Electrospun PCL-Based Vascular Grafts: In Vitro Tests
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning for Graft Fabrication
2.3. Cell Cultures
2.4. Immunofluorescence Staining
2.5. Morphological Analysis
2.6. Cell Shape Analysis
2.7. Proliferation Assay
2.8. Lactate Dehidrogenase (LDH) Activity
2.9. ELISA Assays
2.10. Beta-Galactosidase Staining (SA-b GAL) Staining
2.11. ROS/RNS Assay
2.12. RNA Extraction and First-Strand cDNA Synthesis
2.13. Real-Time PCR
2.14. Statistical Analysis
3. Results
3.1. Scaffold Characterization
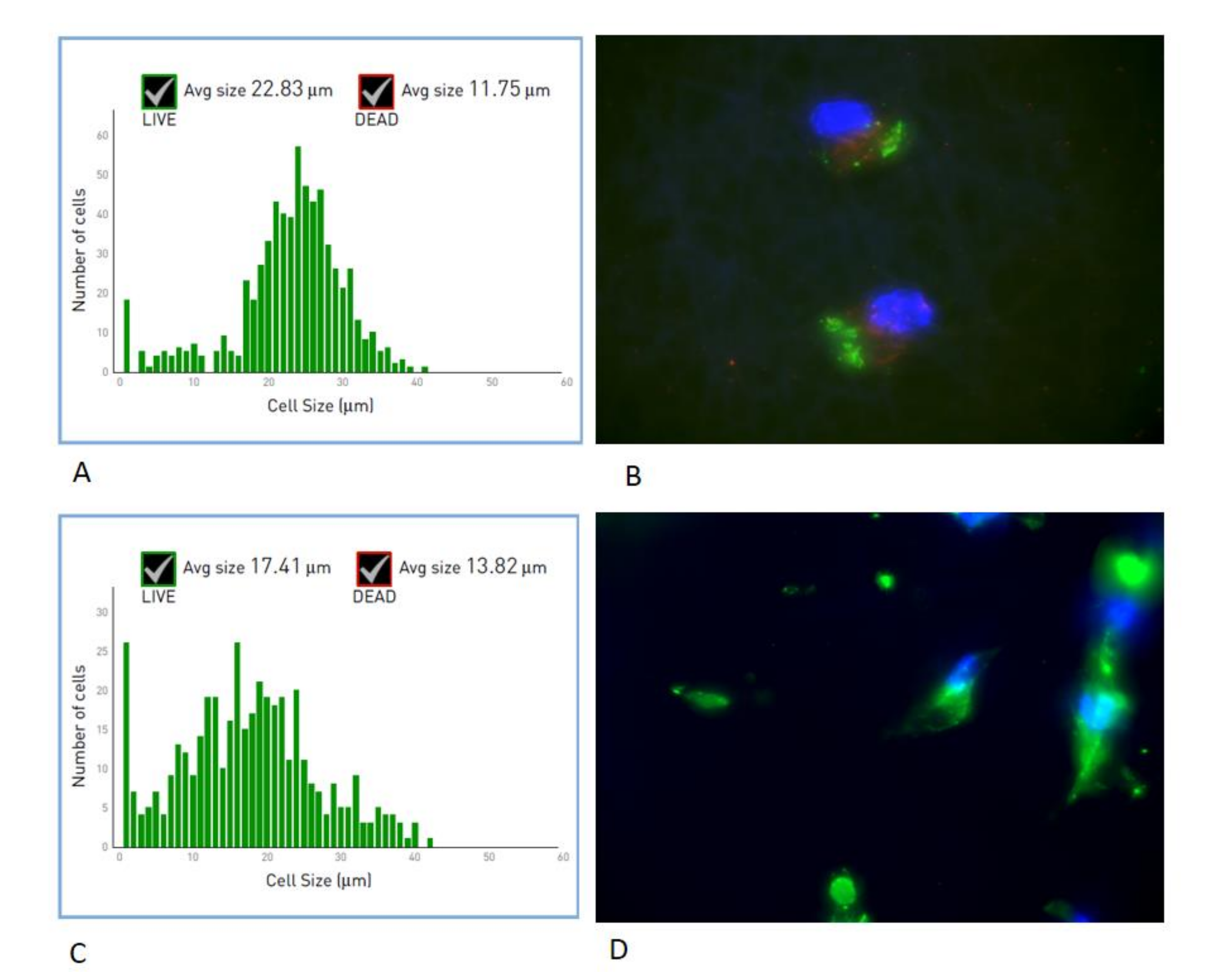
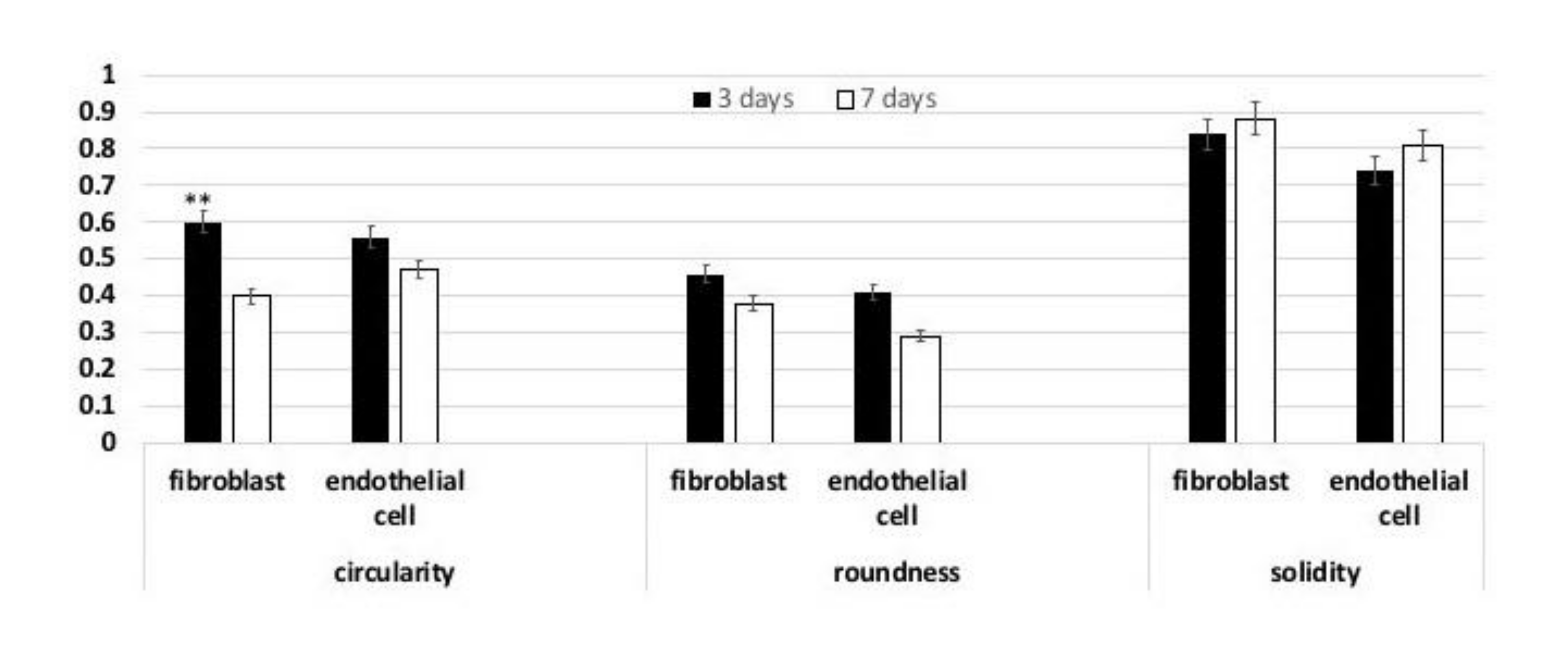
3.2. Cell Shape
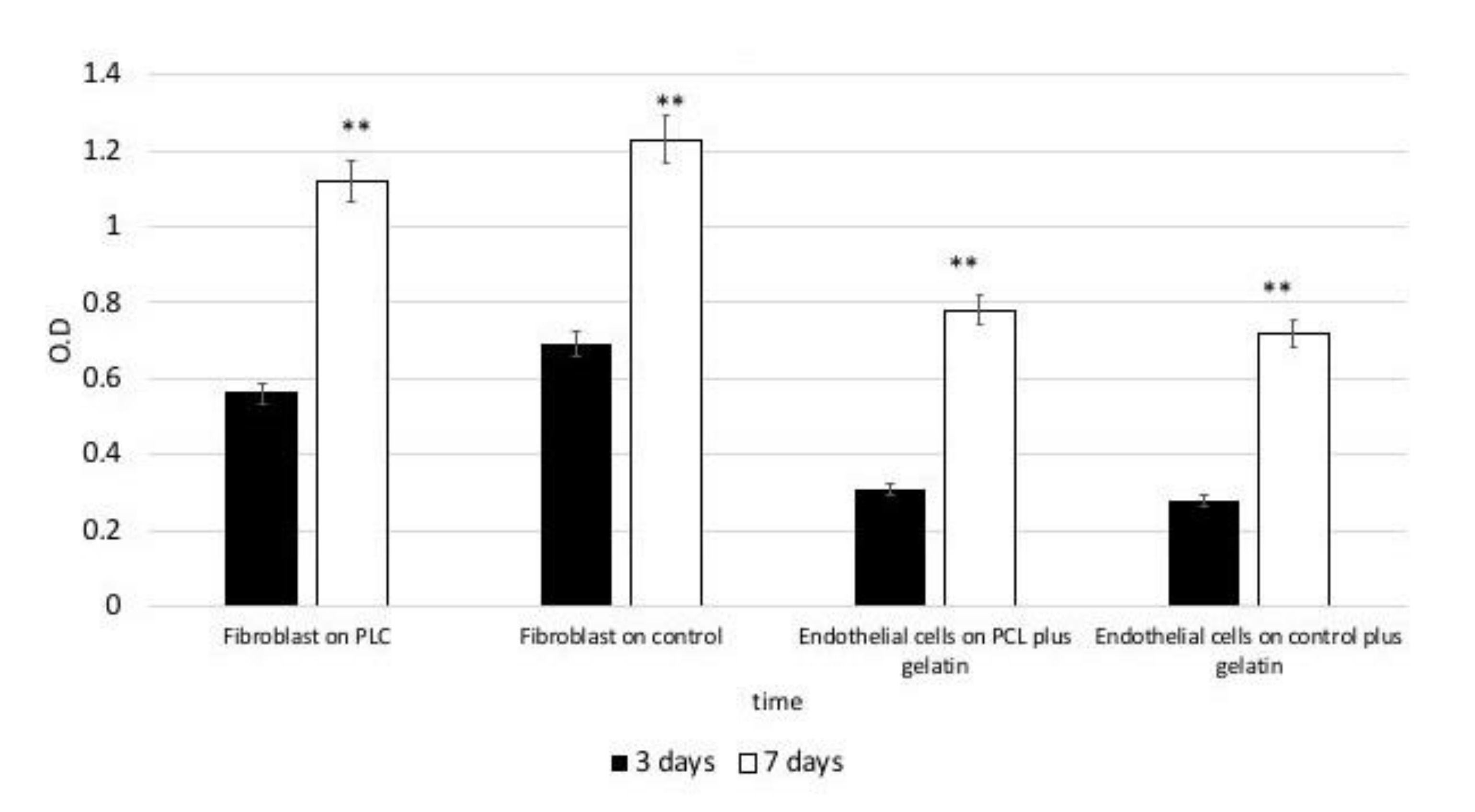
3.3. MTT
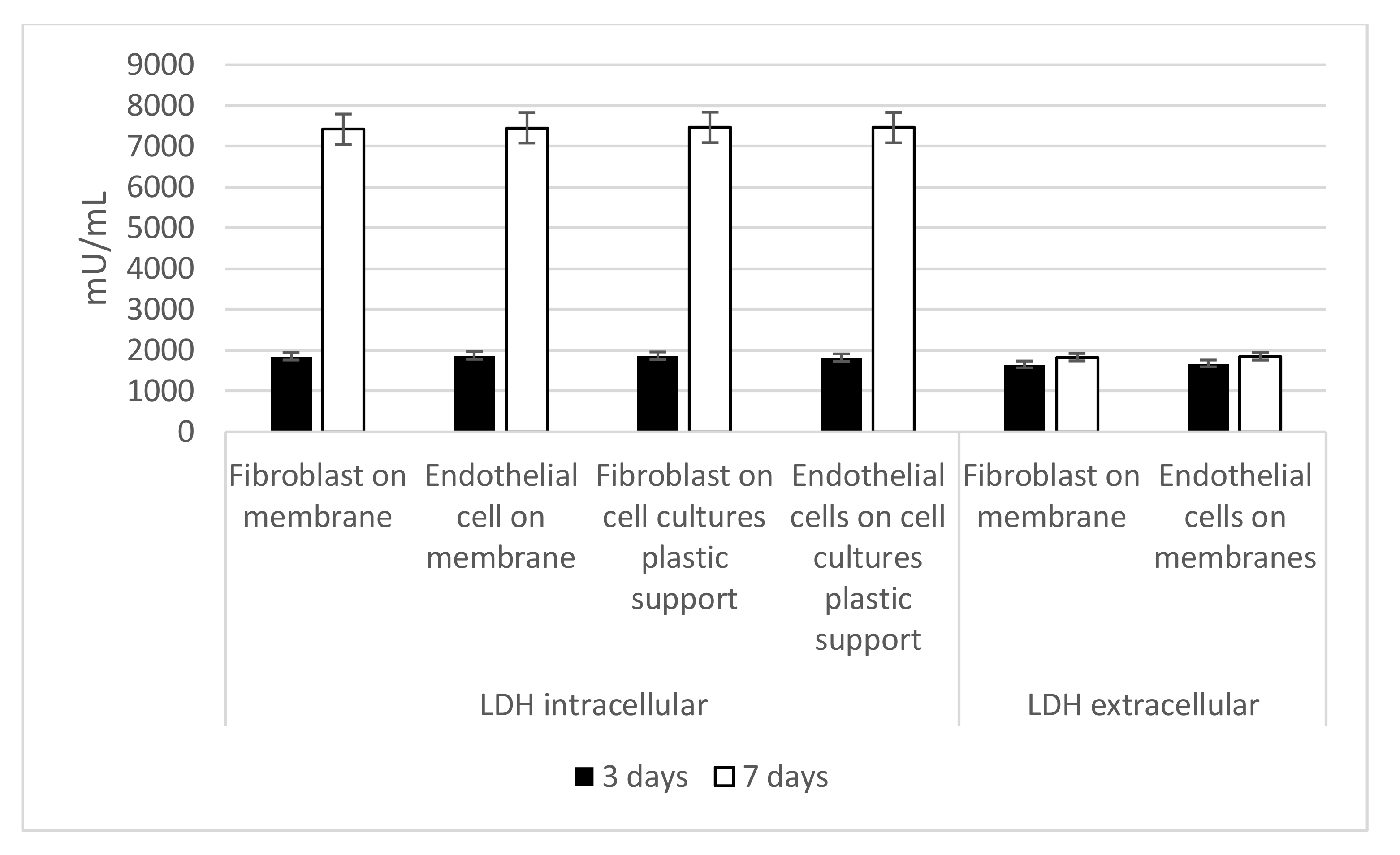
3.4. Intracellular and Extracellular LDH Activity
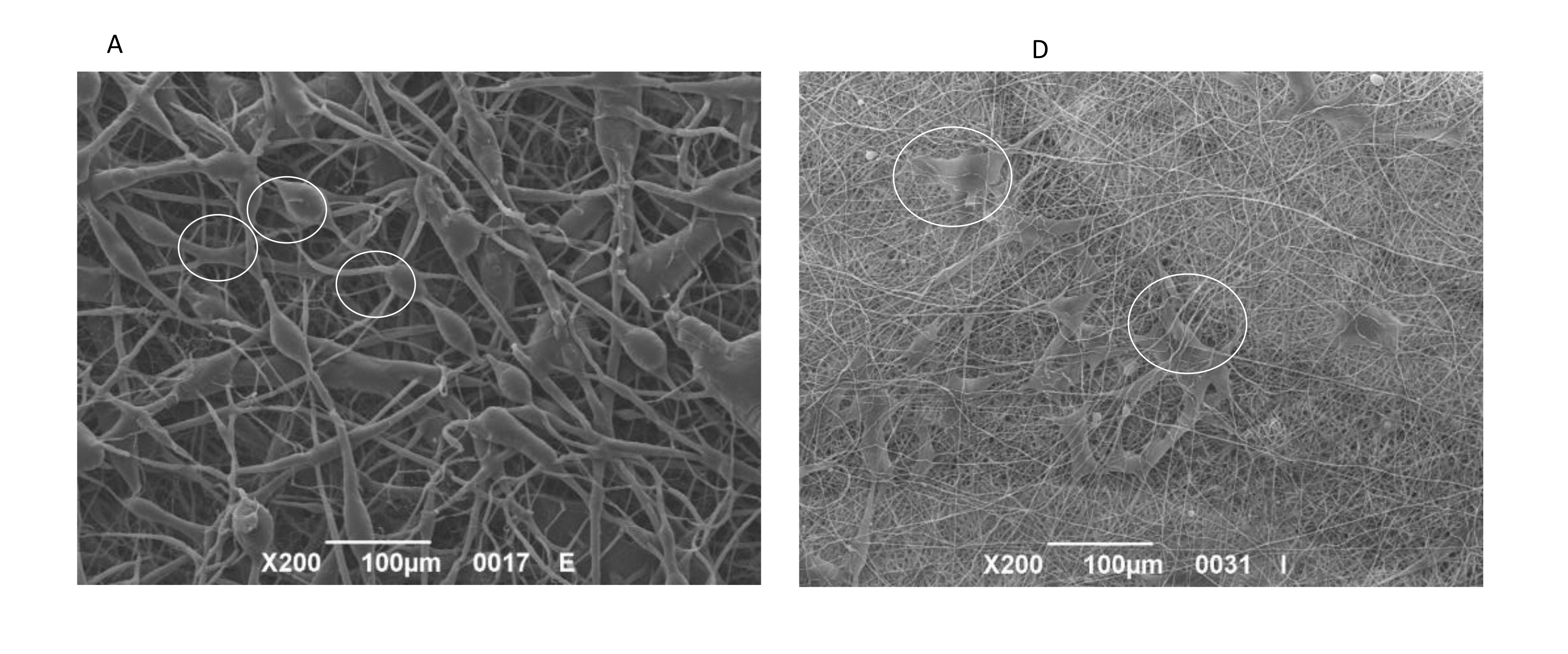
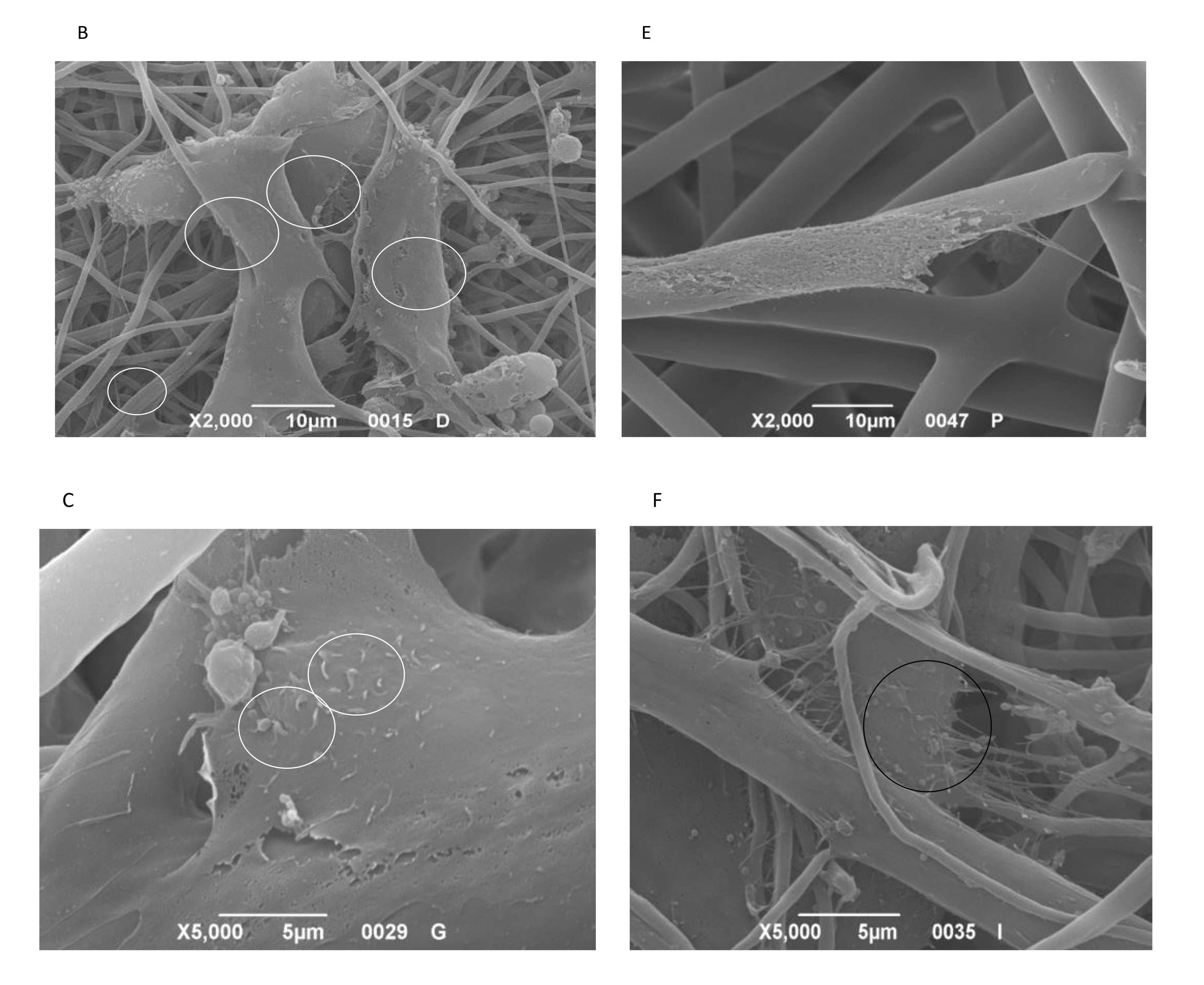
3.5. SEM
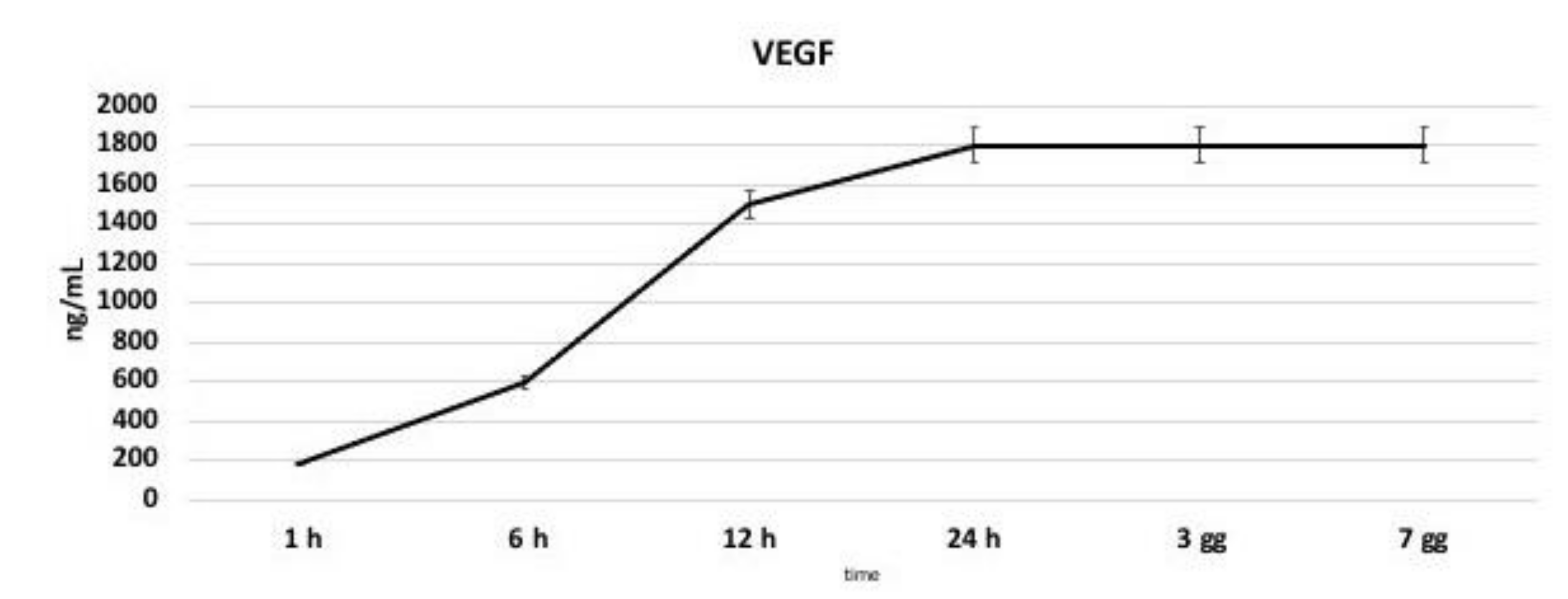
3.6. VEGF Production
3.7. Senescence
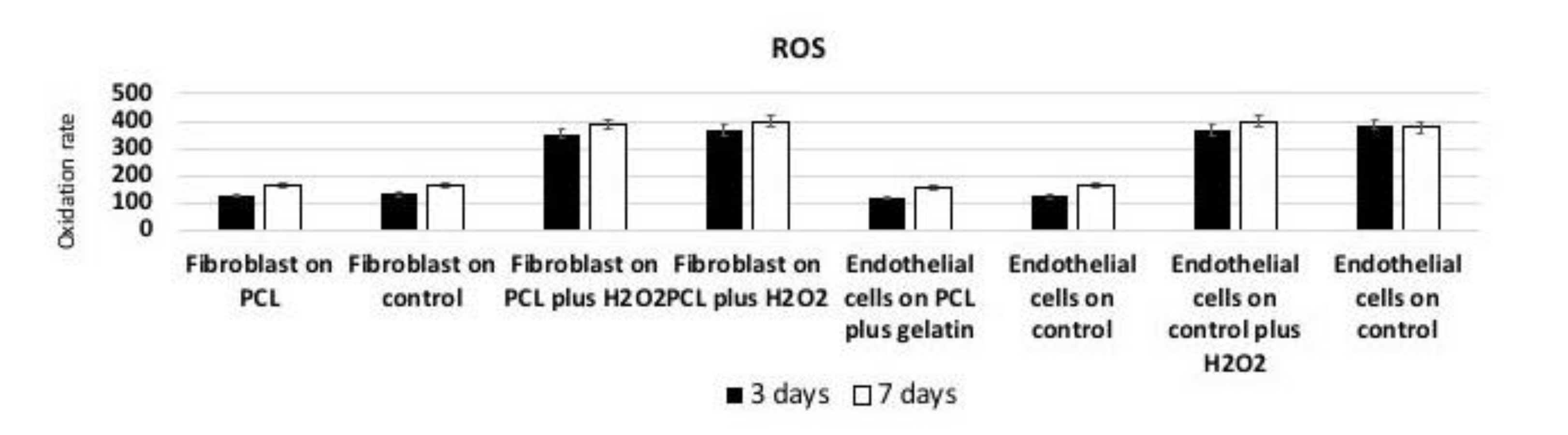
3.8. Reactive Oxygen Species (ROS) Production
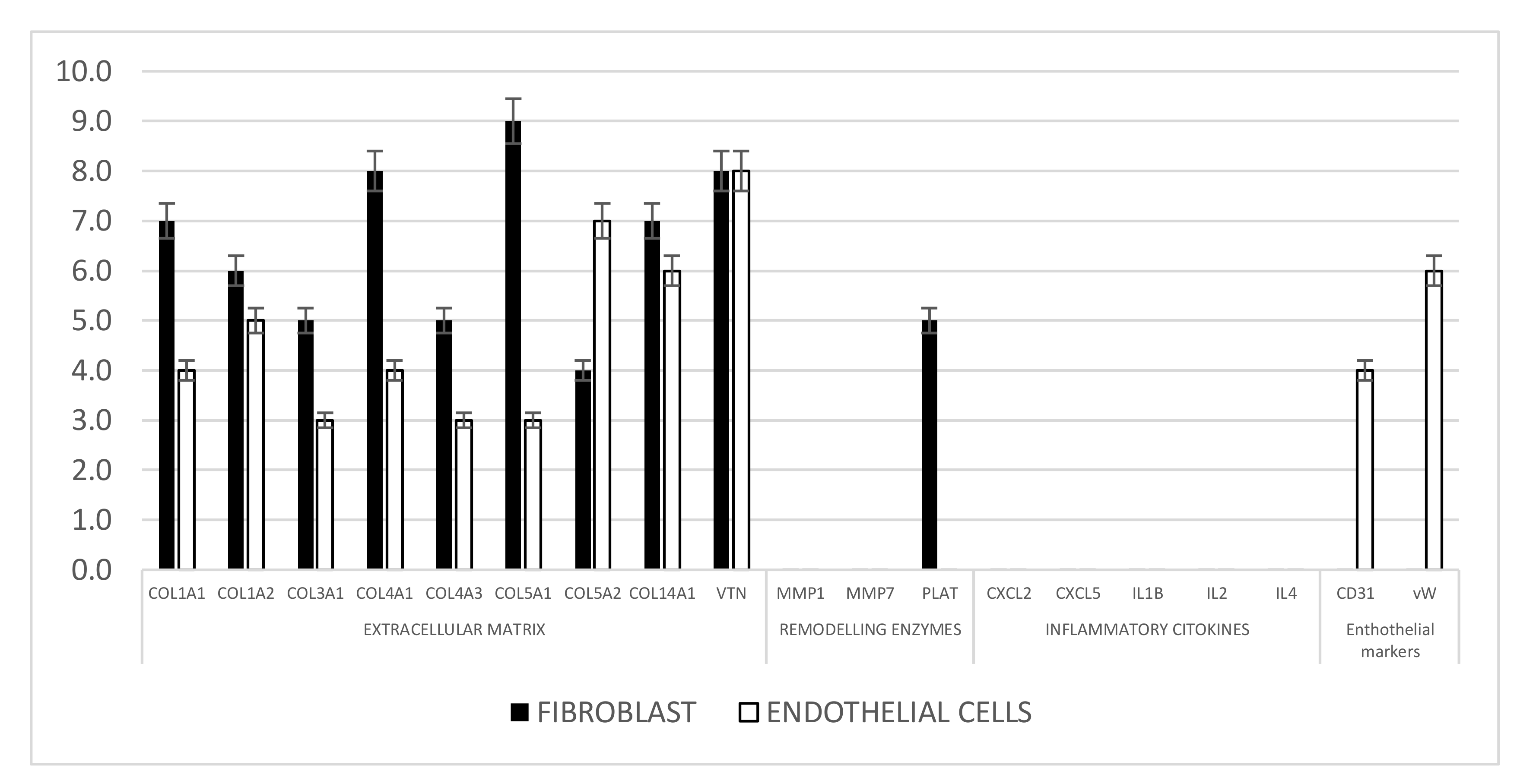
3.9. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smits, P.C.; Chang, C.C.; Chevalier, B.; West, N.E.; Gori, T.; Barbato, E.; Tarantini, G.; Kocka, V.; Achenbach, S.; Dudek, D.; et al. Bioresorbable vascular scaffold versus metallic drug-eluting stent in patients at high risk of restenosis: The COMPARE-ABSORB randomised clinical trial. EuroIntervention 2020, 16, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kang, D.-Y.; Hong, S.-J.; Ahn, J.-M.; Ahn, C.-M.; Park, D.-W.; Kim, J.-S.; Kim, B.-K.; Ko, Y.-G.; Choi, D.; et al. Optical Coherence Tomography for Coronary Bioresorbable Vascular Scaffold Implantation. Circ. Cardiovasc. Interv. 2020, 13, e008383. [Google Scholar] [CrossRef]
- Makkar, R.R.; Cheng, W.; Waksman, R.; Satler, L.F.; Chakravarty, T.; Groh, M.; Abernethy, W.; Russo, M.J.; Heimansohn, D.; Hermiller, J.; et al. Self-expanding intra-annular versus commercially available transcatheter heart valves in high and extreme risk patients with severe aortic stenosis (PORTICO IDE): A randomised, controlled, non-inferiority trial. Lancet 2020, 396, 669–683. [Google Scholar] [CrossRef]
- Akubov, S.J.; Van Mieghem, N.M.; Reardon, M.J.; Serruys, P.W.; Gada, H.; Mumtaz, M.; Deeb, G.M.; Kodali, S.; George, I.; Windecker, S.; et al. Propensity-Matched Comparison of Evolut-R Transcatheter Aortic Valve Implantation with Surgery in Intermediate-Risk Patients (from the SURTAVI Trial). Am. J. Cardiol. 2020, 131. [Google Scholar] [CrossRef]
- Bosiers, M.; Deloose, K.; Callaert, J.; Verbist, J.; Hendriks, J.; Lauwers, P.; Schroë, H.; Lansink, W.; Scheinert, D.; Schmidt, A.; et al. Stent-grafts are the best way to treat complex in-stent restenosis lesions in the superficial femoral artery: 24-month results from a multicenter randomized trial. J. Cardiovasc. Surg. 2020, 61, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Stollwerck, P.L.; Kozlowski, B.; Sandmann, W.; Grabitz, K.; Pfeiffer, T. Long-term dilatation of polyester and expanded polytetrafluoroethylene tube grafts after open repair of infrarenal abdominal aortic aneurysms. J. Vasc. Surg. 2011, 53, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- McQuade, K.; Gable, D.; Pearl, G.; Theune, B.; Black, S. Four-year randomized prospective comparison of percutaneous ePTFE/nitinol self-expanding stent graft versus prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive disease. J. Vasc. Surg. 2010, 52, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Davidovic, L.; Jakovljevic, N.; Radak, D.; Dragas, M.; Ilic, N.; Koncar, I.; Markovic, D. Dacron or ePTFE graft for above-knee femoropopliteal bypass reconstruction. A bi-centre randomised study. Vasa 2010, 39, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bajardi, G.; Pecoraro, F.; Mirabella, D. Efficacy of TachoSil patches in controlling Dacron suture-hole bleeding after abdominal aortic aneurysm open repair. J Cardiothorac. Surg. 2009, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, P.; Sun, X.; Gong, F.; Yang, S.; Shen, L.; Huang, Z.; Wang, C. Synthetic ePTFE Grafts Coated with an Anti-CD133 Antibody-Functionalized Heparin/Collagen Multilayer with Rapid in vivo Endothelialization Properties. ACS Appl. Mater. Interfaces 2013, 5, 7360–7369. [Google Scholar] [CrossRef]
- Mirmohammadsadeghi, A.; Jahannama, N.; Mirmohammadsadeghi, M. Sleep Quality after Coronary Artery Bypass Graft Surgery: Comparing Pulsatile and Nonpulsatile Pump Flow. J. Extra Corpor. Technol. 2020, 52, 314–318. [Google Scholar] [CrossRef]
- Jin, L.; Pan, R.; Huang, L.; Zhang, H.; Jiang, M.; Zhao, H. Family nursing with the assistance of network improves clinical outcome and life quality in patients underwent coronary artery bypass grafting: A consolidated standards of reporting trials-compliant randomized controlled trial. Medicine 2020, 99, e23488. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Salerno, A.; Cesarelli, G.; Pedram, P.; Netti, P.A. Modular Strategies to Build Cell-Free and Cell-Laden Scaffolds towards Bioengineered Tissues and Organs. J. Clin. Med. 2019, 8, 1816. [Google Scholar] [CrossRef] [PubMed]
- Leal, B.B.J.; Wakabayashi, N.; Oyama, K.; Kamiya, H.; Braghirolli, D.I.; Pranke, P. Vascular Tissue Engineering: Polymers and Methodologies for Small Caliber Vascular Grafts. Front Cardiovasc Med. 2021, 7, 592361. [Google Scholar] [CrossRef]
- Liu, R.H.; Ong, C.S.; Fukunishi, T.; Ong, K.; Hibino, N. Review of Vascular Graft Studies in Large Animal Models. Tissue Eng. Part B Rev. 2018, 24, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Cirillo, V.; Ambrosio, L. Bicomponent electrospun scaffolds to design extracellular matrix tissue analogs. Expert Rev. Med. Devices 2015, 13, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Gardin, C.; Ferroni, L.; Latremouille, C.; Chachques, J.C.; Mitrečić, D.; Zavan, B. Recent Applications of Three Dimensional Printing in Cardiovascular Medicine. Cells 2020, 9, 742. [Google Scholar] [CrossRef]
- Cruz-Maya, I.; Varesano, A.; Vineis, C.; Guarino, V. Comparative Study on Protein-Rich Electrospun Fibers for in Vitro Applications. Polymers 2020, 12, 1671. [Google Scholar] [CrossRef] [PubMed]
- Nazarnezhad, S.; Baino, F.; Kim, H.-W.; Webster, T.J.; Kargozar, S. Electrospun Nanofibers for Improved Angiogenesis: Promises for Tissue Engineering Applications. Nanomaterials 2020, 10, 1609. [Google Scholar] [CrossRef]
- Guaccio, A.; Guarino, V.; Perez, M.A.A.-; Cirillo, V.; Netti, P.A.; Ambrosio, L. Influence of electrospun fiber mesh size on hMSC oxygen metabolism in 3D collagen matrices: Experimental and theoretical evidences. Biotechnol. Bioeng. 2011, 108, 1965–1976. [Google Scholar] [CrossRef]
- Johnson, R.; Ding, Y.; Nagiah, N.; Monnet, E.; Tan, W. Coaxially-structured fibres with tailored material properties for vascular graft implant. Mater. Sci. Eng. C 2019, 97, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Wang, J.; Zhang, C.; Yan, H.; Zhu, M.; Wang, K.; Li, C.; Xu, Q.; Kong, D. Effect of Resveratrol on Modulation of Endothelial Cells and Macrophages for Rapid Vascular Regeneration from Electrospun Poly(ε-caprolactone) Scaffolds. ACS Appl. Mater. Interfaces 2017, 9, 19541–19551. [Google Scholar] [CrossRef] [PubMed]
- Bucci, R.; Vaghi, F.; Erba, E.; Romanelli, A.; Gelmi, M.L.; Clerici, F. Peptide grafting strategies before and after electrospinning of nanofibers. Acta Biomater. 2020. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Gradys, A.; Sajkiewicz, P. Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering. Polymers 2020, 12, 2636. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Rault, F.; Lewandowski, M.; Mohsenzadeh, E.; Salaün, F. Electrospun PVDF Nanofibers for Piezoelectric Applications: A Review of the Influence of Electrospinning Parameters on the beta Phase and Crystallinity Enhancement. Polymers 2021, 13, 174. [Google Scholar] [CrossRef]
- Ye, K.; Kuang, H.; You, Z.; Morsi, Y.; Mo, X. Electrospun Nanofibers for Tissue Engineering with Drug Loading and Release. Pharmaceutics 2019, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, Y.; Li, Q.; Turng, L.-S. Artificial small-diameter blood vessels: Materials, fabrication, surface modification, mechanical properties, and bioactive functionalities. J. Mater. Chem. B 2020, 8, 1801–1822. [Google Scholar] [CrossRef] [PubMed]
- Zagho, M.M.; Hussein, E.A.; Elzatahry, A.A. Recent Overviews in Functional Polymer Composites for Biomedical Applications. Polymers 2018, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-Based Electrospun Nanofibers for Biomedical Applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef]
- Lepidi, S.; Grego, F.; Vindigni, V.; Zavan, B.; Tonello, C.; Deriu, G.; Abatangelo, G.; Cortivo, R. Hyaluronan Biodegradable Scaffold for Small-caliber Artery Grafting: Preliminary Results in an Animal Model. Eur. J. Vasc. Endovasc. Surg. 2006, 32, 411–417. [Google Scholar] [CrossRef]
- Pandis, L.; Zavan, B.; Bassetto, F.; Ferroni, L.; Iacobellis, L.; Abatangelo, G.; Lepidi, S.; Cortivo, R.; Vindigni, V. Hyaluronic acid biodegradable material for reconstruction of vascular wall: A preliminary study in rats. Microsurgery 2011, 31, 138–145. [Google Scholar] [CrossRef]
- Zavan, B.; Vindigni, V.; Lepidi, S.; Iacopetti, I.; Avruscio, G.; Abatangelo, G.; Cortivo, R. Neoarteries grown in vivo using a tissue-engineered hyaluronan-based scaffold. FASEB J. 2008, 22, 2853–2861. [Google Scholar] [CrossRef]
- Morganti, C.; Bonora, M.; Marchi, S.; Ferroni, L.; Gardin, C.; Wieckowski, M.R.; Giorgi, C.; Pinton, P.; Zavan, B. Citrate Mediates Crosstalk between Mitochondria and the Nucleus to Promote Human Mesenchymal Stem Cell In Vitro Osteogenesis. Cells 2020, 9, 1034. [Google Scholar] [CrossRef]
- Zavan, B.; Ferroni, L.; Gardin, C.; Sivolella, S.; Piattelli, A.; Mijiritsky, E. Release of VEGF from Dental Implant Improves Osteogenetic Process: Preliminary In Vitro Tests. Materials 2017, 10, 1052. [Google Scholar] [CrossRef]
- Mijiritsky, E.; Ferroni, L.; Gardin, C.; Bressan, E.; Zanette, G.; Piattelli, A.; Zavan, B. Porcine Bone Scaffolds Adsorb Growth Factors Secreted by MSCs and Improve Bone Tissue Repair. Materials 2017, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Zago, M.; Patergnani, S.; Campbell, S.E.; Hébert, L.; Nielsen, M.; Scarpa, C.; Bassetto, F.; Pinton, P.; Zavan, B. Fluorescent Light Energy (FLE) Acts on Mitochondrial Physiology Improving Wound Healing. J. Clin. Med. 2020, 9, 559. [Google Scholar] [CrossRef]
- Gardin, C.; Bosco, G.; Ferroni, L.; Quartesan, S.; Rizzato, A.; Tatullo, M.; Zavan, B. Hyperbaric Oxygen Therapy Improves the Osteogenic and Vasculogenic Properties of Mesenchymal Stem Cells in the Presence of Inflammation In Vitro. Int. J. Mol. Sci. 2020, 21, 1452. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Gardin, C.; Sivolella, S.; Bressan, E.; Zavan, B. A hyaluronan-based scaffold for the in vitro construction of dental pulp-like tissue. Int. J. Mol. Sci. 2015, 16, 4666–4681. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Cirillo, V.; Taddei, P.; Alvarez-Perez, M.A.; Ambrosio, L. Tuning Size Scale and Crystallinity of PCL Electrospun Fibres via Solvent Permittivity to Address hMSC Response. Macromol. Biosci. 2011, 11, 1694–1705. [Google Scholar] [CrossRef]
- Marrese, M.; Guarino, V.; Fasolino, I.; Cirillo, V.; Ambrosio, L. Degradation and early in vitro activity of healthy hepatocytes onto bicomponent electrospun fibers. Int. J. Polym. Mater. 2017, 67, 961–966. [Google Scholar] [CrossRef]
- Marrese, M.; Cirillo, V.; Guarino, V.; Ambrosio, L. Short-Term Degradation of Bi-Component Electrospun Fibers: Qualitative and Quantitative Evaluations via AFM Analysis. J. Funct. Biomater. 2018, 9, 27. [Google Scholar] [CrossRef]
- Cirillo, V.; Clements, B.A.; Guarino, V.; Bushman, J.; Kohn, J.; Ambrosio, L. A comparison of the performance of mono-and bi-component electrospun conduits in a rat sciatic model. Biomaterials 2014, 35, 8970–8982. [Google Scholar] [CrossRef] [PubMed]
- Nemati, S.; Kim, S.J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Xu, T.; Zhang, L. Acellular Small-Diameter Tissue-Engineered Vascular Grafts. Appl. Sci. 2019, 9, 2864. [Google Scholar] [CrossRef]
- Yeo, G.C. A New Vascular Engineering Strategy Using 3D Printed Ice. Trends Biotechnol. 2019, 37, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Bate, T.S.R.; Forbes, S.J.; Callanan, A.J. Controlling Electrospun Polymer Morphology for Tissue Engineering Demonstrated Using hepG2 Cell Line. Vis. Exp. 2020, 159, e61043. [Google Scholar]
- Rose, J.C.; De Laporte, L. Hierarchical Design of Tissue Regenerative Constructs. Adv. Healthc. Mater. 2018, 7, e1701067. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, D.N.; Park, J.S.; Kwon, S.K.; Lee, J.H.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.A. Characterization and preparation of bio-tubular scaffolds for fabricating artificial vascular grafts by combining electrospinning and a 3D printing system. Phys. Chem. Chem. Phys. 2014, 17, 2996–2999. [Google Scholar] [CrossRef]
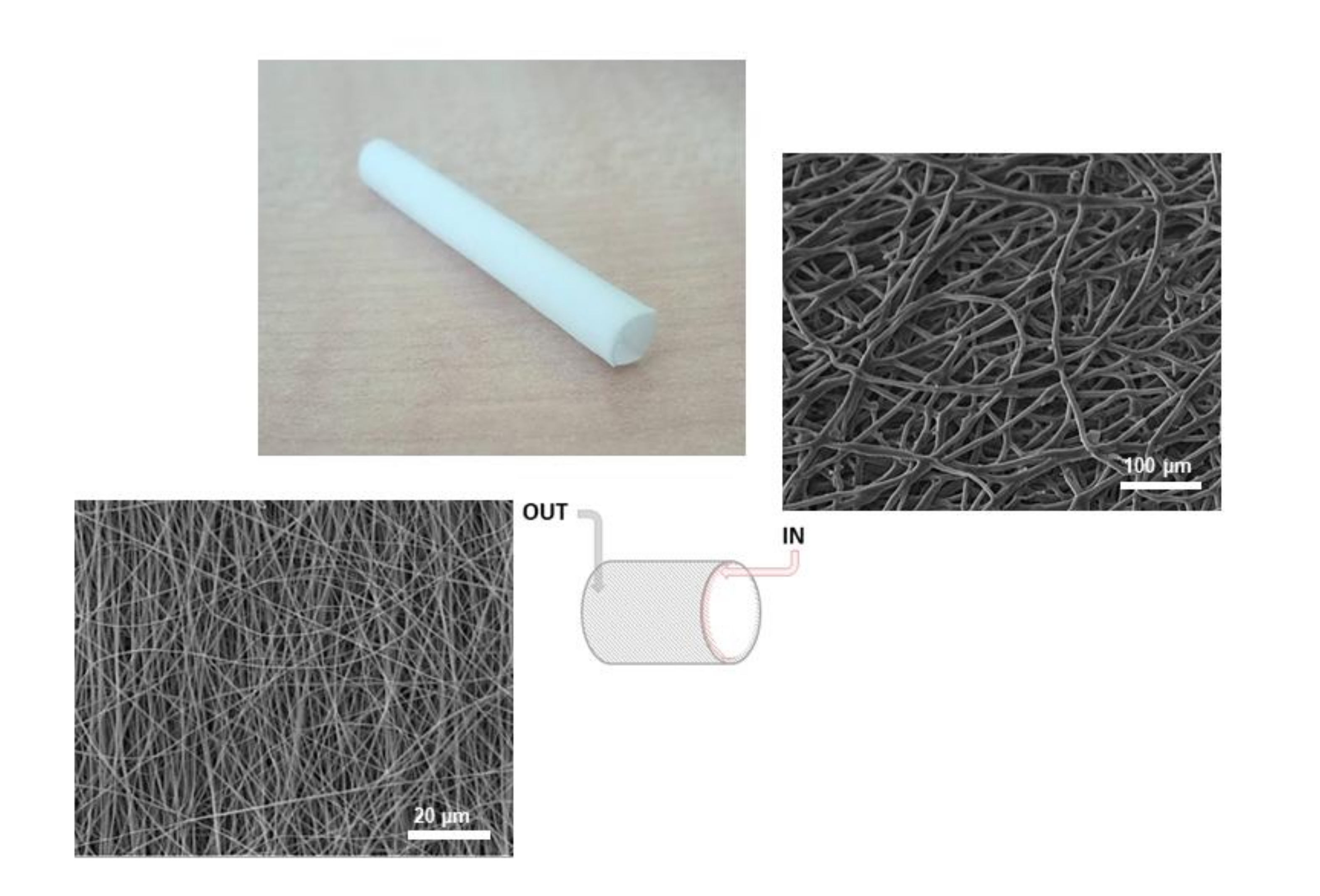

| Layer | Flow Rate (mL/h) | Voltage (kV) | Electrode Gap (mm) |
|---|---|---|---|
| IN | 0.1 | 13 | 130 |
| OUT | 0.5 | 15 | 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavan, B.; Gardin, C.; Guarino, V.; Rocca, T.; Cruz Maya, I.; Zanotti, F.; Ferroni, L.; Brunello, G.; Chachques, J.-C.; Ambrosio, L.; et al. Electrospun PCL-Based Vascular Grafts: In Vitro Tests. Nanomaterials 2021, 11, 751. https://doi.org/10.3390/nano11030751
Zavan B, Gardin C, Guarino V, Rocca T, Cruz Maya I, Zanotti F, Ferroni L, Brunello G, Chachques J-C, Ambrosio L, et al. Electrospun PCL-Based Vascular Grafts: In Vitro Tests. Nanomaterials. 2021; 11(3):751. https://doi.org/10.3390/nano11030751
Chicago/Turabian StyleZavan, Barbara, Chiara Gardin, Vincenzo Guarino, Tiberio Rocca, Iriczalli Cruz Maya, Federica Zanotti, Letizia Ferroni, Giulia Brunello, Juan-Carlos Chachques, Luigi Ambrosio, and et al. 2021. "Electrospun PCL-Based Vascular Grafts: In Vitro Tests" Nanomaterials 11, no. 3: 751. https://doi.org/10.3390/nano11030751
APA StyleZavan, B., Gardin, C., Guarino, V., Rocca, T., Cruz Maya, I., Zanotti, F., Ferroni, L., Brunello, G., Chachques, J.-C., Ambrosio, L., & Gasbarro, V. (2021). Electrospun PCL-Based Vascular Grafts: In Vitro Tests. Nanomaterials, 11(3), 751. https://doi.org/10.3390/nano11030751









