Scholar] [CrossRef]
- Feng, C.; Xue, J.; Yu, X.; Zhai, D.; Lin, R.; Zhang, M.; Xia, L.; Wang, X.; Yao, Q.; Chang, J.; et al. Co-inspired hydroxyapatite-based scaffolds for vascularized bone regeneration. Acta Biomater. 2020. [Google Scholar] [CrossRef]
- Kim, J.W.; Yang, B.E.; Hong, S.J.; Choi, H.G.; Byeon, S.J.; Lim, H.K.; Chung, S.M.; Lee, J.H.; Byun, S.H. Bone Regeneration Capability of 3D Printed Ceramic Scaffolds. Int. J. Mol. Sci. 2020, 21, 4837. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, E.J.; Davydov, A.V.; Frukhbeyen, S.; Seppala, J.E.; Takagi, S.; Chow, L.; Alimperti, S. Biofabrication of 3D printed hydroxyapatite composite scaffolds for bone regeneration. Biomed. Mater. 2020. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, L.; Gao, H.; Shi, X.; Wang, Y. In Situ Formation of Hexagon-like Column Array Hydroxyapatite on 3D-Plotted Hydroxyapatite Scaffolds by Hydrothermal Method and Its Effect on Osteogenic Differentiation. ACS Appl. Bio Mater. 2020, 3, 1753–1760. [Google Scholar] [CrossRef]
- Wei, Y.; Gao, H.; Hao, L.; Shi, X.; Wang, Y. Constructing a Sr(2+)-Substituted Surface Hydroxyapatite Hexagon-Like Microarray on 3D-Plotted Hydroxyapatite Scaffold to Regulate Osteogenic Differentiation. Nanomaterials 2020, 10, 1672. [Google Scholar] [CrossRef]
- Chi, H.; Chen, G.; He, Y.; Chen, G.; Tu, H.; Liu, X.; Yan, J.; Wang, X. 3D-HA Scaffold Functionalized by Extracellular Matrix of Stem Cells Promotes Bone Repair. Int. J. Nanomed. 2020, 15, 5825–5838. [Google Scholar] [CrossRef]
- Lim, H.K.; Hong, S.J.; Byeon, S.J.; Chung, S.M.; On, S.W.; Yang, B.E.; Lee, J.H.; Byun, S.H. 3D-Printed Ceramic Bone Scaffolds with Variable Pore Architectures. Int. J. Mol. Sci. 2020, 21, 6942. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992. [Google Scholar] [CrossRef]
- Meng, H.; Xue, M.; Xia, T.; Ji, Z.; Tarn, D.Y.; Zink, J.I.; Nel, A.E. Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model. Acs Nano 2011, 5, 4131–4144. [Google Scholar] [CrossRef]
- Shadjou, N.; Hasanzadeh, M. Bone tissue engineering using silica-based mesoporous nanobiomaterials: Recent progress. Mater. Sci. Eng. C 2015, 55, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chen, Z.; Farnaghi, S.; Friis, T.; Mao, X.; Xiao, Y.; Wu, C. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomater. 2016, 30, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, P.; Nie, W.; Peng, C.; Wang, J. Incorporation of dexamethasone-loaded mesoporous silica nanoparticles into mineralized porous biocomposite scaffolds for improving osteogenic activity. Int. J. Biol. Macromol. 2020, 149, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.S.; Alvarez Echazu, M.I.; Olivetti, C.E.; Desimone, M.F. Synthesis and Characterization of Ibandronate-Loaded Silica Nanoparticles and Collagen Nanocomposites. Curr. Pharm. Biotechnol. 2015, 16, 661–667. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Hu, Y.; Yuan, W.; Qiu, X.; Jiang, T.; Xia, C.; Xiong, L.; Li, F.; Gao, Y. EDTA-Modified 17beta-Estradiol-Laden Upconversion Nanocomposite for Bone-Targeted Hormone Replacement Therapy for Osteoporosis. Theranostics 2020, 10, 3281–3292. [Google Scholar] [CrossRef]
- Lei, L.; Liu, Z.; Yuan, P.; Jin, R.; Wang, X.; Jiang, T.; Chen, X. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J. Mater. Chem. B 2019. [Google Scholar] [CrossRef]
- Amoozgar, Z.; Yeo, Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Karpov, T.E.; Peltek, O.O.; Muslimov, A.R.; Tarakanchikova, Y.V.; Grunina, T.M.; Poponova, M.S.; Karyagina, A.S.; Chernozem, R.V.; Pariy, I.O.; Mukhortova, Y.R.; et al. Development of Optimized Strategies for Growth Factor Incorporation onto Electrospun Fibrous Scaffolds To Promote Prolonged Release. ACS Appl. Mater. Interfaces 2020, 12, 5578–5592. [Google Scholar] [CrossRef]
- He, S.; Lin, K.F.; Fan, J.J.; Hu, G.; Dong, X.; Zhao, Y.N.; Song, Y.; Guo, Z.S.; Bi, L.; Liu, J. Synergistic Effect of Mesoporous Silica and Hydroxyapatite in Loaded Poly(DL-lactic-co-glycolic acid) Microspheres on the Regeneration of Bone Defects. Biomed. Res. Int. 2016, 2016, 9824827. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Xu, Y.; Feng, P.; Xu, L.; Peng, S.; Deng, Y. Co-enhance bioactive of polymer scaffold with mesoporous silica and nano-hydroxyapatite. J. Biomater. Sci. Polym. Ed. 2019, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.F.; Gomide, V.S.; Da Silva Junior, A.C.; Goes, A.M.; De Sousa, E.M. An in situ synthesis of mesoporous SBA-16/hydroxyapatite for ciprofloxacin release: In vitro stability and cytocompatibility studies. J. Mater. Sci. Mater. Med. 2014, 25, 2527–2540. [Google Scholar] [CrossRef]
- Tamburaci, S.; Kimna, C.; Tihminlioglu, F. Bioactive diatomite and POSS silica cage reinforced chitosan/Na-carboxymethyl cellulose polyelectrolyte scaffolds for hard tissue regeneration. Mater. Sci. Eng. C 2019. [Google Scholar] [CrossRef]
- Gaihre, B.; Lecka-Czernik, B.; Jayasuriya, A.C. Injectable nanosilica-chitosan microparticles for bone regeneration applications. J. Biomater. Appl. 2017. [Google Scholar] [CrossRef]
- Khatami, N.; Khoshfetrat, A.B.; Khaksar, M.; Zamani, A.R.N.; Rahbarghazi, R. Collagen-alginate-nano-silica microspheres improved the osteogenic potential of human osteoblast-like MG-63 cells. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef]
- Sun, J.L.; Jiao, K.; Song, Q.; Ma, C.F.; Ma, C.; Tay, F.R.; Niu, L.N.; Chen, J.H. Intrafibrillar silicified collagen scaffold promotes in-situ bone regeneration by activating the monocyte p38 signaling pathway. Acta Biomater. 2017, 354–365. [Google Scholar] [CrossRef]
- Dai, C.; Guo, H.; Lu, J.; Shi, J.; Wei, J.; Liu, C. Osteogenic evaluation of calcium/magnesium-doped mesoporous silica scaffold with incorporation of rhBMP-2 by synchrotron radiation-based muCT. Biomaterials 2011, 32, 8506–8517. [Google Scholar] [CrossRef]
- Peng, X.Y.; Hu, M.; Liao, F.; Yang, F.; Ke, Q.F.; Guo, Y.P.; Zhu, Z.H. La-Doped mesoporous calcium silicate/chitosan scaffolds for bone tissue engineering. Biomater. Sci. 2019, 7, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Xia, L.; Chen, Z.; Lv, F.; Zhu, H.; Wei, F.; Han, S.; Chang, J.; Xiao, Y.; Wu, C. Europium-doped mesoporous silica nanosphere as an immune-modulating osteogenesis/angiogenesis agent. Biomaterials 2017, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Peng, X.Y.; Yang, F.; Ke, Q.F.; Zhu, Z.H.; Guo, Y.P. Gadolinium-doped mesoporous calcium silicate/chitosan scaffolds enhanced bone regeneration ability. Mater. Sci. Eng. C 2019, 104, 109999. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Coelho, P.G.; Kang, B.S.; Sul, Y.T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef]
- Ding, Y.; Yuan, Z.; Liu, P.; Cai, K.; Liu, R. Fabrication of strontium-incorporated protein supramolecular nanofilm on titanium substrates for promoting osteogenesis. Mater. Sci. Eng. C 2020, 111, 110851. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z. Involvement of FAK/P38 Signaling Pathways in Mediating the Enhanced Osteogenesis Induced by Nano-Graphene Oxide Modification on Titanium Implant Surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef]
- Prabakaran, S.; Rajan, M.; Lv, C.; Meng, G. Lanthanides-Substituted Hydroxyapatite/Aloe vera Composite Coated Titanium Plate for Bone Tissue Regeneration. Int. J. Nanomed. 2020, 15, 8261–8279. [Google Scholar] [CrossRef]
- Chen, L.; Mou, S.; Li, F.; Zeng, Y.; Sun, Y.; Horch, R.E.; Wei, W.; Wang, Z.; Sun, J. Self-Assembled Human Adipose-Derived Stem Cell-Derived Extracellular Vesicle-Functionalized Biotin-Doped Polypyrrole Titanium with Long-Term Stability and Potential Osteoinductive Ability. ACS Appl. Mater. Interfaces 2019, 11, 46183–46196. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; He, L.; Huang, H.; Weng, J. Bio-surface coated titanium scaffolds with cancellous bone-like biomimetic structure for enhanced bone tissue regeneration. Acta Biomater. 2020, 114, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Ouyang, Z.; Tan, Y.; Wu, H.; Liu, Y. Activating macrophages for enhanced osteogenic and bactericidal performance by Cu ion release from micro/nano-topographical coating on a titanium substrate. Acta Biomater. 2019, 100, 415–426. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, Y.; Xu, W.; Weng, Z.; Cao, F.; Wan, X.; Cui, T.; Yu, Y.; Liao, L.; Wang, X. Tremella-Like ZnO@Col-I-Decorated Titanium Surfaces with Dual-Light-Defined Broad-Spectrum Antibacterial and Triple Osteogenic Properties. ACS Appl. Mater. Interfaces 2020, 12, 30044–30051. [Google Scholar] [CrossRef]
- Fu, X.; Liu, P.; Zhao, D.; Yuan, B.; Xiao, Z.; Zhou, Y.; Yang, X.; Zhu, X.; Tu, C.; Zhang, X. Effects of Nanotopography Regulation and Silicon Doping on Angiogenic and Osteogenic Activities of Hydroxyapatite Coating on Titanium Implant. Int. J. Nanomed. 2020, 15, 4171–4189. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef] [PubMed]
- Vrancken, B.; Thijs, L.; Kruth, J.P.; Van Humbeeck, J. Microstructure and mechanical properties of a novel β titanium metallic composite by selective laser melting. Acta Mater. 2014, 68, 150–158. [Google Scholar] [CrossRef]
- Wang, J.C.; Liu, Y.J.; Qin, P.; Liang, S.X.; Sercombe, T.B.; Zhang, L.C. Selective laser melting of Ti–35Nb composite from elemental powder mixture: Microstructure, mechanical behavior and corrosion behavior. Mater. Sci. Eng. 2019, 760, 214–224. [Google Scholar] [CrossRef]
- Leong, S.S.; Edith, W.F.; Yee, Y.W. Selective laser melting of titanium alloy with 50 wt% tantalum: Effect of laser process parameters on part quality. Int. J. Refract. Met. Hard Mater. 2018, 77, 120–127. [Google Scholar]
- Cho, H.R.; Roh, T.S.; Shim, K.W.; Kim, Y.O.; Lew, D.H.; Yun, I.S. Skull Reconstruction with Custom Made Three-Dimensional Titanium Implant. Arch. Craniofac.Surg. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Lim, J.Y.; Yun, I.S.; Kim, J.S.; Woo, S.H.; Kim, D.S.; Shim, K.W. Cranioplasty Enhanced by Three-Dimensional Printing: Custom-Made Three-Dimensional-Printed Titanium Implants for Skull Defects. J. Craniofac. Surg. 2016, 27, 943–949. [Google Scholar] [CrossRef]
- Lee, Y.W.; You, H.J.; Jung, J.A.; Kim, D.W. Mandibular reconstruction using customized three-dimensional titanium implant. Arch. Craniofac. Surg. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Hamid, K.S.; Parekh, S.G.; Adams, S.B. Salvage of Severe Foot and Ankle Trauma With a 3D Printed Scaffold. Foot Ankle Int. 2016, 37, 433–439. [Google Scholar] [CrossRef]
- Williams, L.R.; Fan, K.F.; Bentley, R.P. Custom-made titanium cranioplasty: Early and late complications of 151 cranioplasties and review of the literature. Int. J. Oral Maxillofac. Surg. 2015, 44, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Wu, L.; Zhou, C.; Fan, H.; Gou, M.; Li, Z.; Zhang, B.; Lei, H.; Sun, H.; Liang, J.; et al. 3D printed titanium scaffolds with homogeneous diamond-like structures mimicking that of the osteocyte microenvironment and its bone regeneration study. Biofabrication 2020. [Google Scholar] [CrossRef]
- Maietta, S.; Gloria, A.; Improta, G.; Richetta, M.; Martorelli, M. A Further Analysis on Ti6Al4V Lattice Structures Manufactured by Selective Laser Melting. J. Healthc. Eng. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ye, H.; Fang, J.; Zhong, C.; Yao, J.; Park, J.; Lu, X.; Ren, F. Engineering High-Resolution Micropatterns Directly onto Titanium with Optimized Contact Guidance to Promote Osteogenic Differentiation and Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 43888–43901. [Google Scholar] [CrossRef]
- Saruta, J.; Sato, N.; Ishijima, M.; Okubo, T.; Hirota, M.; Ogawa, T. Disproportionate Effect of Sub-Micron Topography on Osteoconductive Capability of Titanium. Int. J. Mol. Sci. 2019, 20, 4027. [Google Scholar] [CrossRef] [PubMed]
- Gorgin Karaji, Z.; Jahanmard, F.; Mirzaei, A.H.; Van der Wal, B.; Amin Yavari, S. A multifunctional silk coating on additively manufactured porous titanium to prevent implant-associated infection and stimulate bone regeneration. Biomed. Mater. 2020, 15, 065016. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Y.; Xu, D.; Gong, K. The Calcium Phosphate Modified Titanium Implant Combined With Platelet-Rich Plasma Treatment Promotes Implant Stabilization in an Osteoporotic Model. J. Craniofac. Surg. 2020. [Google Scholar] [CrossRef]
- Teng, F.Y.; Tai, I.C.; Ho, M.L.; Wang, J.W.; Weng, L.W.; Wang, Y.J.; Wang, M.W.; Tseng, C.C. Controlled release of BMP-2 from titanium with electrodeposition modification enhancing critical size bone formation. Mater. Sci. Eng. C 2019, 105, 109879. [Google Scholar] [CrossRef]
- Hung, C.C.; Chaya, A.; Liu, K.; Verdelis, K.; Sfeir, C. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway. Acta Biomater. 2019, 98, 246–255. [Google Scholar] [CrossRef]
- Hamushan, M.; Cai, W.; Zhang, Y.; Lou, T.; Zhang, S.; Zhang, X.; Cheng, P.; Zhao, C.; Han, P. High-purity magnesium pin enhances bone consolidation in distraction osteogenesis model through activation of the VHL/HIF-1alpha/VEGF signaling. J. Biomater. Appl. 2020, 35, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Tie, D.; Guan, R.; Liu, H.; Cipriano, A.; Liu, Y.; Wang, Q.; Huang, Y.; Hort, N. An in vivo study on the metabolism and osteogenic activity of bioabsorbable Mg-1Sr alloy. Acta Biomater. 2016, 29, 455–467. [Google Scholar] [CrossRef]
- Niu, J.; Xiong, M.; Guan, X.; Zhang, J.; Huang, H.; Pei, J.; Yuan, G. The in vivo degradation and bone-implant interface of Mg-Nd-Zn-Zr alloy screws: 18 months post-operation results. Corros. Sci. 2016, 113, 183–187. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Esteban-Lucia, M.; Martinez-Campos, E.; Mohedano, M.; Arrabal, R.; Blawert, C.; Zheludkevich, M.L.; Matykina, E. PEO coatings design for Mg-Ca alloy for cardiovascular stent and bone regeneration applications. Mater. Sci. Eng. C 2019, 105, 110026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ni, N.; Su, Y.; Miao, H.; Tang, Z.; Ji, Y.; Wang, Y.; Gao, H.; Ju, Y.; Sun, N.; et al. Targeting Local Osteogenic and Ancillary Cells by Mechanobiologically Optimized Magnesium Scaffolds for Orbital Bone Reconstruction in Canines. ACS Appl. Mater. Interfaces 2020, 12, 27889–27904. [Google Scholar] [CrossRef]
- Perumal, G.; Ramasamy, B.; Nandkumar, A.M.; Dhanasekaran, S.; Ramasamy, S.; Doble, M. Bilayer nanostructure coated AZ31 magnesium alloy implants: In vivo reconstruction of critical-sized rabbit femoral segmental bone defect. Nanomedicine 2020, 29, 102232. [Google Scholar] [CrossRef]
- Lin, Z.; Zhao, Y.; Chu, P.K.; Wang, L.; Pan, H.; Zheng, Y.; Wu, S.; Liu, X.; Cheung, K.M.C.; Wong, T.; et al. A functionalized TiO2/Mg2TiO4 nano-layer on biodegradable magnesium implant enables superior bone-implant integration and bacterial disinfection. Biomaterials 2019, 219, 119372. [Google Scholar] [CrossRef]
- Bessa-Goncalves, M.; Silva, A.M.; Bras, J.P.; Helmholz, H.; Luthringer-Feyerabend, B.J.C.; Willumeit-Romer, R.; Barbosa, M.A.; Santos, S.G. Fibrinogen and magnesium combination biomaterials modulate macrophage phenotype, NF-kB signaling and crosstalk with mesenchymal stem/stromal cells. Acta Biomater. 2020, 114, 471–484. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Wang, L.; Yuan, G.; Dai, K.; Pei, J.; Hao, Y. Dual modulation of bone formation and resorption with zoledronic acid-loaded biodegradable magnesium alloy implants improves osteoporotic fracture healing: An in vitro and in vivo study. Acta Biomater. 2018, 65, 486–500. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Sapari, M.A.B.; Nai, M.L.S.; Meenashisundaram, G.K.; Gupta, M. Additive manufacturing of magnesium–zinc–zirconium (ZK) alloys via capillary-mediated binderless three-dimensional printing—ScienceDirect. Mater. Des. 2019, 169, 107683. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.A.; Fockaert, L.I.; Pouran, B.; Tumer, N.; Schroder, K.U.; Mol, J.M.C.; Weinans, H.; et al. Additively manufactured biodegradable porous magnesium. Acta Biomater. 2018, 67, 378–392. [Google Scholar] [CrossRef]
- Zhu, D.; Su, Y.; Young, M.L.; Ma, J.; Zheng, Y.; Tang, L. Biological Responses and Mechanisms of Human Bone Marrow Mesenchymal Stem Cells to Zn and Mg Biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 27453–27461. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Y.; Xu, X.; Lu, Y.; Chen, L.; Li, D.; Dai, Y.; Kang, Y.; Yu, K. Investigation on the microstructure, mechanical properties, in vitro degradation behavior and biocompatibility of newly developed Zn-0.8%Li-(Mg, Ag) alloys for guided bone regeneration. Mater. Sci. Eng. C 2019, 99, 1021–1034. [Google Scholar] [CrossRef]
- Montani, M.; Demir, A.G.; Mostaed, E.; Vedani, M.; Previtali, B. Processability of pure Zn and pure Fe by SLM for biodegradable metallic implant manufacturing. Rapid Prototyp. J. 2017. [Google Scholar] [CrossRef]
- Li, Y.; Pavanram, P.; Zhou, J.; Lietaert, K.; Bobbert, F.S.L.; Kubo, Y.; Leeflang, M.A.; Jahr, H.; Zadpoor, A.A. Additively manufactured functionally graded biodegradable porous zinc. Biomater. Sci. 2020, 8, 2404–2419. [Google Scholar] [CrossRef] [PubMed]
- Lietaert, K.; Zadpoor, A.A.; Sonnaert, M.; Schrooten, J.; Weber, L.; Mortensen, A.; Vleugels, J. Mechanical properties and cytocompatibility of dense and porous Zn produced by laser powder bed fusion for biodegradable implant applications. Acta Biomater. 2020, 110, 289–302. [Google Scholar] [CrossRef]
- Jakhmola, A.; Vecchione, R.; Gentile, F.; Profeta, M.; Manikas, A.C.; Battista, E.; Celentano, M.; Onesto, V.; Netti, P.A. Experimental and theoretical study of biodirected green synthesis of gold nanoflowers. Mater. Today Chem. 2019, 14. [Google Scholar] [CrossRef]
- Jakhmola, A.; Vecchione, R.; Onesto, V.; Gentile, F.; Profeta, M.; Battista, E.; Manikas, A.C.; Netti, P.A. A theoretical and experimental study on L-tyrosine and citrate mediated sustainable production of near infrared absorbing twisted gold nanorods. Mat. Sci. Eng. C 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Celentano, M.; Jakhmola, A.; Profeta, M.; Battista, E.; Guarnieri, D.; Gentile, F.; Netti, P.A.; Vecchione, R. Diffusion limited green synthesis of ultra-small gold nanoparticles at room temperature. Colloid Surf. A 2018, 558, 548–557. [Google Scholar] [CrossRef]
- Russo, T.; Gloria, A.; De Santis, R.; D’Amora, U.; Balato, G.; Vollaro, A.; Oliviero, O.; Improta, G.; Triassi, M.; Ambrosio, L. Preliminary focus on the mechanical and antibacterial activity of a PMMA-based bone cement loaded with gold nanoparticles. Bioact. Mater. 2017, 2, 156–161. [Google Scholar] [CrossRef]
- Liao, J.; Shi, K.; Jia, Y.; Wu, Y.; Qian, Z. Gold nanorods and nanohydroxyapatite hybrid hydrogel for preventing bone tumor recurrence via postoperative photothermal therapy and bone regeneration promotion. Bioact. Mater. 2021, 6, 2221–2230. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, P.; Zhao, Q.; Shen, K.; Qiu, Y.; Xiao, Y.; Yuan, Q.; Zhang, Y. Dual Functional Monocytes Modulate Bactericidal and Anti-Inflammation Process for Severe Osteomyelitis Treatment. Small 2020, 16, e2002301. [Google Scholar] [CrossRef]
- Sanchez-Casanova, S.; Martin-Saavedra, F.M.; Escudero-Duch, C.; Uceda, M.I.F.; Prieto, M.; Arruebo, M.; Acebo, P.; Fabiilli, M.L.; Franceschi, R.T.; Vilaboa, N. Local delivery of bone morphogenetic protein-2 from near infrared-responsive hydrogels for bone tissue regeneration. Biomaterials 2020, 241. [Google Scholar] [CrossRef]
| Type | Advantages | Drawbacks |
|---|---|---|
| nHA/polymer composites |
| Not custom-made |
| 3D printed nHA-based inorganic nanomaterials |
| Immature design and manufacturing methods [35] |
| MSNs | Seldom used alone in bone regeneration | |
| Ti-based nanomaterials | ||
| Mg-based biomaterials |
|
|
| Zn-based biomaterials |
|
|
| AuNPs |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Cui, S.; Luo, D.; Liu, Y. Novel Inorganic Nanomaterial-Based Therapy for Bone Tissue Regeneration. Nanomaterials 2021, 11, 789. https://doi.org/10.3390/nano11030789
Fu Y, Cui S, Luo D, Liu Y. Novel Inorganic Nanomaterial-Based Therapy for Bone Tissue Regeneration. Nanomaterials. 2021; 11(3):789. https://doi.org/10.3390/nano11030789
Chicago/Turabian StyleFu, Yu, Shengjie Cui, Dan Luo, and Yan Liu. 2021. "Novel Inorganic Nanomaterial-Based Therapy for Bone Tissue Regeneration" Nanomaterials 11, no. 3: 789. https://doi.org/10.3390/nano11030789
APA StyleFu, Y., Cui, S., Luo, D., & Liu, Y. (2021). Novel Inorganic Nanomaterial-Based Therapy for Bone Tissue Regeneration. Nanomaterials, 11(3), 789. https://doi.org/10.3390/nano11030789