Novel Nanoarchitectures Based on Lignin Nanoparticles for Electrochemical Eco-Friendly Biosensing Development
Abstract
1. Introduction
2. Experimental Methods
2.1. Reagents and Apparatus
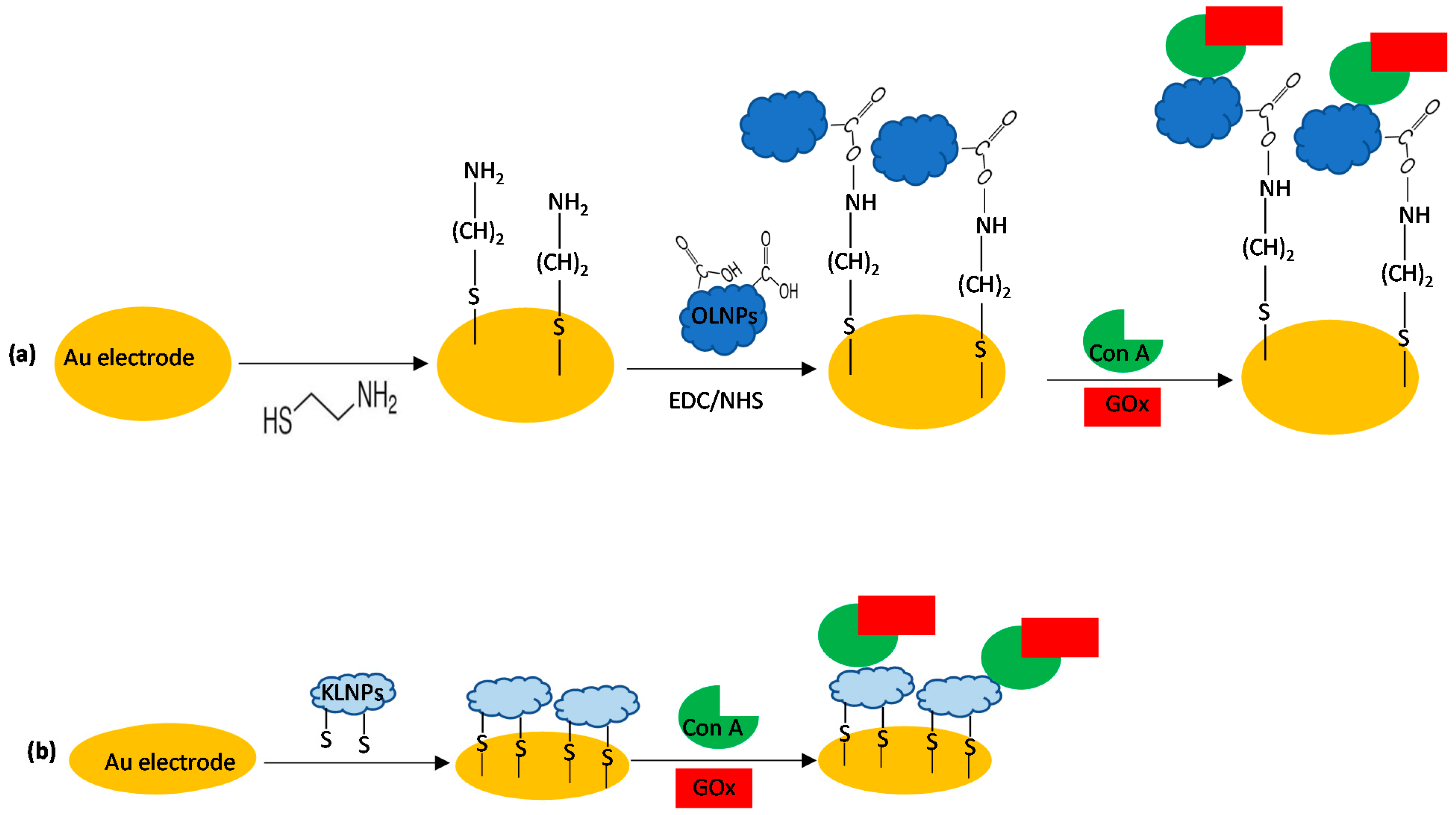
2.2. Preparation of Gold-Modified Electrodes
2.2.1. Layer-by-Layer Nanoassembly
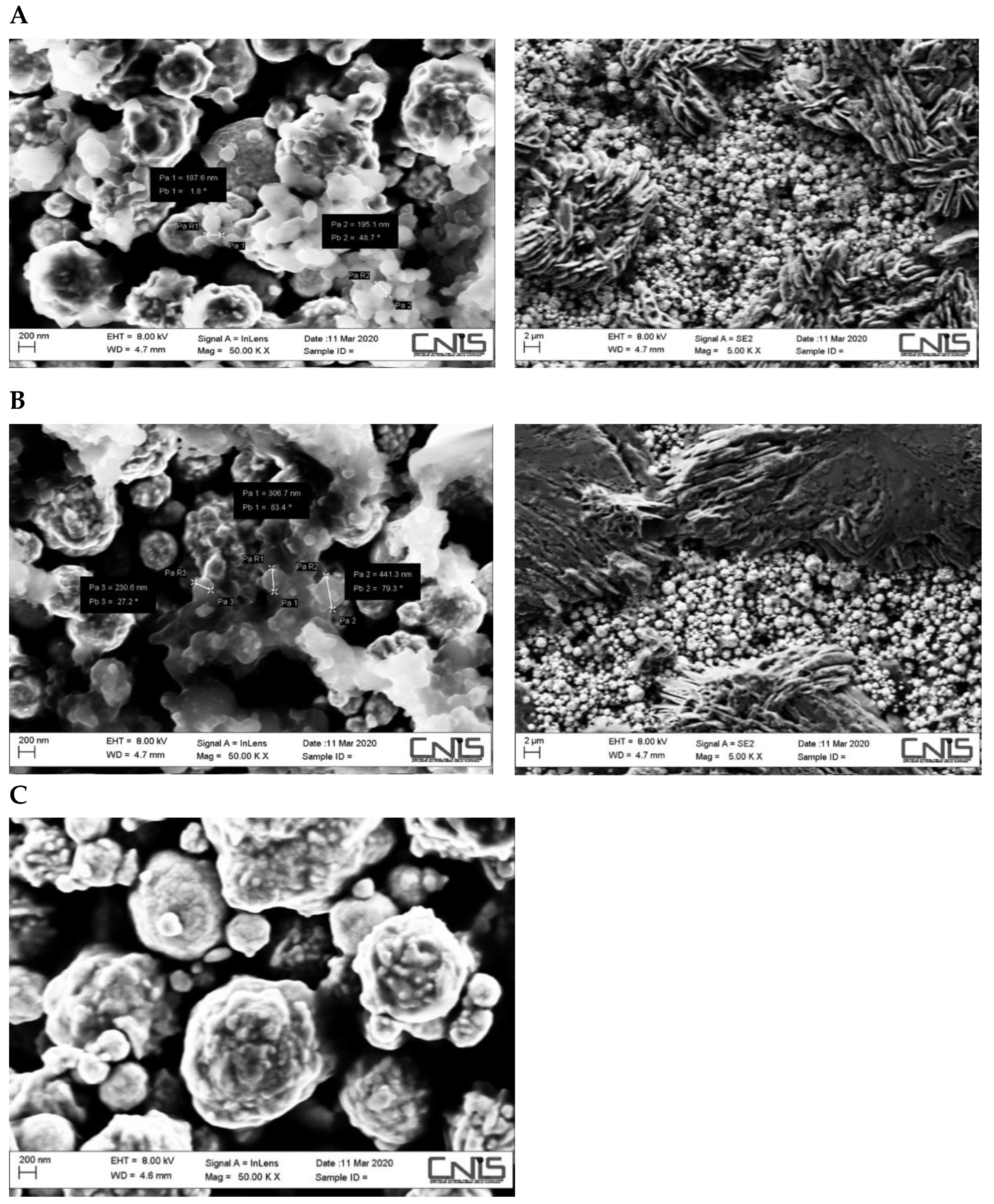
2.3. SEM Experiments
3. Results and Discussion
3.1. SEM Characterization
3.2. Electrochemical Characterization
3.2.1. Cyclic Voltammetry Characterization
3.2.2. Electrochemical Impedance Spectroscopy Characterization
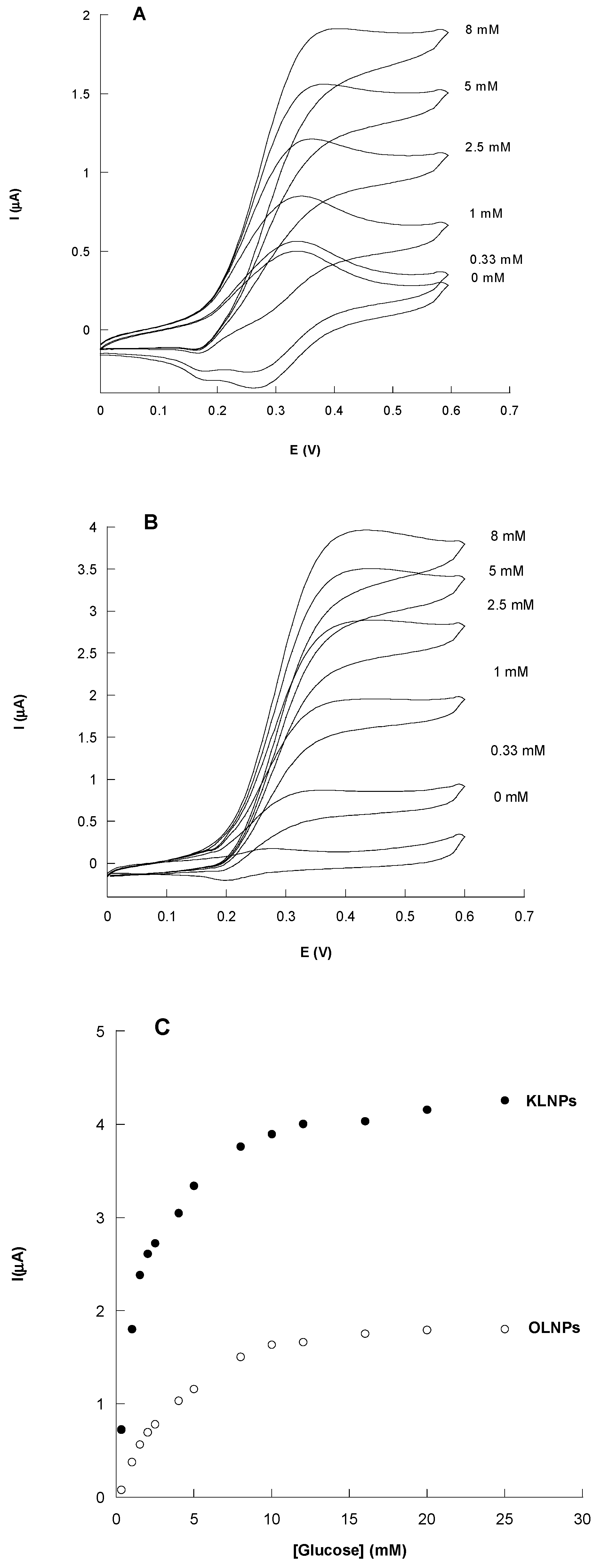
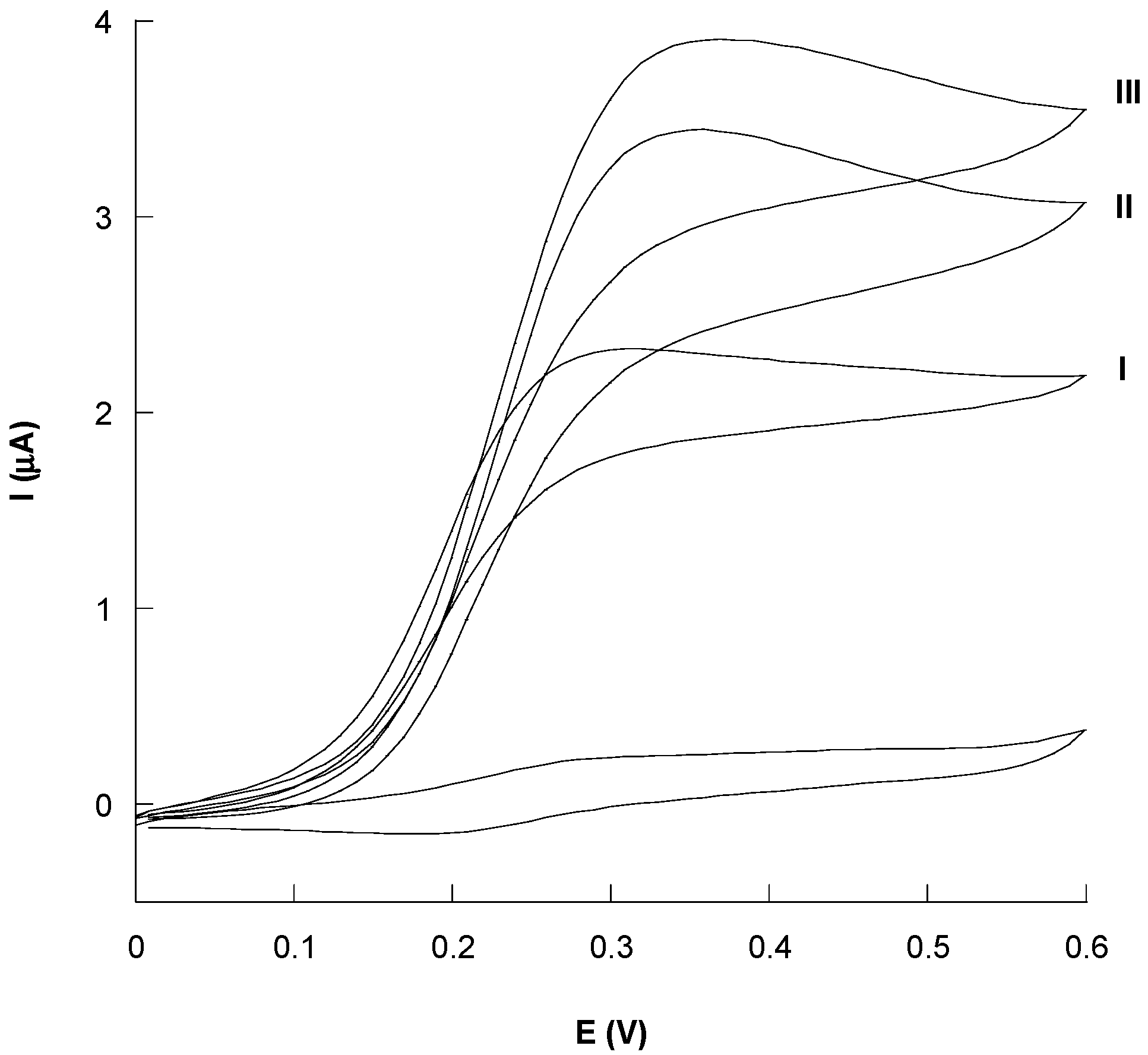
3.3. Catalytic Behavior of Au/SAMCys/OLNPs/Con A/GOx and Au/KLNPs/Con A/GOx Platforms
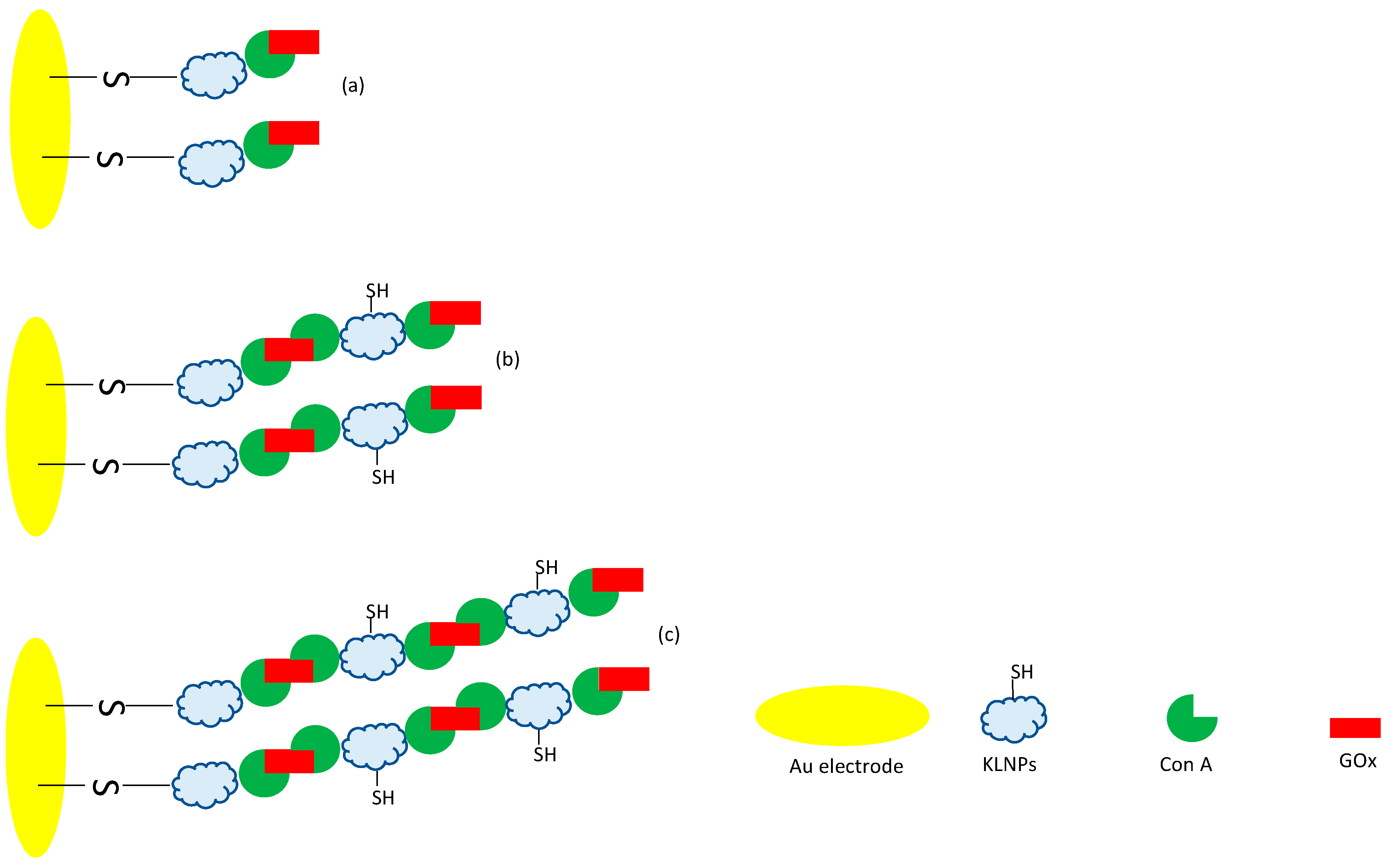
3.4. LbL Functionalization of the Au/KLNPs/Con A/GOx Platform
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Guo, Q.; Cui, D. Recent advances in nanotechnology applied to biosensors. Sensors 2009, 9, 1033–1053. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Ju, H.; Zhang, X.; Wang, J. (Eds.) Nanomaterials for immunosensors and immunoassays. In NanoBiosensing, Biological and Medical Physics; Biomedical Engineering; Springer: New York, NY, USA, 2011; pp. 425–452. [Google Scholar]
- Bishop, K.J.M.; Wilmer, C.E.; Soh, S.; Grzybowski, B.A. Nanoscale forces and their uses in self-assembly. Small 2009, 5, 1600–1630. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ju, H. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134. [Google Scholar] [CrossRef] [PubMed]
- Putzbach, W.; Ronkainen, N. Immobilization techniques in the fabrication of nanomaterial-based electrochemical biosensors: A review. Sensors 2013, 13, 4811–4840. [Google Scholar] [CrossRef]
- Subramoney, S. Carbon Nanotubes. In Encyclopedia of Materials: Science and Technology 2006, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2001; pp. 1–8. [Google Scholar]
- Wan, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar]
- Le Goff, A.; Holzinger, M.; Cosnier, S. Enzymatic biosensors based on SWCNT-conducting polymer electrodes. Analyst 2011, 136, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, K.; Burghard, M. Biosensors based on carbon nanotubes. Anal. Bioanal. Chem. 2006, 385, 452–468. [Google Scholar] [CrossRef]
- Gruner, G. Carbon nanotube transistors for biosensing applications. Anal. Bioanal. Chem. 2006, 384, 322–335. [Google Scholar] [CrossRef]
- Besteman, K.; Lee, J.O.; Wiertz, F.G.M.; Heering, H.A.; Dekker, C. Enzyme-coated carbon nanotubes as single-molecule biosensors. Nano Lett. 2003, 3, 727–730. [Google Scholar] [CrossRef]
- Valentini, F.; Carbone, M.; Palleschi, G. Carbon nanostructured materials for applications in nano-medicine, cultural heritage, and electrochemical biosensors. Anal. Bioanal. Chem. 2013, 405, 451–465. [Google Scholar] [CrossRef]
- Vamvakaki, V.; Chaniotakis, N.A. Carbon nanostructures as transducers in biosensors. Sens. Actuators B Chem. 2007, 126, 193–197. [Google Scholar] [CrossRef]
- Liu, S.; Guo, X. Carbon nanomaterials field-effect-transistor-based biosensors. NPG Asia Mater. 2012, 4, e23. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent advances in graphene-based biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef]
- Zhu, Z. An overview of carbon nanotubes and graphene for biosensing applications. Nano-Micro Lett. 2017, 9, 25. [Google Scholar] [CrossRef]
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef]
- Ma, H.; Wu, D.; Cui, Z.; Li, Y.; Zhang, Y.; Du, B.; Wei, Q. Graphene-based optical and electrochemical biosensors: A review. Anal. Lett. 2012, 46, 1–17. [Google Scholar] [CrossRef]
- Ratinac, K.R.; Yang, W.; Gooding, J.J.; Thordarson, P.; Braet, F. Graphene and related materials in electrochemical sensing. Electroanalysis 2011, 23, 803–826. [Google Scholar] [CrossRef]
- Carbone, M.; Gorton, L.; Antiochia, R. An overview of the latest graphene-based sensors for glucose detection: The effects of graphene defects. Electroanalysis 2015, 27, 16–31. [Google Scholar] [CrossRef]
- Li, Y.; Schluesener, H.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Bollella, P.; Schultz, C.; Favero, G.; Mazzei, F.; Ludwig, R.; Gorton, L.; Antiochia, R. Green synthesis and characterization of gold and silver nanoparticles and their application for development of a third generation lactose biosensor. Electroanalysis 2017, 29, 77–86. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Algar, W.R.; Tavares, A.J.; Krull, U.J. Beyond labels: A review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal. Chim. Acta 2010, 673, 1–25. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Nanowire-based biosensors. Anal. Chem. 2006, 78, 4260–4269. [Google Scholar] [CrossRef]
- Arora, P.; Sindhu, A.; Dilbaghi, N.; Chaudhury, A. Engineered multifunctional nanowires as novel biosensing tools for highly sensitive detection. Appl. Nanosci. 2013, 3, 363–372. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
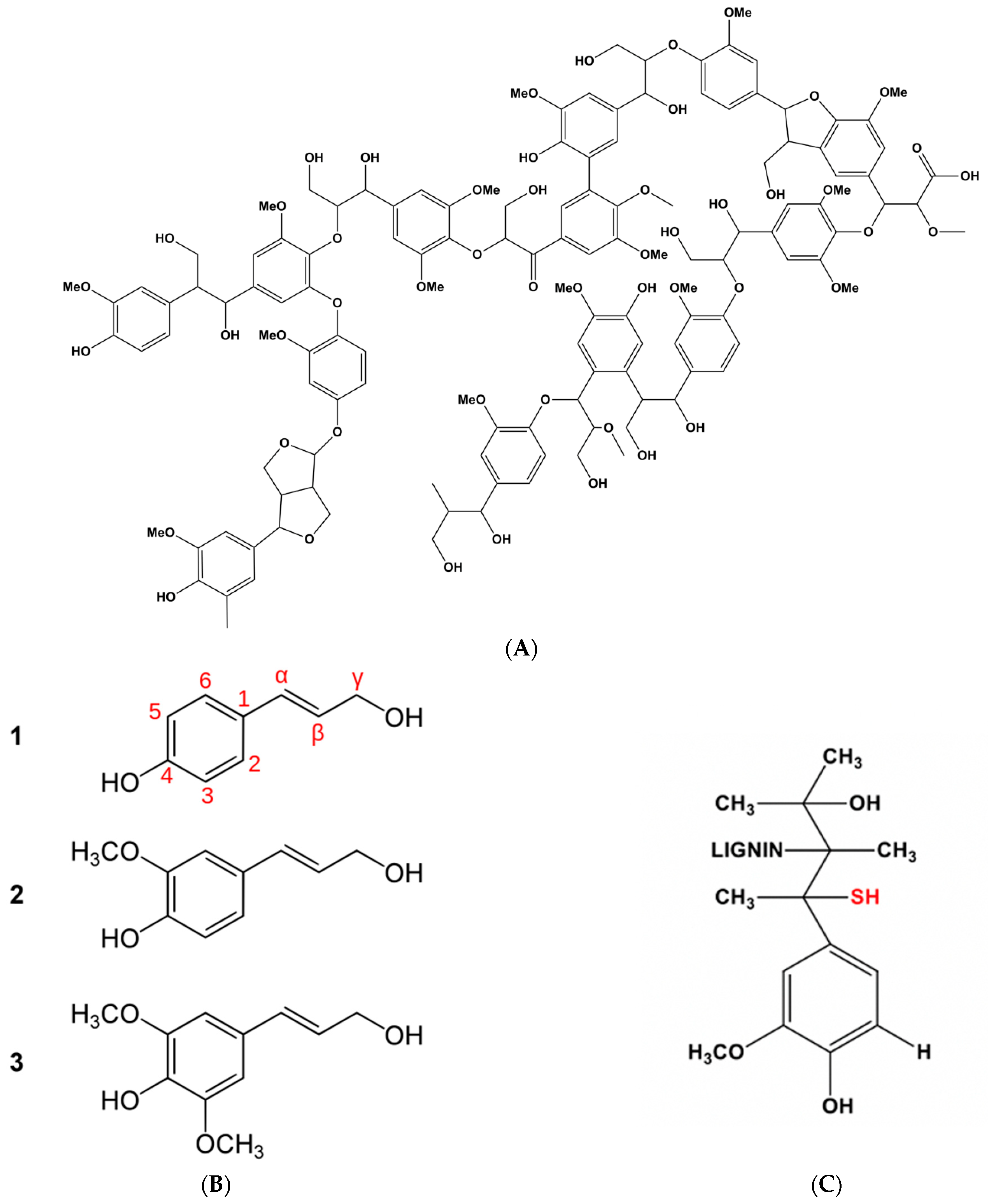
- Chauhan, P.S. Lignin nanoparticles: Eco-friendly and versatile tool for new era. Bioresour. Technol. Rep. 2020, 9, 100374. [Google Scholar] [CrossRef]
- Zhao, W.; Simmons, B.; Singh, S.; Ragauskas, A.; Cheng, G. From lignin association to nano-/micro-particle preparation: Extracting higher value of lignin. Green Chem. 2016, 18, 5693–5700. [Google Scholar] [CrossRef]
- Beisl, S.; Miltner, A.; Friedi, A. Lignin from micro- to nanosize: Production methods. Int. J. Mol. Sci. 2017, 18, 1244. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards. Lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Ma, R.; Guo, M.; Zhang, X. Recent advances in oxidative valorization of lignin. Catal. Today 2018, 302, 50–60. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Bertoglio, F.; Owczarek, J.S.; Bruni, G.; Kozanecki, M.; Kenny, J.M.; Torre, L.; Visai, L.; Puglia, D. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydr. Polym. 2018, 181, 275–284. [Google Scholar] [CrossRef]
- Yearla, S.R.; Padmasree, K. Preparation and characterisation of lignin nanoparticles: Evaluation of their potential as antioxidants and UV protectants. J. Exp. Nanosci. 2016, 11, 289–302. [Google Scholar] [CrossRef]
- Wang, B.; Sun, D.; Wang, H.M.; Yuan, T.Q.; Sun, R.C. Green and facile preparation of regular lignin nanoparticles with high yield and their natural broad-spectrum sunscreens. ACS Sustain. Chem. Eng. 2019, 7, 2658–2666. [Google Scholar] [CrossRef]
- Feldman, D. Lignin nanocomposites. J. Macromol. Sci. Part. A 2016, 53, 382–387. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B. In Vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, R.; Hu, L.Q.; Zou, Z.F.; Si, C.L. Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.L.; Hult, E.L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Gong, W.; Ran, Z.; Ye, F.; Zhao, G. Lignin from bamboo shoot shells as an activator and novel immobilizing support for a-amylase. Food Chem. 2017, 228, 455–462. [Google Scholar] [CrossRef]
- Sipponen, M.K.; Farooq, M.; Koivisto, J.; Pellis, A.; Seitsonen, J.; Österberg, M. Spatially confined lignin nanospheres for biocatalytic ester synthesis in aqueous media. Nat. Commun. 2018, 9, 2300. [Google Scholar] [CrossRef]
- Glasser, W.G. About making lignin great again—Some lessons from the past. Front. Chem. 2019, 7, 565. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Botta, L.; Bollella, P.; Antiochia, R.; Crucianelli, M.; Saladino, R. Layer by layer supported laccase on lignin nanoparticles catalyzes the selective oxidation of alcohols to aldehydes. Catal. Sci. Technol. 2019, 15, 4125–4134. [Google Scholar] [CrossRef]
- Capecchi, E.; Piccinino, D.; Delfino, I.; Bollella, P.; Antiochia, R.; Saladino, R. Functionalized tyrosinase-lignin nanoparticles as sustainable catalysts for the oxidation of phenols. Nanomaterials 2018, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, E.; Gallay, P.; Galicia, L.; Eguìlaz, M.; Rivas, G. Nanoarchitectures based on multi-walled carbon nanotubes non-covalently functionalized with Concanavalin A: A new building-block with supramolecular recognition properties for the development of electrochemical biosensors. Sens. Actuators B Chem. 2019, 292, 254–262. [Google Scholar] [CrossRef]
- Capecchi, E.; Piccinino, D.; Tomaino, E.; Bizzarri, B.M.; Polli, F.; Antiochia, R.; Mazzei, F.; Saladino, R. Lignin nanoparticles are renewable and functional platforms for the concanavalin a oriented immobilization of glucose oxidase-peroxidase in cascade biosensing. RSC Adv. 2020, 48, 29031–29042. [Google Scholar] [CrossRef]
- Frommhagen, M.; Mutte, S.K.; Westphal, A.H.; Koetsier, M.J.; Hinz, S.W.A.; Visser, J.; Vincken, J.-P.; Weijers, D.; van Berkel, W.J.H.; Gruppen, H.; et al. Boosting LPMO-driven lignocellulose degradation by polyphenol oxidase-activated lignin building blocks. Biotechnol. Biofuels 2017, 10, 121. [Google Scholar] [CrossRef]
- Nasrullah, A.; Bhat, A.H.; Khan, S.A. Comprehensive approach on the structure, production, processing, and application of lignin. In Lignocellulosic Fibre and Biomass-Based Composite Materials Processing, Properties and Applications; Woodhead Publishing: Cambridge, UK, 2017; pp. 165–178. [Google Scholar]
- Ashter, S.A. Chemistry of Cellulosic Polymer, in Technology and Applications of Polymers Derived from Biomass; William Andrew: New York, NY, USA, 2018; pp. 57–74. [Google Scholar]
- Beisl, S.; Friedl, A.; Miltner, A. Lignin from micro- to nanosize: Applications. Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Nano-structured lignina as green antioxidant and UV shielding ingredient for sunscreen applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Bao, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Lignin valorization: Lignin nanoparticles as high-value bio-additive for multifunctional nanocomposites. Biotechnol. Biofuels 2017, 10, 192. [Google Scholar] [CrossRef]
- Capecchi, E.; Piccinino, D.; Bizzarri, B.M.; Avitabile, D.; Pelosi, C.; Colantonio, C.; Calabrò, G.; Saladino, R. Enzyme lignin nanocapsules are sustainable catalysts and vehicles for the preparation of unique polyvalent bioinks. Macromolecules 2019, 20, 1975–1988. [Google Scholar] [CrossRef]
- Boland, S.; Foster, K.; Leech, D. A stability comparison of redox-active layers produced by chemical coupling of an osmium redox complex to pre-functionalized gold and carbon electrodes. Electrochim. Acta 2009, 54, 1986–1991. [Google Scholar] [CrossRef]
- Parvathy, G.; Sethulekshmi, A.S.; Javan, J.S.; Raman, A.; Saritha, A. Lignin based nano-composites: Synthesis and applications. Process Safety Environ. Prot. 2021, 145, 395–410. [Google Scholar]
- Ganesh, V.; Pal, S.K.; Kumar, S.; Lakshminarayanan, V. Self-assembled monolayers (SAMs) of alkoxycyanobiphenyl thiols on gold-A study of electron transfer reaction using cyclic voltammetry and electrochemical impedance spectroscopy. J. Colloid Interface Sci. 2006, 296, 195–203. [Google Scholar] [CrossRef]
- Oldham, K.B. Analytical expressions for the reversible Randles-Sevcik function. J. Electroanal. Chem. Interfacial Electrochem. 1979, 105, 373–375. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An extended method for the practical evaluation of the standard rate constant from cyclic voltammetric data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Bollella, P.; Mazzei, F.; Favero, G.; Fusco, G.; Ludwig, R.; Gorton, L.; Antiochia, R. Improved DET communication between cellobiose dehydrogenase and a gold electrode modified with a rigid self-assembled monolayer and green metal nanoparticles: The role of an ordered nanostructuration. Biosens. Bioelectron. 2017, 88, 196–203. [Google Scholar] [CrossRef]
- Shrivastava, A. Method for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, J.-J.; Chen, H.-Y. Electrochemical biosensors based on layer-by-layer assemblies. Electroanalysis 2006, 18, 1737–1748. [Google Scholar] [CrossRef]
- Wu, B.-Y.; Hou, S.-H.; Yin, F.; Li, J.; Zhao, Z.-X.; Huang, J.-D.; Chen, Q. Amperometric glucose biosensor based on layer-by-layer assembly of multilayer films composed of chitosan, gold nanoparticles and glucose oxidase modified Pt electrode. Bios. Bioelectron. 2007, 22, 838–844. [Google Scholar] [CrossRef]
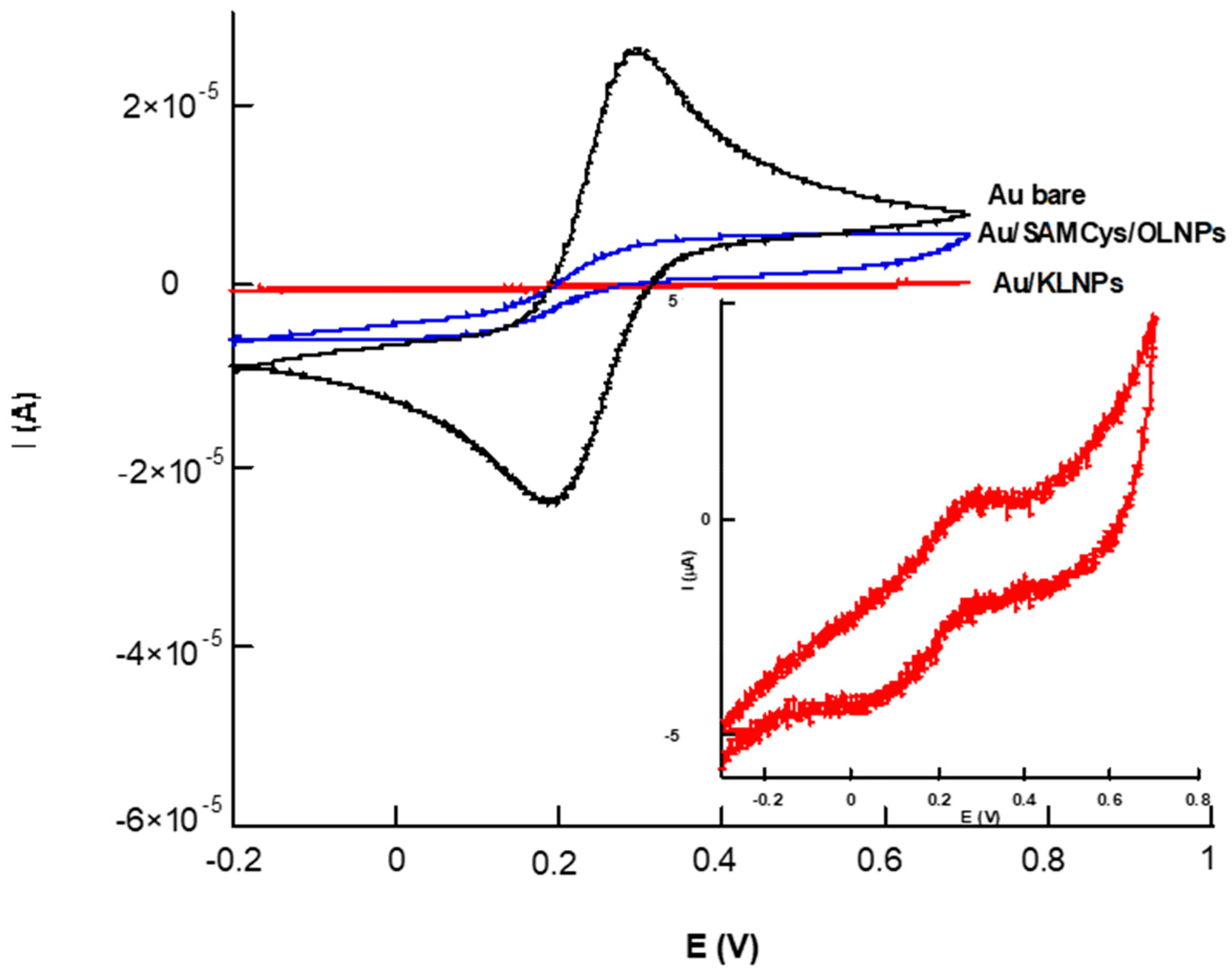
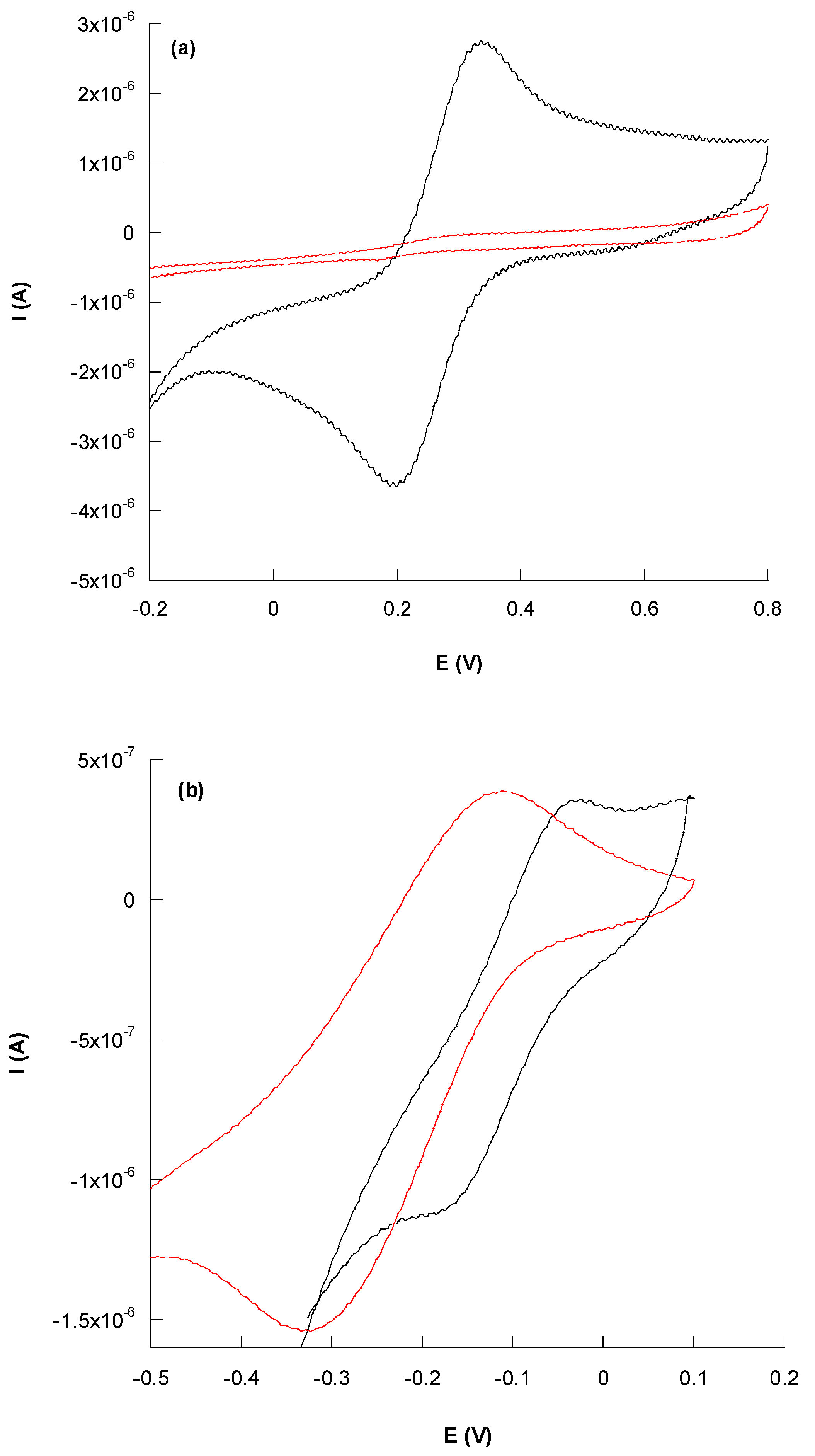
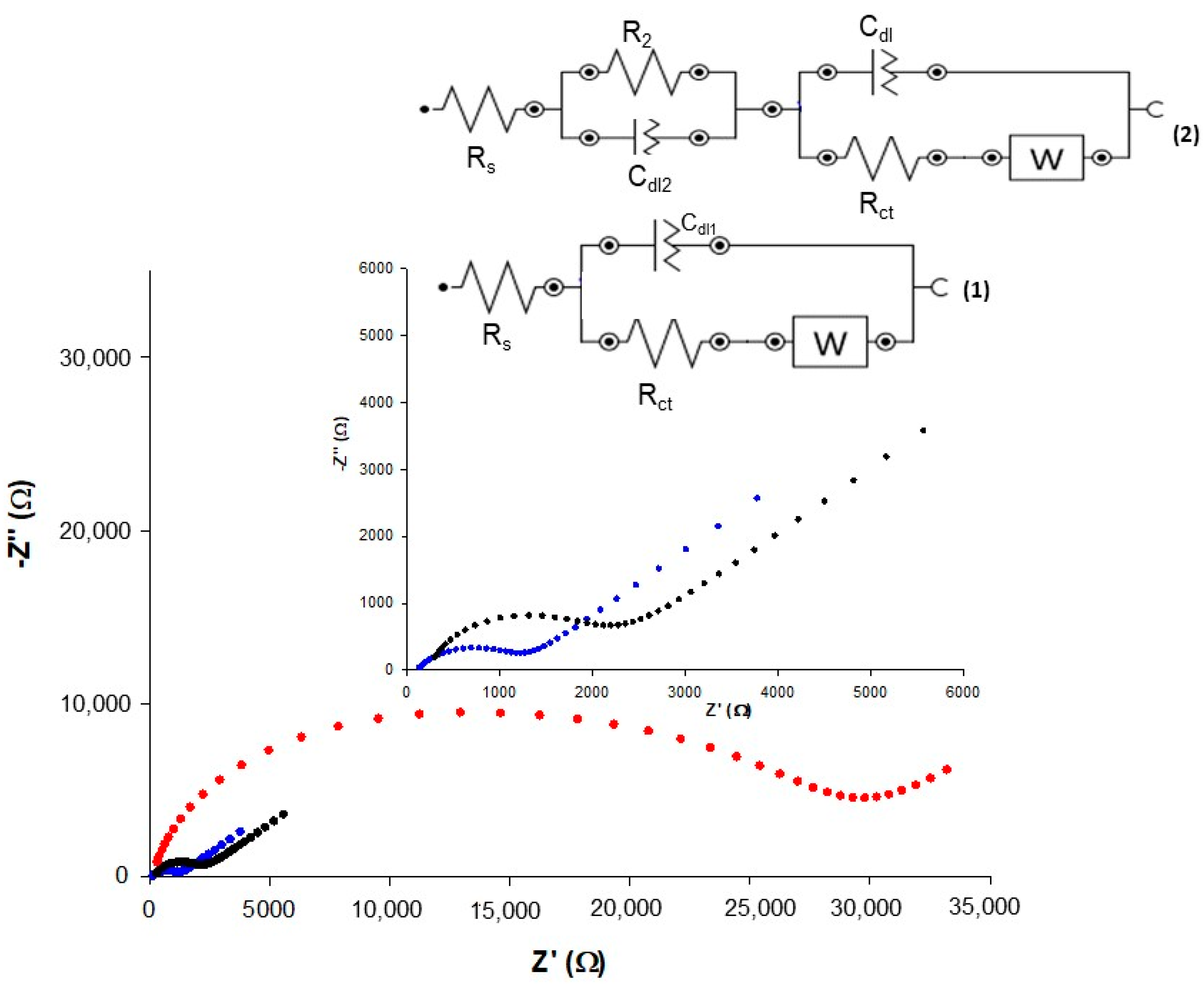
| Electrochemical Platform | Ae (mm2) | k0 (10−3 cm s−1) | ρ |
|---|---|---|---|
| Au bare | 5.3 | 3.97 ± 0.79 | 0.75 |
| Au/SAMcys/OLNPs | 2.6 | 1.96 ± 0.34 | 0.37 |
| Au/KLNPs | 0.8 | 0.16 ± 0.36 | 0.11 |
| Electrochemical Platform | Imax (µA) | KMapp (mM) | Linear Range (mM) | Sensitivity (µA/mM cm2) | LOD (mM) | R2 |
|---|---|---|---|---|---|---|
| Au/SAMCys/OLNPs/Con A/GOx | 2.43 ± 0.11 | 2.30 ± 0.42 | 0.33–2.5 | 4.53 ± 0.467 | 0.11 | 0.968 |
| Au/KLNPs/Con A/GOx | 4.58 ± 0.04 | 1.37 ± 0.06 | 0.15–2.5 | 13.74 ± 1.84 | 0.05 | 0.947 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortolini, C.; Capecchi, E.; Tasca, F.; Pofi, R.; Venneri, M.A.; Saladino, R.; Antiochia, R. Novel Nanoarchitectures Based on Lignin Nanoparticles for Electrochemical Eco-Friendly Biosensing Development. Nanomaterials 2021, 11, 718. https://doi.org/10.3390/nano11030718
Tortolini C, Capecchi E, Tasca F, Pofi R, Venneri MA, Saladino R, Antiochia R. Novel Nanoarchitectures Based on Lignin Nanoparticles for Electrochemical Eco-Friendly Biosensing Development. Nanomaterials. 2021; 11(3):718. https://doi.org/10.3390/nano11030718
Chicago/Turabian StyleTortolini, Cristina, Eliana Capecchi, Federico Tasca, Riccardo Pofi, Mary Anna Venneri, Raffaele Saladino, and Riccarda Antiochia. 2021. "Novel Nanoarchitectures Based on Lignin Nanoparticles for Electrochemical Eco-Friendly Biosensing Development" Nanomaterials 11, no. 3: 718. https://doi.org/10.3390/nano11030718
APA StyleTortolini, C., Capecchi, E., Tasca, F., Pofi, R., Venneri, M. A., Saladino, R., & Antiochia, R. (2021). Novel Nanoarchitectures Based on Lignin Nanoparticles for Electrochemical Eco-Friendly Biosensing Development. Nanomaterials, 11(3), 718. https://doi.org/10.3390/nano11030718











