Chitosan-Based Nanomaterials as Valuable Sources of Anti-Leishmanial Agents: A Systematic Review
Abstract
1. Introduction
2. Methods
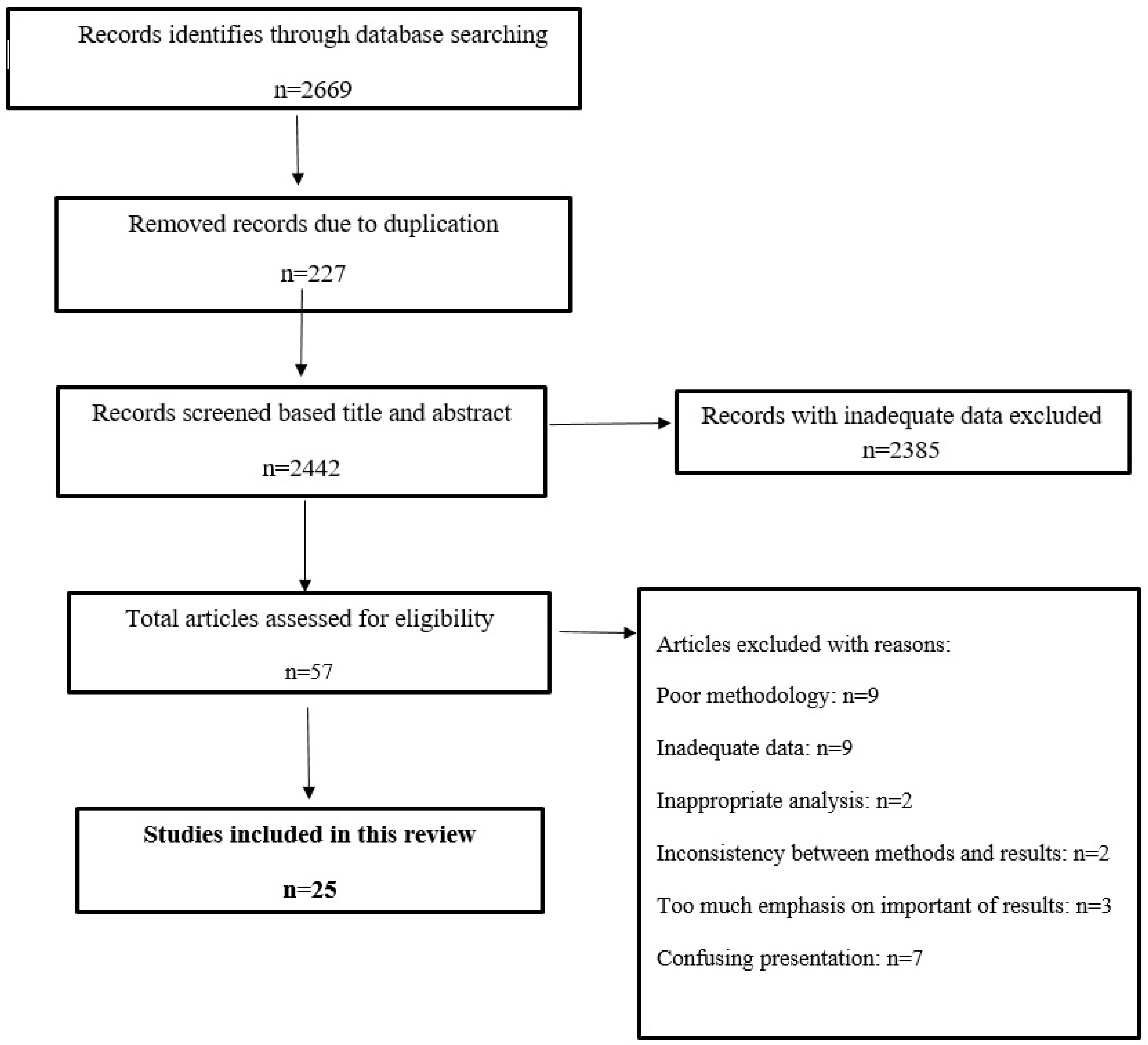
2.1. Database Search
2.2. Quality Assessment and Article Selection
2.3. Data Extraction
3. Results and Discussion
3.1. Chitosan Treatments In Vitro
3.2. Chitosan Treatments In Vivo
3.3. Treatments Using Chitosan as Vehicle
3.4. Possible Antimicrobial Mechanisms of Chitosan
3.5. Cytotoxicity Effects of Chitosan
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Urbanization: An increasing risk factor for leishmaniasis. Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 2002, 77, 365–370. [Google Scholar]
- Pearson, R.D.; Sousa, A.Q. Clinical spectrum of leishmaniasis. Clin Infect Dis. 1996, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, N.C. Cutaneous leishmaniasis: Clinical dermatology Review article. Clin. Exp. Dermatol. Clin. Dermatol. 2000, 25, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical syndromes and treatment. Qjm: Int. J. Med. 2014, 107, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Scorza, B.M.; Carvalho, E.M.; Wilson, M.E. Cutaneous Manifestations of Human and Murine Leishmaniasis. Int. J. Mol. Sci. 2017, 18, 1296. [Google Scholar] [CrossRef]
- Bi, K.; Chen, Y.; Zhao, S.; Kuang, Y.; Wu, C.-H.J. Current Visceral Leishmaniasis Research: A Research Review to Inspire Future Study. BioMed Res. Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- WHO Expert Committee on the Control of the Leishmaniases. Meeting, World Health Organization. In Proceedings of the Control of the Leishmaniases: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, Switzerland, 22–26 March 2010; World Health Organization: Copenhagen, Denmark, 2010. [Google Scholar]
- Monzote, L. Current treatment of leishmaniasis: A review. Open Antimicrob. Agents J. 2009, 1, 9–19. [Google Scholar] [CrossRef]
- Santos, D.O.; Coutinho, C.E.R.; Madeira, M.F.; Bottino, C.G.; Vieira, R.T.; Nascimento, S.B.; Bernardino, A.; Bourguignon, S.C.; Corte-Real, S.; Pinho, R.T.; et al. Leishmaniasis treatment—a challenge that remains: A review. Parasitol. Res. 2008, 103, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.F.; Schubach, A.O.; Martins, M.M.; Passos, S.L.; Oliveira, R.V.; Marzochi, M.C.; Andrade, C.A. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop. 2011, 118, 87–96. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef]
- Guan, G.; Azad, A.K.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological Effects and Applications of Chitosan and Chito-Oligosaccharides. Front. Physiol. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Rozman, N.A.S.; Tong, W.Y.; Leong, C.R.; Tan, W.N.; Hasanolbasori, M.A.; Abdullah, S.Z. Potential Antimicrobial Applications of Chitosan Nanoparticles (ChNP). J. Microbiol. Biotechnol. 2019, 29, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Varshosaz, J.; Arbabi, B.; Pestehchian, N.; Saberi, S.; Delavari, M. Chitosan-titanium dioxide-glucantime nanoassemblies effects on promastigote and amastigote of Leishmania major. Int. J. Biol. Macromol. 2018, 107, 212–221. [Google Scholar] [CrossRef]
- Mammeri, M.; Chevillot, A.; Thomas, M.; Polack, B.; Julien, C.; Marden, J.-P.; Auclair, E.; Vallée, I.; Adjou, K.T. Efficacy of chitosan, a natural polysaccharide, against Cryptosporidium parvum in vitro and in vivo in neonatal mice. Exp. Parasitol. 2018, 194, 1–8. [Google Scholar] [CrossRef]
- Torabi, N.; Dobakhti, F.; Faghihzadeh, S.; Haniloo, A. In vitro and in vivo effects of chitosan-praziquantel and chitosan-albendazole nanoparticles on Echinococcus granulosus Metacestodes. Parasitol. Res. 2018, 117, 2015–2023. [Google Scholar] [CrossRef]
- Teimouri, A.; Azami, S.J.; Keshavarz, H.; Esmaeili, F.; Alimi, R.; Mavi, S.A.; Shojaee, S. Anti-Toxoplasma activity of various molecular weights and concentrations of chitosan nanoparticles on tachyzoites of RH strain. Int. J. Nanomed. 2018, 13, 1341–1351. [Google Scholar] [CrossRef]
- Cheraghipour, K.; Masoori, L.; Ezzatkhah, F.; Salimikia, I.; Amiri, S.; Makenali, A.S.; Taherpour, F.; Mahmoudvand, H. Effect of chitosan on Toxoplasma gondii infection: A systematic review. Parasite Epidemiol. Control. 2020, 11, e00189. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Singh, P.K.; Pawar, V.K.; Jaiswal, A.K.; Singh, Y.; Srikanth, C.H.; Chaurasia, M.; Bora, H.K.; Raval, K.; Meher, J.G.; Gayen, J.R.; et al. Chitosan coated PluronicF127 micelles for effective delivery of Amphotericin B in experimental visceral leishmaniasis. Int. J. Biol. Macromol. 2017, 105, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, M.; Esboei, B.R.; Mousavi, P.; Fakhar, M.; Akhoundi, B. Potent antileishmanial activity of chitosan against Iranian strain of Leishmania major (MRHO/IR/75/ER): In vitro and in vivo assay. J. Vector Borne Dis. 2018, 55, 111. [Google Scholar] [CrossRef]
- Lima, D.D.S.; Gullon, B.; Cardelle-Cobas, A.; Brito, L.M.; Rodrigues, K.A.F.; Quelemes, P.V.; Ramos-Jesus, J.; Arcanjo, D.D.R.; Plácido, A.; Batziou, K.; et al. Chitosan-based silver nanoparticles: A study of the antibacterial, antileishmanial and cytotoxic effects. J. Bioact. Compat. Polym. 2017, 32, 397–410. [Google Scholar] [CrossRef]
- Kunjachan, S.; Gupta, S.; Dwivedi, A.K.; Dube, A.; Chourasia, M.K. Chitosan-based macrophage-mediated drug targeting for the treatment of experimental visceral leishmaniasis. J. Microencapsul. 2011, 28, 301–310. [Google Scholar] [CrossRef]
- Tripathi, P.; Jaiswal, A.K.; Dube, A.; Mishra, P.R. Hexadecylphosphocholine (Miltefosine) stabilized chitosan modified Ampholipospheres as prototype co-delivery vehicle for enhanced killing of L. donovani. Int. J. Biol. Macromol. 2017, 105, 625–637. [Google Scholar] [CrossRef]
- Karam, T.K.; Ortega, S.; Nakamura, T.U.; Auzély-Velty, R.; Nakamura, C.V. Development of chitosan nanocapsules containing essential oil of Matricaria chamomilla L. for the treatment of cutaneous leishmaniasis. Int. J. Biol. Macromol. 2020, 162, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Dwivedi, P.; Khatik, R.; Jaiswal, A.K.; Dube, A.; Shukla, P.; Mishra, P.R. Development of 4-sulfated N -acetyl galactosamine anchored chitosan nanoparticles: A dual strategy for effective management of Leishmaniasis. Colloids Surf. B Biointerfaces 2015, 136, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Feizabadi, E.; Zavaran Hosseini, A.; Soudi Sara, K. Studying the role of chitosan nanoparticle loaded with Leishmania major Secretory and excretory antigens on the number of apoptotic macrophages in parasite sensitive mouse. Daneshvar Med. Basic Clin. Res. J. 2020, 26, 9–18. [Google Scholar]
- Khan, M.; Shereen, M.A.; Khokhar, M.; Kamil, A.; Rahman, H. A novel effective therapeutic approach for treatment of Leishmania tropica through Miltefosine Loaded Chitosan Nanoparticles. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Riezk, A.; Van Bocxlaer, K.; Yardley, V.; Murdan, S.; Croft, S.L. Activity of Amphotericin B-Loaded Chitosan Nanoparticles against Experimental Cutaneous Leishmaniasis. Molecules 2020, 25, 4002. [Google Scholar] [CrossRef]
- Rahimi, M.; Tabaei, S.J.S.; Ziai, S.A.; Sadri, M. Anti-Leishmanial Effects of Chitosan-Polyethylene Oxide Nanofibers Containing Berberine: An Applied Model for Leishmania Wound Dressing. Iran. J. Med. Sci. 2020, 45, 286–297. [Google Scholar]
- Chaubey, P.; Mishra, B.; Mudavath, S.L.; Patel, R.R.; Chaurasia, S.; Sundar, S.; Suvarna, V.; Monteiro, M. Mannose-conjugated curcumin-chitosan nanoparticles: Efficacy and toxicity assessments against Leishmania donovani. Int. J. Biol. Macromol. 2018, 111, 109–120. [Google Scholar] [CrossRef]
- Cabral, F.V.; Pelegrino, M.T.; Sauter, I.P.; Seabra, A.B.; Cortez, M.; Ribeiro, M.S. Nitric oxide-loaded chitosan nanoparticles as an innovative antileishmanial platform. Nitric Oxide-Biol. Chem. 2019, 93, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, F.; Motazedian, M.H.; Asgari, Q.; Morowvat, M.H.; Molaei, M.; Heli, H. Paromomycin-loaded mannosylated chitosan nanoparticles: Synthesis, characterization and targeted drug delivery against leishmaniasis. Acta Trop. 2019, 197, 105072. [Google Scholar] [CrossRef]
- Seyyed Tabaei, S.J.; Rahimi, M.; Akbaribazm, M.; Ziai, S.A.; Sadri, M.; Shahrokhi, S.R.; Rezaei, M.S. Chitosan-based nano-scaffolds as antileishmanial wound dressing in BALB/c mice treatment: Characterization and design of tissue regeneration. Iran J. Basic Med. Sci. 2020, 23, 788–799. [Google Scholar]
- Malli, S.; Pomel, S.; Dennemont, I.; Loiseau, P.M.; Bouchemal, K. Combination of amphotericin B and chitosan platelets for the treatment of experimental cutaneous leishmaniasis: Histological and immunohistochemical examinations. J. Drug Deliv. Sci. Technol. 2019, 50, 34–41. [Google Scholar] [CrossRef]
- Malli, S.; Pomel, S.; Ayadi, Y.; Deloménie, C.; Da Costa, A.; Loiseau, P.M.; Bouchemal, K. Topically Applied Chitosan-Coated Poly(isobutylcyanoacrylate) Nanoparticles Are Active Against Cutaneous Leishmaniasis by Accelerating Lesion Healing and Reducing the Parasitic Load. ACS Appl. Bio Mater. 2019, 2, 2573–2586. [Google Scholar] [CrossRef]
- Gupta, P.K.; Jaiswal, A.K.; Asthana, S.; Verma, A.; Kumar, V.; Shukla, P.; Dwivedi, P.; Dube, A.; Mishra, P.R. Self Assembled Ionically Sodium Alginate Cross-Linked Amphotericin B Encapsulated Glycol Chitosan Stearate Nanoparticles: Applicability in Better Chemotherapy and Non-Toxic Delivery in Visceral Leishmaniasis. Pharm. Res. 2015, 32, 1727–1740. [Google Scholar] [CrossRef]
- Siqueira-Neto, J.L.; Song, O.-R.; Oh, H.; Sohn, J.-H.; Yang, G.; Nam, J.; Jang, J.; Cechetto, J.; Lee, C.B.; Moon, S.; et al. Antileishmanial High-Throughput Drug Screening Reveals Drug Candidates with New Scaffolds. PLoS Negl. Trop. Dis. 2010, 4, e675. [Google Scholar] [CrossRef]
- Sharlow, E.R.; Close, D.; Shun, T.; Leimgruber, S.; Reed, R.; Mustata, G.; Wipf, P.; Johnson, J.; O’Neil, M.; Grogl, M.; et al. Identification of Potent Chemotypes Targeting Leishmania major Using a High-Throughput, Low-Stringency, Computationally Enhanced, Small Molecule Screen. PLoS Negl. Trop. Dis. 2009, 3, e540. [Google Scholar] [CrossRef]
- St George, S.; Bishop, J.V.; Titus, R.G.; Selitrennikoff, C.P. Novel compounds active against Leishmania major. Antimicrob. Agents Chemother. 2006, 50, 474–479. [Google Scholar] [CrossRef][Green Version]
- Fumarola, L.; Spinelli, R.; Brandonisio, O. In vitro assays for evaluation of drug activity against Leishmania spp. Res. Microbiol. 2004, 155, 224–230. [Google Scholar] [CrossRef]
- De Muylder, G.; Ang, K.K.; Chen, S.; Arkin, M.R.; Engel, J.C.; McKerrow, J.H. A screen against Leishmania intracellular amastigotes: Comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl. Trop. Dis. 2011, 5, e1253. [Google Scholar] [CrossRef]
- Vermeersch, M.; Da Luz, R.I.; Toté, K.; Timmermans, J.-P.; Cos, P.; Maes, L. In Vitro Susceptibilities of Leishmania donovani Promastigote and Amastigote Stages to Antileishmanial Reference Drugs: Practical Relevance of Stage-Specific Differences. Antimicrob. Agents Chemother. 2009, 53, 3855–3859. [Google Scholar] [CrossRef]
- Ribeiro, T.G.; Franca, J.R.; Fuscaldi, L.L.; Santos, M.L.; Duarte, M.C.; Lage, P.S.; Martins, V.T.; Costa, L.E.; Fernandes, S.O.; Cardoso, V.N.; et al. An optimized nanoparticle delivery system based on chitosan and chondroitin sulfate molecules reduces the toxicity of amphotericin B and is effective in treating tegumentary leishmaniasis. Int. J. Nanomed. 2014, 9, 5341–5353. [Google Scholar]
- Asthana, S.; Jaiswal, A.K.; Gupta, P.K.; Pawar, V.K.; Dube, A.; Chourasia, M.K. Immunoadjuvant Chemotherapy of Visceral Leishmaniasis in Hamsters Using Amphotericin B-Encapsulated Nanoemulsion Template-Based Chitosan Nanocapsules. Antimicrob. Agents Chemother. 2013, 57, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Samani, S.; Bahraini, D.; Shokri, J.; Kamali-Sarvestani, E.; Baezegar-Jalali, M.; Samiei, A.; Danesh-Bahreini, M.A.; Barzegar-Jalali, M. Nanovaccine for leishmaniasis: Preparation of chitosan nanoparticles containing Leishmania superoxide dismutase and evaluation of its immunogenicity in BALB/c mice. Int. J. Nanomed. 2011, 6, 835–842. [Google Scholar] [CrossRef]
- Mehrizi, T.Z.; Ardestani, M.S.; Hoseini, M.H.M.; Khamesipour, A.; Mosaffa, N.; Ramezani, A. Novel nano-sized chitosan amphotericin B formulation with considerable improvement against Leishmania major. Nanomedicine 2018, 13, 3129–3147. [Google Scholar] [CrossRef]
- Moreno, E.; Schwartz, J.; Larrea, E.; Conde, I.; Font, M.; Sanmartín, C.; Irache, J.M.; Espuelas, S. Assessment of β-lapachone loaded in lecithin-chitosan nanoparticles for the topical treatment of cutaneous leishmaniasis in L. major infected BALB/c mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 2003–2012. [Google Scholar] [CrossRef]
- Mehrizi, T.Z.; Ardestani, M.S.; Hoseini, M.H.M.; Khamesipour, A.; Mosaffa, N.; Ramezani, A. Novel Nanosized Chitosan-Betulinic Acid Against Resistant Leishmania Major and First Clinical Observation of such parasite in Kidney. Sci. Rep. 2018, 8, 11759. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Lalatsa, A.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Garrett, N.L.; Moger, J.; Guarro, J.; Capilla, J.; Ballesteros, M.P.; Schätzlein, A.G.; et al. Oral Particle Uptake and Organ Targeting Drives the Activity of Amphotericin B Nanoparticles. Mol. Pharm. 2015, 12, 420–431. [Google Scholar] [CrossRef]
- Nagy, A.; Harrison, A.; Sabbani, S.; Munson Jr, R.S.; Dutta, P.K.; Waldman, W.J. Silver nanoparticles embedded in zeolite membranes: Release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011, 6, 1833. [Google Scholar]
- Suman Gupta, N. Visceral leishmaniasis: Experimental models for drug discovery. Indian J. Med. Res. 2011, 133, 27–39. [Google Scholar] [PubMed]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed]
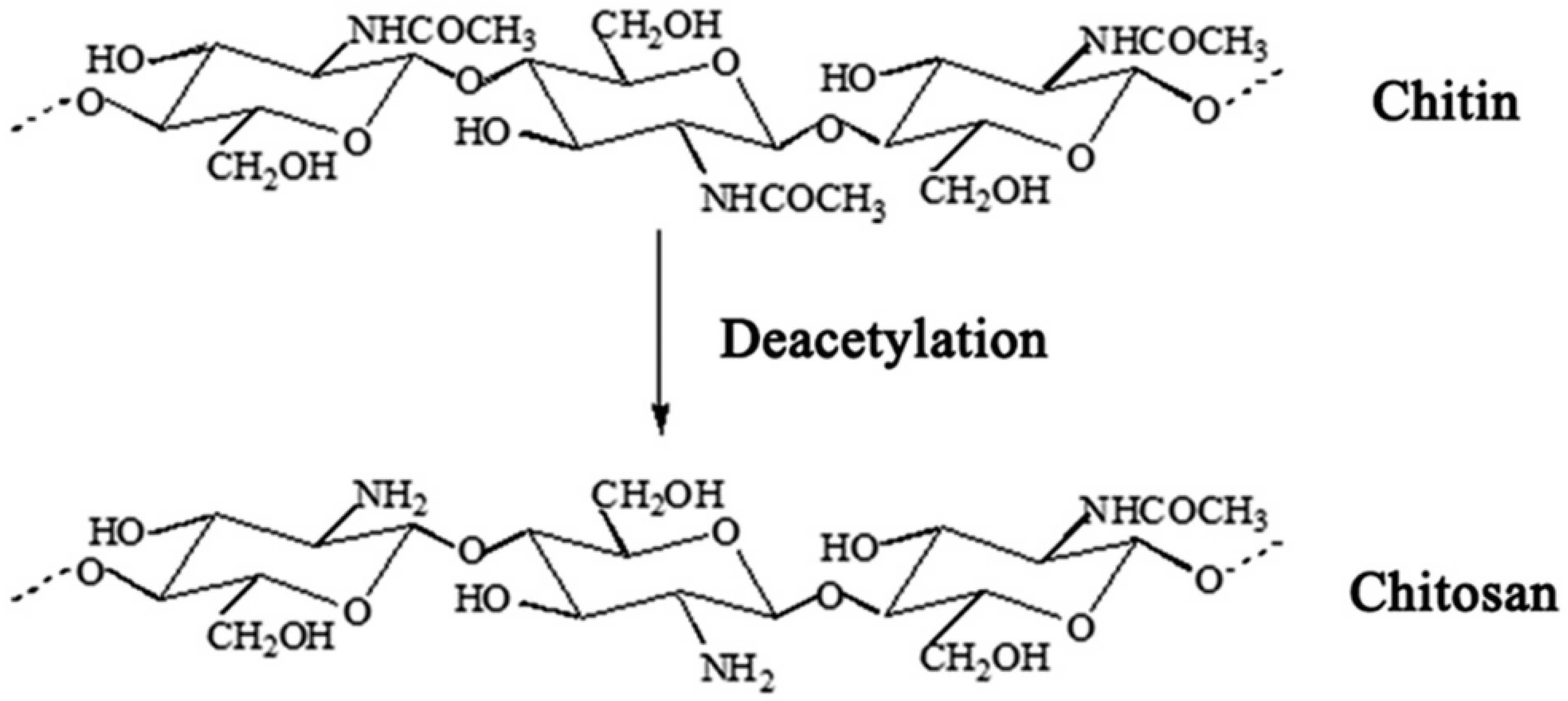
| Type of Chitosan | Combined with the Drug | Year of Publication | Parasite Form | Leishmania spp. | Concentration | Time | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|
| AmB-loaded pluronic F127 (PF 127) micelles coated with chitosan (Cs-PF-AmB-M) | Amphotericin B (AmpB) | 2017 | Promastigotes | Leishmania donovani | 0.03, 0.05, 0.1, 0.2, 0.4, 0.8 µg/mL | 72 h | Experiments have shown that Cs-PF-AmB-M at a dose of 0.049 µg/mL may reduce parasitic load by 50%; whereas PF-AmB-M at a dose of 0.08 µg/mL reduced parasitic load by 50%. | [30] |
| Chitosan | - | 2018 | Promastigotes | L. major | 50, 100, 200, 400 μg/mL | 30, 60, 120 and 180 min | The results showed that chitosan at the concentrations of 200 and 400 μg/mL after 180 min killed 100% of promastigote. | [31] |
| Chitosan-based silver nanoparticles | - | 2017 | Promastigotes and amastigotes | L. amazonensis | 0.42 to 27µg | 48 h | The results showed that this compound has potent anti-leishmanial effects against promastigote and amastigote stages of L. amazonensis after 48 h exposure, with IC50 values ranging from 0.422 to 2120 μg/mL. | [32] |
| Chitosan microparticles | Doxorubicin hydrochloride (DOX) | 2011 | Promastigotes | L. donovani | 0.03, 0.08, 0.13 and 0.2 mg/mL | 20 h | The results showed that the greatest effect of these microparticles was in the first 60 min and caused nonspecific activation of phagocytosis in macrophages. | [33] |
| Chitosan anchored nanostructured lipid carriers (NLC) | Miltefosine (HePC- hexadecyl phosphocholine) and amphotericin B (AmB) | 2017 | Amastigotes | L. donovani | 50, 100, 250, 500, 1000 ng/mL | 4 h | The results showed that the highest effect of these nanoparticles was at a concentration of 1000 ng/mL, which killed more than 80% of amastigotes, while AmB alone reduced the parasitic load by about 60%. | [34] |
| Chitosan nanocapsules containing essential oil of Matricaria chamomilla (NCEO) | - | 2020 | Promastigotes | L. amazonensis | 0.1–1000 μg/mL | 48 h | The results showed that IC50 NCEO was 7.18 ± 0.7 μg/mL against promastigotes and 14.29 ± 1.01 μg/mL against amastigotes. | [35] |
| Chitosan nanoparticles (CNPs) and, 4-SO4GalNAc modified chitosan nanoparticles (SCNP) | Amphotericin B (AmpB) | 2015 | Amastigotes | L. donovani | 0.05, 0.1, 0.2. 0.4. 0.8 (µg/mL) | 24 h | The results showed that AmB-SCNPs and AmB–CNPs had a better effect in comparison with amphotericin B and more than 80% of their lethality was recorded, while for amphotericin B 70% lethality have been recorded. | [36] |
| Chitosan nanoparticles | - | 2019 | Amastigotes | L. major | 5–250 µL/mL | 48 h | Chitosan coupled with L. major secretory and excretory proteins can increase the ability of infected macrophages to remove parasites by reducing apoptosis. | [37] |
| Chitosan nanoparticles | Miltefosine | 2020 | Promastigotes and amastigotes | L. tropica | 100 µL/mL | 72 h | The results showed that IC50 value for promastigote and amastigote forms of L. tropica was 0.07 ± 0.05 µL/mL and 0.09 ± 0.02 µL/mL, respectively, | [38] |
| Chitosan nanoparticles and sodium tripolyphosphate (TPP) | Amphotericin B (AmB) | 2020 | Amastigotes | L. major and L. mexicana | 1 mg/mL | 7 days | The results showed that EC50 value of AmB-CH-TPP for L. major and L. mexicana amastigotes was 0.14 ± 0.09 µg/mL and 0.5 ± 0.01 µg/mL, respectively. | [39] |
| Chitosan-polyethylene oxide nanofibers containing berberine | - | 2020 | Promastigotes and amastigote | L. major | 0.01–50 μg/mL | 24, 48, 72 h | The results showed that this compound has potent anti-leishmanial effects against promastigotes and amastigotes of L. major with IC50 values ranging from 0.197 to 1.023 μg/mL. | [40] |
| Curcumin-loaded mannose-functionalized chitosan nanoparticles (Cur-MCN) | - | 2018 | Amastigotes | L. donovani | 0.05–2.0 mg/L | 72 h | The results showed that Cur-MCN at the concentration of 0.518 ± 0.01 mg/L reduced 50% of amastigotes; also, no toxic effect on macrophages was observed in the use of Cur-MCN. | [41] |
| Encapsulate S-nitroso-mercaptosuccinic acid into chitosan nanoparticles (NONPs) | - | 2019 | Promastigotes and amastigotes | L. amazonensis | 25, 50, 75, 100, 200, 400 µM | 24 h | Experiments on amastigotes and promastigotes of L. amazonensis showed that NONPs reduced 65% of the parasitic load at a dose of 200 µM and killed 85% of promastigotes at a dose of 75 µM. These nanoparticles also reduced the number of amastigotes from 8.5 ± 1.2 in the control group to 4.5 ± 0.4 per 300 macrophages and reduced the infection rate from 76.2 ± 7.1 to 63.7 ± 5.4. | [42] |
| Mannosylated chitosan (MCS) with dextran (dex) | Paromomycin (PM) | 2019 | Amastigotes | L. major | 5, 10, 20, 40, 80, 160, 320 μg/mL | 24, 48 h | The results showed that this compound has no cytotoxicity on macrophages and at a dose of 5 μg/mL reduced more than 60% of the parasitic load inside macrophages. | [43] |
| Nanosized chitosan-betulinic acid | - | 2020 | Promastigotes and amastigote | L. major | 20 μg/mL | 48 h | The results showed that BK20 (20 μg/mL) was effective to kill the parasite by 86% compared to negative control group. The infection rate and the mean number of amastigotes per each macrophage were found to be 73% and 7%, respectively. | [44] |
| Oleoyl chitosan and α-cyclodextrin (α-CD) | - | 2019 | Amastigotes | L. major | 100 μL | 4 days | The results showed that the use of oleoyl chitosan/α-CD platelets at a dose of 60.24 ± 4.42 μg/mL killed 50% of amastigotes. | [45] |
| Poly (isobutylcyano acrylate) nanoparticles coated with chitosan (Cs-NPs) | Amphotericin B-deoxycholate (AmB-DOC) | 2019 | Promastigotes and amastigote | L. major | 20 mg/mL | 10 min, 20 min, 30 min, 1 h, or 2 h | The IC50 values for L. major promastigote and axenic amastigote forms were 1.14 ± 0.11 μg/mL and 0.53 ± 0.07 μg/mL, respectively. | [46] |
| Sodium alginate-glycol chitosan stearate nanoparticles (SA-GCS-NP) | Amphotericin B (AmB) | 2015 | Amastigotes | L. donovani | 10 ng/mL | 48 h | The IC50 values of AmB-SAGCS-NP and AmB for amastigotes of L. donovani were 0.128 ± 0.024 μg/mL and 0.214 ± 0.06 μg/mL, respectively. | [47] |
| Type of Chitosan | Combined with the Drug | Method | Administration | Animal | Leishmania spp. | Dose | Time | Outcome | Year of Publication | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| AmB-loaded pluronic F127 (PF 127) micelles coated with chitosan (Cs-PF-AmB-M) | Amphotericin B (AmB) | Film hydration method | Intraperitoneal | Syrian golden hamster | Leishmania donovani | 1 mg/kg | 5 days | The results showed that Cs-PF-AmB-M and PF-AmB-M significantly reduced the parasite load; also, the number of amastigotes was significantly reduced by 52.67 ± 17.24. | 2017 | [30] |
| Chitosan | - | - | Topically | BALB/c mice | Leishmania major | 200, 400 μg/mL | 28 days | Chitosan reduced the size of the lesion from 10.7 ± 3.24 mm in the control group to 1.05 ± 1.02 mm on day 28 at a dose of 400 μg/mL. | 2018 | [31] |
| Chitosan microparticles | Doxorubicin hydrochloride (DOX) | - | Intraperitoneal | Golden hamsters | Leishmania donovani | 500 mg/kg | 7 days | The results showed that this compound killed 78.2 ± 10.4% of amastigotes. | 2011 | [33] |
| Chitosan anchored nanostructured lipid carriers (NLC) | Miltefosine (HePC- hexadecyl phosphocholine) and amphotericin B (AmB) | - | Intravenous | Naive hamsters | Leishmania donovani | 1 mg/kg | 5 days | The results showed that HePC-AmB-CNLCs could reduce the parasitic load by 88.14 ± 4.12%, while tween 80-AmB-CNLCs and AmB reduced the parasite load by 70.91 ± 3.5% and 53.26 ± 2.5%, respectively. | 2017 | [34] |
| Chitosan nanocapsule (CNC) | Amphotericin B | Emulsification n-solvent evaporation | Intraperitoneal | Syrian golden hamsters | Leishmania donovani | 1 mg of drug/kg | 30 days | The results showed that this compound killed 86.1 ± 2.08% of Leishmania amastigotes. | 2013 | [55] |
| Chitosan nanoparticles | - | Ionotropic gelation process | Subcutaneously | BALB/c mice | Leishmania major | 5 μg/50 μL | 3 weeks | The results showed that injection of this compound in BALB/c mice could activate TH1 cells and IgG2a and eradicate Leishmania with cell-mediated immunity. | 2011 | [56] |
| Chitosan nanoparticles | Amphotericin B (AmpB) | Polyelectrolyte complexes technique | Intravenous | BALB/c mice | Leishmania amazonensis | 100 μL/kg | 10 days | The results showed that the combined use of chitosan and chondroitin sulfate nanoparticles with amphotericin B can significantly reduce the lesion size and parasitic load and also provide higher levels of IFN-γ and IL-12. | 2014 | [54] |
| Chitosan nanoparticles (CNPs) and, 4-SO4GalNAc modified chitosan nanoparticles (SCNP) | Amphotericin B (AmpB) | Ionic gelation | Intravenous | Wistar rats | Leishmania donovani | 1 mg/kg | 0.5, 1, 2, 4, 6 and 24 h | The results showed that the use of AmB-SCNPs reduced the load of parasites in the spleen by 75.30 ± 3.76%, but the use of AmB-CNPs and amphotericin B alone kills 63.89 ± 3.44% and 47.56 ± 2.37% of parasites. | 2015 | [36] |
| Chitosan nanoparticles and sodium tripolyphosphate (TPP) | Amphotericin B (AmB) | Dextran sulphate aqueous solution | Intravenous | BALB/c mice | Leishmania major | 1.25, 2.5, 5 mg/kg | 10 days | AmB-CH-TPP at a dose of 5 mg/kg reduced the size of the lesion by 83% and also reduce the parasitic load by 99%, but CH-TPP only reduced 35% of the lesion size and 65% of parasitic load. | 2020 | [39] |
| Chitosan platelets | Amphotericin B-deoxycholate | - | Intralesional | BALB/c mice | Leishmania major | 100 μL/kg | 13 days | The results showed that the use of AmB-DOC and the chitosan platelets caused thickening and dry scales on the lesion, which indicated improvement; granuloma spread in these mice is more limited and the number of infected macrophages is less than the use of AmB-DOC. | 2019 | [45] |
| Curcumin-loaded mannose-functionalized chitosan nanoparticles (Cur-MCN) | - | - | Intraperitoneal | Hamster | Leishmania donovani | 50 mg/kg | 5 days | The results showed that Cur-MCN have more anti-leishmaniasis properties than curcumin alone and are also more efficient at drug delivery than Cur-CN (curcumin-loaded unconjugated chitosan nanoparticles). Cur-MCN were able to reduce the parasitic load in the spleen by 94.20% and the number of amastigotes from 1647 ± 125.2 in the control group to 112 ± 32.2 per 500 macrophages. | 2018 | [41] |
| Nano chitosan | Amphotericin B | - | Intralesional | BALB/c mice | Leishmania major | 5, 7, 10 mg/kg | 3 weeks | The results showed that this compound improved the lesion and reduce its diameter to 0 mm and killed 81% of amastigotes; additionally, no mortality was reported in mice after using this compound; while using amphotericin B alone, 10% of mice died, and no toxicity or side effects were reported. | 2018 | [57] |
| Poly (isobutylcyano acrylate) nanoparticles coated with chitosan (Cs-NPs) | Amphotericin B-deoxycholate (AmB-DOC) | - | Topically | BALB/c mice | Leishmania major | 100 μL/kg | 13 days | The results showed that topical application of this compound with or without AmB-DOC on the skin of L. major mice could cause a slight improvement of the CL lesion; also, the collected skin samples showed that this combination reduces the parasitic load. | 2019 | [46] |
| Sodium alginate-glycol chitosan stearate nanoparticles (SA-GCS-NP) | Amphotericin B (AmB) | Ionotropic complexation method | Intraperitoneally | Syrian golden hamsters | Leishmania donovani | 5, 10, 20 mg/kg | 5 days | The results showed that AmB-SAGCS-NP reduced 70.21 ± 3.46% of the parasitic load, while AmB kills only 53.24 ± 2.84% of amastigotes. | 2015 | [47] |
| β-lapachone (βLP) in lecithin-chitosan nanoparticles (NP) | - | - | Topically | BALB/c mice | Leishmania major | 20 mg/kg | 21 days | The use of these nanoparticles in CL reduced the number of amastigotes from 46 to 11 per 100 macrophages; also, these nanoparticles reduced the size of the lesion from 61.2 ± 21.2 mm2 to 35.7 ± 29.4 mm2. | 2015 | [58] |
| Nanosized chitosan-betulinic acid | - | Drug adsorption and phase separation | Intraperitoneally | BALB/c mice | Leishmania major | 20 mg/kg | 28 days | The lesion size in positive control group (GUL200) was negligibly decreased to 1.2 mm; also, in B20 mg/kg and K12.5 mg/kg receiver mice, the lesion size was slightly decreased, while in the group of BK20 mg/kg, the lesion size was considerably decreased and reached to zero (p < 0.001) | 2018 | [59] |
| Chitosan-based nano-scaffolds | - | Electrospinning method | Topically | BALB/c mice | Leishmania major | 20 wt% | 28 days | This compound significantly reduced skin ulcer diameter (p = 0.000), parasite burden (p = 0.003), changes in the epidermis (p = 0.023), and dermis (p = 0.032); indicated significantly strong effectiveness of the produced nano-scaffolds against Leishmania ulcers. | 2020 | [44] |
| N-Palmitoyl-N-monomethyl-N,N-dimethyl-N,N,N-trimethyl6-O-glycol chitosan nanoparticles (GCPQ) | Amphotericin B | - | Orally | BALB/c mice | Leishmania infantum | 5 mg/kg | 10 days | AmB-GCPQ nanoparticles demonstrated higher efficacy compared with parenteral liposomal AmB. | 2015 | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlMohammed, H.I.; Khudair Khalaf, A.; E. Albalawi, A.; Alanazi, A.D.; Baharvand, P.; Moghaddam, A.; Mahmoudvand, H. Chitosan-Based Nanomaterials as Valuable Sources of Anti-Leishmanial Agents: A Systematic Review. Nanomaterials 2021, 11, 689. https://doi.org/10.3390/nano11030689
AlMohammed HI, Khudair Khalaf A, E. Albalawi A, Alanazi AD, Baharvand P, Moghaddam A, Mahmoudvand H. Chitosan-Based Nanomaterials as Valuable Sources of Anti-Leishmanial Agents: A Systematic Review. Nanomaterials. 2021; 11(3):689. https://doi.org/10.3390/nano11030689
Chicago/Turabian StyleAlMohammed, Hamdan I., Amal Khudair Khalaf, Aishah E. Albalawi, Abdullah D. Alanazi, Parastoo Baharvand, Ali Moghaddam, and Hossein Mahmoudvand. 2021. "Chitosan-Based Nanomaterials as Valuable Sources of Anti-Leishmanial Agents: A Systematic Review" Nanomaterials 11, no. 3: 689. https://doi.org/10.3390/nano11030689
APA StyleAlMohammed, H. I., Khudair Khalaf, A., E. Albalawi, A., Alanazi, A. D., Baharvand, P., Moghaddam, A., & Mahmoudvand, H. (2021). Chitosan-Based Nanomaterials as Valuable Sources of Anti-Leishmanial Agents: A Systematic Review. Nanomaterials, 11(3), 689. https://doi.org/10.3390/nano11030689








