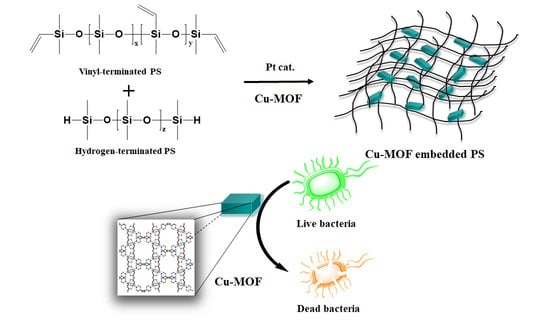
Robust Copper Metal–Organic Framework-Embedded Polysiloxanes for Biomedical Applications: Its Antibacterial Effects on MRSA and In Vitro Cytotoxicity
Abstract
1. Introduction
2. Materials and Methods
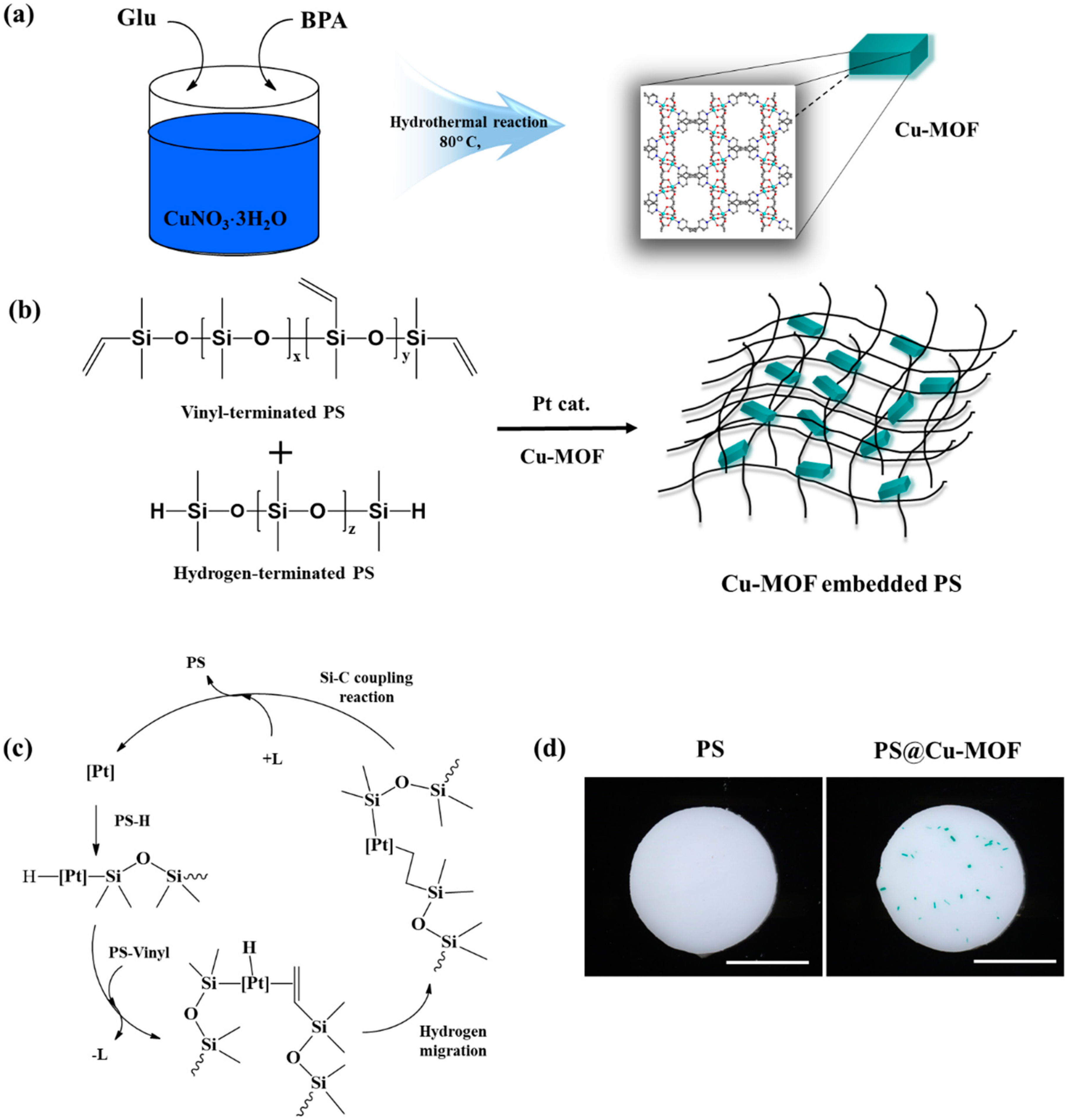
2.1. Preparation of the Cu-MOF
2.2. Preparation of PS and the Cu-MOF-Embedded PS (PS@Cu-MOF)
2.3. Instrumentation
2.4. Mechanical Properties of the PS@Cu-MOF
2.5. Degradation and Metal Ion Release Tests
2.6. Antibacterial Tests
2.7. Cytotoxicity Assays
3. Results and Discussion
3.1. Preparation of the Cu-MOF-Embedded PS (PS@Cu-MOF)
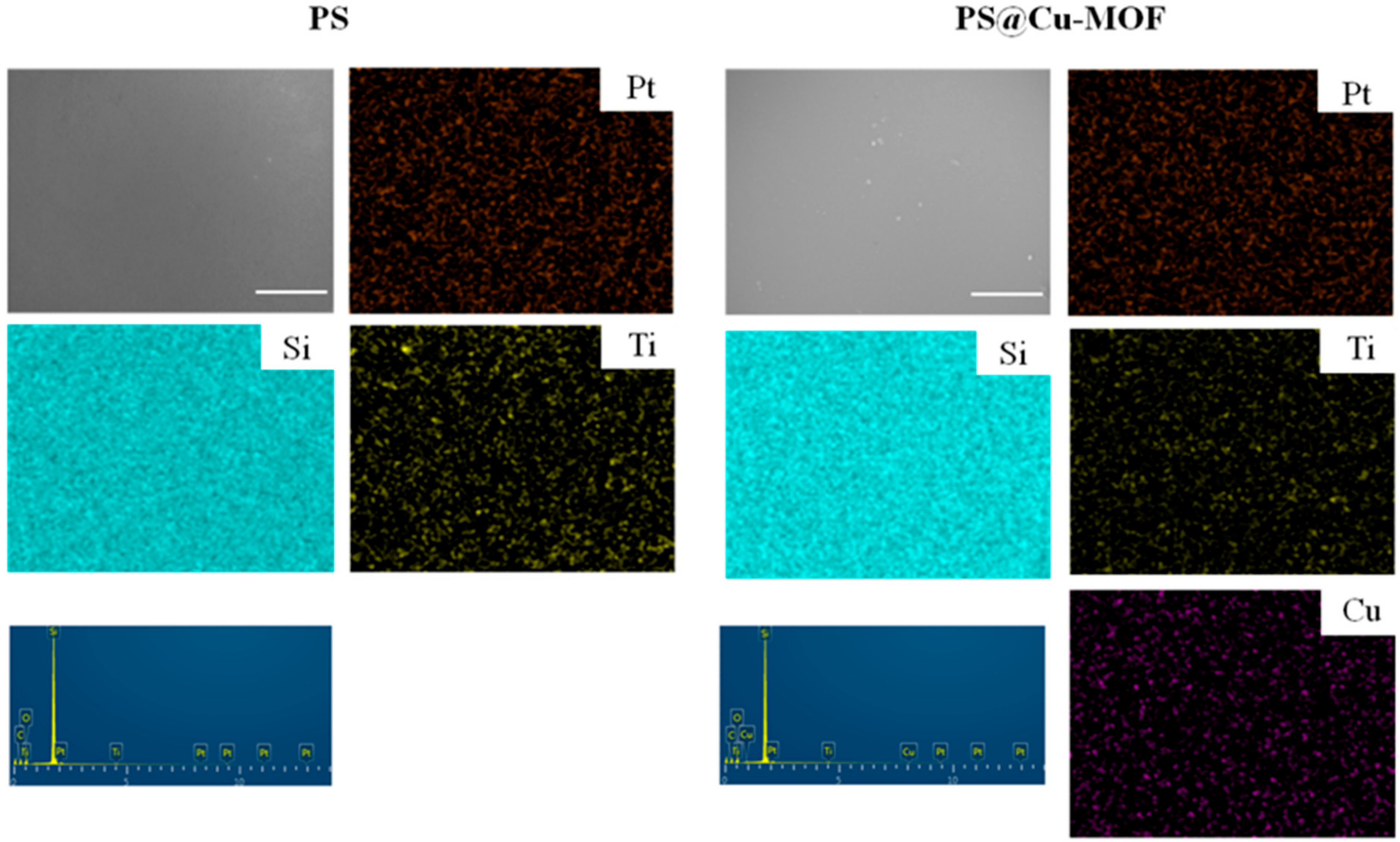
3.2. Characterization of the PS@Cu-MOF
3.3. Mechanical Properties of PS@Cu-MOF
3.4. Antibacterial Activity Tests
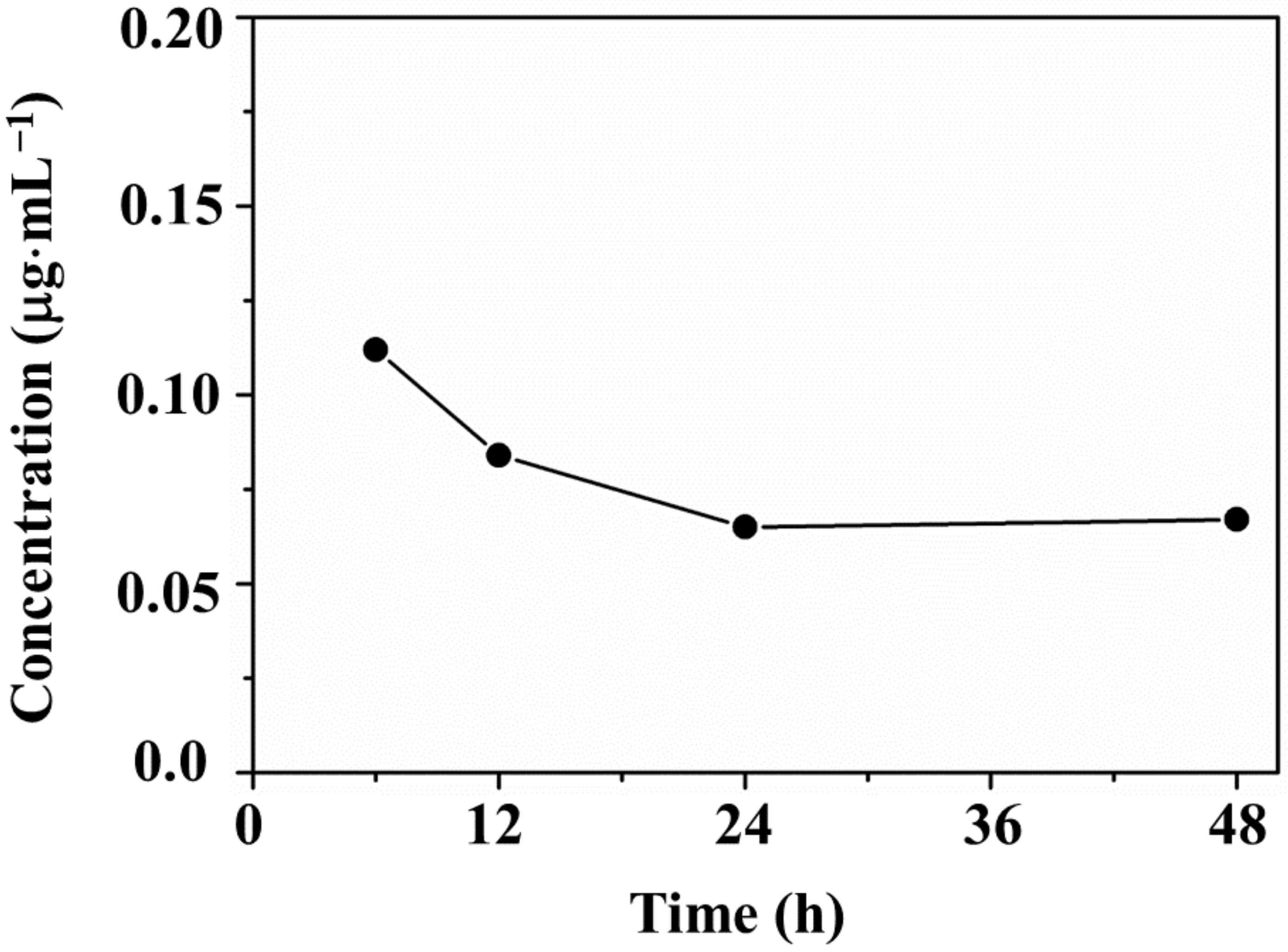
3.5. Ion Release Tests
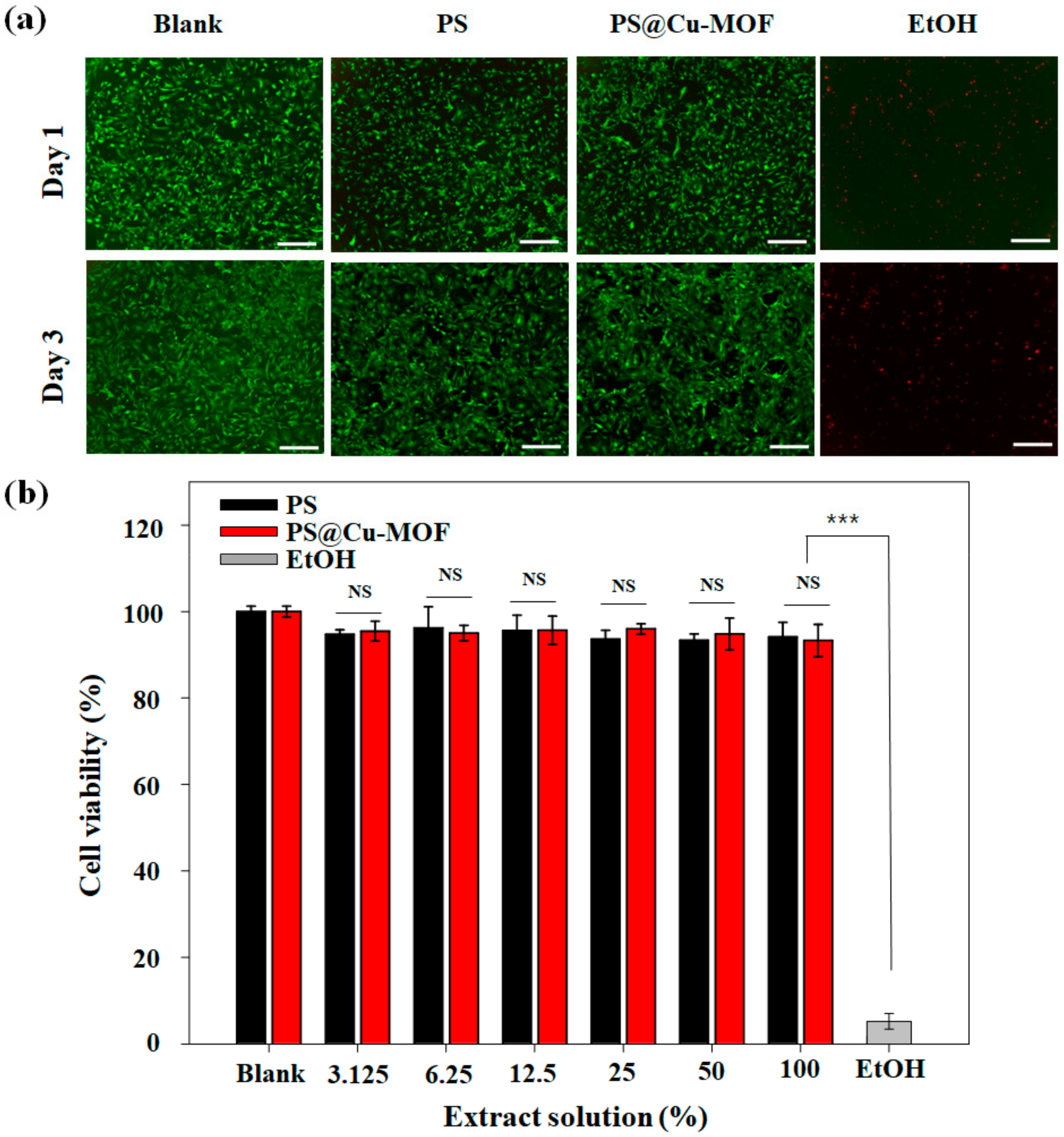
3.6. Cytotoxicity of PS@Cu-MOF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.Z.S.; Cooper, S.L. Recent advances in antimicrobial dendrimers. Adv. Mater. 2000, 12, 843–846. [Google Scholar] [CrossRef]
- Gabriel, G.J.; Som, A.; Madkour, A.E.; Eren, T.; Tew, G.N. Infectious disease: Connecting innate immunity to biocidal polymers. Mater. Sci. Eng. R. Rep. 2007, 57, 28–64. [Google Scholar] [CrossRef] [PubMed]
- Hadjesfandiari, N.; Yu, K.; Mei, Y.; Kizhakkedathu, J.N. Polymer brush-based approaches for the development of infection-resistant surfaces. J. Mater. Chem. B 2014, 2, 4946–4978. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef]
- Zhu, T.; Cui, Y.; Zhang, M.; Zhao, D.; Liu, G.; Ding, J. Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioact. Mater. 2020, 5, 584–601. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, L.; Kleshcheva, K. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Rupa, S.; Dutta, B.; Singh, S.P.; Rathor, A. Research article case report: Silicone implant in augmentation of saddle nose. Int. J. Recent Sci. Res. 2013, 4, 1661–1662. [Google Scholar]
- Elist, J.J.; Shirvanian, V.; Lemperle, G. Surgical treatment of penile deformity due to curvature using a subcutaneous soft silicone implant: Case report. Open J. Urol. 2014, 4, 91–97. [Google Scholar] [CrossRef]
- Beigbeder, A.; Labruyère, C.; Viville, P.; Pettitt, M.E.; Callow, M.E.; Callow, J.A. Surface and fouling-release properties of silicone/organomodified montmorillonite coatings. J. Adhes. Sci. Tech. 2011, 25, 1689–1700. [Google Scholar] [CrossRef]
- Mark, J.E. Some interesting things about polysiloxanes. Acc. Chem. Res. 2004, 37, 946–953. [Google Scholar] [CrossRef]
- Hogg, A.; Aellen, T.; Uhl, S.; Graf, B.; Keppner, H.; Tardy, Y.; Burger, J. Ultra-thin layer packaging for implantable electronic devices. J. Micromech. Microeng. 2013, 23, 075001. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric biomaterials for medical implants and devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Hassler, C.; von Metzen, R.P.; Ruther, P.; Stieglitz, T. Characterization of parylene C as an encapsulation material for implanted neural prostheses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 266–274. [Google Scholar] [CrossRef]
- Van Zele, D.; Heymans, O. Breast implants. A review. Acta Chir. Belg. 2004, 104, 158–164. [Google Scholar] [CrossRef]
- Lo, R.; Li, P.Y.; Saati, S.; Agrawal, R.; Humayun, M.S.; Meng, E. A refillable microfabricated drug delivery device for treatment of ocular diseases. Lab Chip 2008, 8, 1027–1030. [Google Scholar] [CrossRef]
- Lo, R.; Li, P.Y.; Saati, S.; Agrawal, R.N.; Humayun, M.S.; Meng, E. A passive MEMS drug delivery pump for treatment of ocular diseases. Biomed. Microdevices 2009, 11, 959–970. [Google Scholar] [CrossRef]
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Rahimi, A.; Mashak, A. Review on rubbers in medicine: Natural, silicone and polyurethane rubbers. Plast. Rubber Compos. 2013, 42, 223–230. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Howlader, M.M.R.; Deen, M.J.; Haddara, Y.M.; Selvaganapathy, P.R. Polymer integration for packaging of implantable sensors. Sens. Actuat. B Chem. 2014, 202, 758–778. [Google Scholar] [CrossRef]
- Kugel, A.; Chisholm, B.; Ebert, S.; Jepperson, M.; Jarabek, L.; Stafslien, S. Antimicrobial polysiloxane polymers and coatings containing pendant. Polym. Chem. 2010, 1, 442–452. [Google Scholar] [CrossRef]
- Islas, M.S.; Medina, J.J.M.; López Tévez, L.L.; Rojo, T.; Lezama, L.; Merino, M.G.; Calleros, L.; Cortes, M.A.; Puyol, R.M.; Echeverría, G.A.; et al. Antitumoral, antihypertensive, antimicrobial, and antioxidant effects of an octanuclear copper (II)-telmisartan complex with an hydrophobic nanometer hole. Inorg. Chem. 2014, 53, 5724–5737. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; Guedes da Silva, M.F.C.; Król, J.; Oliveira, M.C.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Bioactive silver–organic networks assembled from 1,3,5-triaza-7-phosphaadamantane and flexible cyclohexanecarboxylate blocks. Inorg. Chem. 2016, 55, 1486–1496. [Google Scholar] [CrossRef]
- Wang, L.; Yang, W.; Zhu, W.; Guan, X.; Xie, Z.; Sun, Z.-M. A nanosized {Ag@Ag12} “molecular windmill” templated by polyoxometalates anions. Inorg. Chem. 2014, 53, 11584–11588. [Google Scholar] [CrossRef] [PubMed]
- Chojnowski, J.; Fortuniak, W.; Rościszewski, P.; Werel, W.; Łukasiak, J.; Kamysz, W.; Hałasa, R. Polysilsesquioxanes and oligosilsesquioxanes substituted by alkylammonium salts as antibacterial biocides. J. Inorg. Organomet. Polym. Mater. 2006, 16, 219–230. [Google Scholar] [CrossRef]
- Mansouri, J.; Truong, V.K.; MacLaughlin, S.; Mainwaring, D.E.; Moad, G.; Dagley, I.J.; Ivanova, E.P.; Crawford, R.J.; Chen, V. Polymerization-Induced Phase Segregation and Self-Assembly of Siloxane Additives to Provide Thermoset Coatings with a Defined Surface Topology and Biocidal and Self-Cleaning Properties. Nanomaterials 2019, 9, 1610. [Google Scholar] [CrossRef] [PubMed]
- Mizerska, U.; Fortuniak, W.; Chojnowski, J.; Halasa, R.; Konopacka, A.; Werel, W. Polysiloxane cationic biocides with imidazolium salt (ImS) groups, synthesis and antibacterial properties. Eur. Polym. J. 2009, 45, 779–787. [Google Scholar] [CrossRef]
- Mizerska, U.; Fortuniak, W.; Chojnowski, J.; Turecka, K.; Konopacka, A.; Werel, W. Antimicrobial siloxane statistical and graft copolymers substituted with t-butylamine and t-butylammonium biocidal functions. J. Inorg. Organomet. Polym. Mater. 2010, 20, 554–563. [Google Scholar] [CrossRef][Green Version]
- Rozga-Wijas, K.; Mizerska, U.; Fortuniak, W.; Chojnowski, J.; Halasa, R.; Werel, W. Quaternary ammonium salts (QAS) modified polysiloxane biocide supported on silica materials. J. Inorg. Organomet. Polym. Mater. 2007, 17, 605–613. [Google Scholar] [CrossRef]
- Chisholm, B.J.; Majumdar, P. Antimicrobial Polysiloxane Materials Containing Metal Species. U.S. Patent 8,709,394 B2, 29 April 2014. [Google Scholar]
- Yilgor, E.; Nugay, I.I.; Bakan, M.; Yilgor, I. Antibacterial silicone-urea/organoclay nanocomposites. Silicon 2009, 1, 183–190. [Google Scholar] [CrossRef]
- Prasse, M.; Christofferson, A.J.; Elbourne, A.; Cheeseman, S.; Shi, Y.; Rolland, M.; Cozzolino, D.; Chapman, J.; McConville, C.F.; Crawford, R.J.; et al. Conformationally tuned antibacterial oligomers target the peptidoglycan of Gram-positive bacteria. J. Colloid Interface Sci. 2020, 580, 850–862. [Google Scholar]
- Liu, Q.; Ning, D.; Li, W.-J.; Du, X.-M.; Wang, Q.; Li, Y.; Ruan, W.-J. A point-of-care diagnostics logic detector based on glucose oxidase immobilized lanthanide functionalized metal—Organic frameworks. Analyst 2019, 114, 1916–1922. [Google Scholar] [CrossRef]
- Sava Gallis, D.F.; Butler, K.S.; Agola, J.O.; Pearce, C.J.; McBride, A.A. Antibacterial countermeasures via metal–organic framework-supported sustained therapeutic release. ACS Appl. Mater. Interfaces 2019, 11, 7782–7791. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ye, J.; Zhang, D.; Xie, R.; Bogale, R.F.; Sun, Y.; Zhao, L.; Zhao, Q.; Ning, G. Silver carboxylate metal-organic frameworks with highly antibacterial activity and biocompatibility. J. Inorg. Biochem. 2014, 138, 114–121. [Google Scholar] [CrossRef]
- Wang, S.; Yan, F.; Ren, P.; Li, Y.; Wu, Q.; Fang, X.; Chen, F.; Wang, C. Incorporation of metal-organic frameworks into electrospun chitosan/poly (vinyl alcohol) nanofibrous membrane with enhanced antibacterial activity for wound dressing application. Int. J. Biol. Macromol. 2020, 158, 9–17. [Google Scholar] [CrossRef]
- Rubin, H.N.; Neufeld, B.H.; Reynolds, M.M. Surface-anchored metal—Organic framework—Cotton material for tunable antibacterial copper delivery. ACS Appl. Mater. Interfaces 2018, 10, 15189–15199. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Li, H.; Duan, X.; Liu, G.; Liu, X. Photochemical synthesis and characterization of Ag/TiO2 nanotube composites. J. Mater. Sci. 2008, 43, 1669–1676. [Google Scholar] [CrossRef]
- Zeng, H.; Zhao, C.; Qiu, J.; Yang, Y.; Chen, G. Preparation and optical properties of silver nanoparticles induced by a femtosecond laser irradiation. J. Cryst. Growth 2007, 300, 519–522. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Q.; Wang, Y.; Zhang, Q.; Qiao, X. Synthesis of pyridinium polysiloxane for antibacterial coating in supercritical carbon dioxide. J. Appl. Polym. Sci. 2015, 132, 41723. [Google Scholar] [CrossRef]
- Jońca, J.; Tukaj, C.; Werel, W.; Mizerska, U.; Fortuniak, W.; Chojnowski, J. Bacterial membranes are the target for antimicrobial polysiloxane-methacrylate copolymer. J. Mater. Sci. Mater. Med. 2016, 27, 55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gwon, K.; Han, I.; Lee, S.; Kim, Y.; Lee, D.N. Novel metal—Organic framework-based photocrosslinked hydrogel system for efficient antibacterial applications. ACS Appl. Mater. Interface 2020, 12, 20234–20242. [Google Scholar] [CrossRef]
- Jo, J.H.; Kim, H.-C.; Huh, S.; Kim, Y.; Lee, D.N. Antibacterial activities of Cu-MOFs containing glutarates and bipyridyl ligands. Dalton Trans. 2019, 48, 8084–8093. [Google Scholar] [CrossRef]
- Hwang, I.H.; Bae, J.M.; Kim, W.-S.; Jo, Y.D.; Kim, C.; Kim, Y.; Kim, S.-J.; Huh, S. Bifunctional 3D Cu-MOFs containing glutarates and bipyridylligands: Selective CO2sorption and heterogeneous catalysis. Dalton Trans. 2012, 41, 12759. [Google Scholar] [CrossRef]
- Gwon, K.; Jo, E.-J.; Sahu, A.; Lee, J.Y.; Kim, M.-G.; Tae, G. Improved near infrared-mediated hydrogel formation using diacrylated Pluronic F127-coated upconversion nanoparticles. Mater. Sci. Eng. C 2018, 90, 77–84. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, J.; Wang, L.; Wang, R.; Liu, Z.; Zhou, R. An enzyme-mediated in situ hydrogel based on polyaspartamide derivatives for localized drug delivery and 3D scaffolds. RSC Adv. 2016, 6, 101334–101346. [Google Scholar] [CrossRef]
- Siew, W.Y.; Abu Bakar, N.H.H.; Abu Bakar, M. The influence of green synthesis on the formation of various copper benzene-1,3,5-tricarboxylate compounds. Inorg. Chim. Acta 2018, 482, 53–61. [Google Scholar] [CrossRef]
- Meister, T.K.; Riener, K.; Gigler, P.; Stohrer, J.; Herrmann, W.A.; Kühn, F.E. Platinum catalysis revisited—Unraveling principles of catalytic olefin hydrosilylation. ACS Catal. 2016, 6, 1274–1284. [Google Scholar] [CrossRef]
- Nakajima, Y.; Shimada, S. Hydrosilylation reaction of olefins: Recent advances and perspectives. RSC Adv. 2015, 5, 20603–20616. [Google Scholar] [CrossRef]
- Ferreira, P.; Carvalho, Á.; Ruivo Correia, T.; Paiva Antunes, B.; Joaquim Correia, I.; Alves, P. Functionalization of polydimethylsiloxane membranes to be used in the production of voice prostheses. Sci. Technol. Adv. Mater. 2013, 14, 055006. [Google Scholar] [CrossRef] [PubMed]
- Al-Taweel, S.S.; Saud, H.R. New route for synthesis of pure anatase TiO2 nanoparticles via utrasoundassisted sol-gel method. J. Chem. Pharm. Res. 2016, 8, 620–626. [Google Scholar]
- Fan, J.; Huang, J.; Yan, M.; Gong, Z.; Gao, L.; Chen, Y. Thermoplastic multifunctional polysiloxane-based materials from broad gradient-transition multiphase separation. J. Mater. Chem. A 2020, 8, 16376–16384. [Google Scholar] [CrossRef]
- González-Rivera, J.; Iglio, R.; Barillaro, G.; Duce, C.; Tinè, M.R. Structural and Thermoanalytical Characterization of 3D Porous PDMS Foam Materials: The Effect of Impurities Derived from a Sugar Templating Process. Polymers 2018, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Staszek, M.; Siegel, J.; Kolářová, K.; Rimpelová, S.; Švorčík, V. Formation and antibacterial action of Pt and Pd nanoparticles sputtered into liquid. Micro Nano Lett. 2014, 9, 778–781. [Google Scholar] [CrossRef]
- Wanag, A.; Rokicka, P.; Kusiak-Nejman, E.; Kapica-Kozar, J.; Wrobel, R.J.; Markowska-Szczupak, A.; Morawski, A.W. Antibacterial properties of TiO2 modified with reduced graphene oxide. Ecotoxicol. Environ. Saf. 2018, 147, 788–793. [Google Scholar] [CrossRef]
- Tu, Q.; Zhang, Q.; Wang, Y.; Jiao, J.; Peng, T.; Wang, J. Antibacterial properties of poly(dimethylsiloxane) surfaces modified with graphene oxide-catechol composite. Prog. Org. Coat. 2019, 127, 247–253. [Google Scholar] [CrossRef]


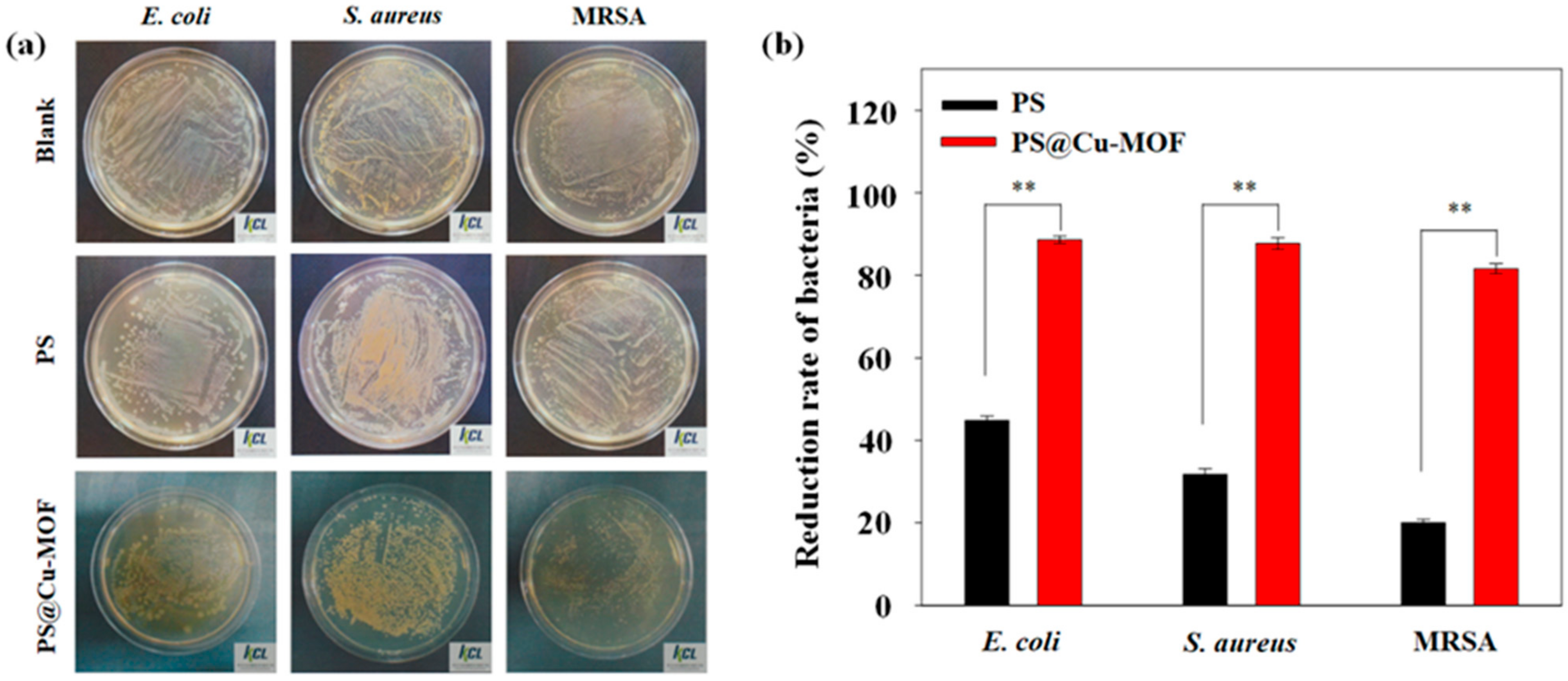
| Test Items | Test Results a,b | |||
|---|---|---|---|---|
| Early Conc. (cfu⋅mL−1) | After 24 h, Conc. (cfu⋅mL−1) | Reduction Rate of Bacteria (%) | ||
| E. coli | blank | 3.4 × 105 | 9.8 × 106 | - |
| PS | 3.4 × 105 | 5.4 × 106 | 44.8 | |
| blank | 1.2 × 105 | 3.8 × 106 | - | |
| PS@Cu-MOF | 1.2 × 105 | 4.3 × 105 | 88.6 | |
| S. aureus | blank | 3.6 × 105 | 9.1 × 106 | - |
| PS | 3.6 × 105 | 6.2 × 106 | 31.8 | |
| blank | 1.6 × 105 | 2.2 × 106 | - | |
| PS@Cu-MOF | 1.6 × 105 | 2.7 × 105 | 87.7 | |
| MRSA | blank | 3.6 × 105 | 9.0 × 106 | - |
| PS | 3.6 × 105 | 7.2 × 106 | 20.0 | |
| blank | 3.0 × 105 | 9.8 × 105 | - | |
| PS@Cu-MOF | 3.0 × 105 | 1.8 × 105 | 81.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwon, K.; Kim, Y.; Cho, H.; Lee, S.; Yang, S.-H.; Kim, S.-J.; Lee, D.N. Robust Copper Metal–Organic Framework-Embedded Polysiloxanes for Biomedical Applications: Its Antibacterial Effects on MRSA and In Vitro Cytotoxicity. Nanomaterials 2021, 11, 719. https://doi.org/10.3390/nano11030719
Gwon K, Kim Y, Cho H, Lee S, Yang S-H, Kim S-J, Lee DN. Robust Copper Metal–Organic Framework-Embedded Polysiloxanes for Biomedical Applications: Its Antibacterial Effects on MRSA and In Vitro Cytotoxicity. Nanomaterials. 2021; 11(3):719. https://doi.org/10.3390/nano11030719
Chicago/Turabian StyleGwon, Kihak, Youngmee Kim, Hyunjun Cho, Seonhwa Lee, So-Hyeon Yang, Sung-Jin Kim, and Do Nam Lee. 2021. "Robust Copper Metal–Organic Framework-Embedded Polysiloxanes for Biomedical Applications: Its Antibacterial Effects on MRSA and In Vitro Cytotoxicity" Nanomaterials 11, no. 3: 719. https://doi.org/10.3390/nano11030719
APA StyleGwon, K., Kim, Y., Cho, H., Lee, S., Yang, S.-H., Kim, S.-J., & Lee, D. N. (2021). Robust Copper Metal–Organic Framework-Embedded Polysiloxanes for Biomedical Applications: Its Antibacterial Effects on MRSA and In Vitro Cytotoxicity. Nanomaterials, 11(3), 719. https://doi.org/10.3390/nano11030719