Simulation of BNNSs Dielectrophoretic Motion under a Nanosecond Pulsed Electric Field
Abstract
1. Introduction
2. Simulation Methods
2.1. Theoretical Study of Dielectrophoretic Motion under a Nanosecond Pulsed Electric Field
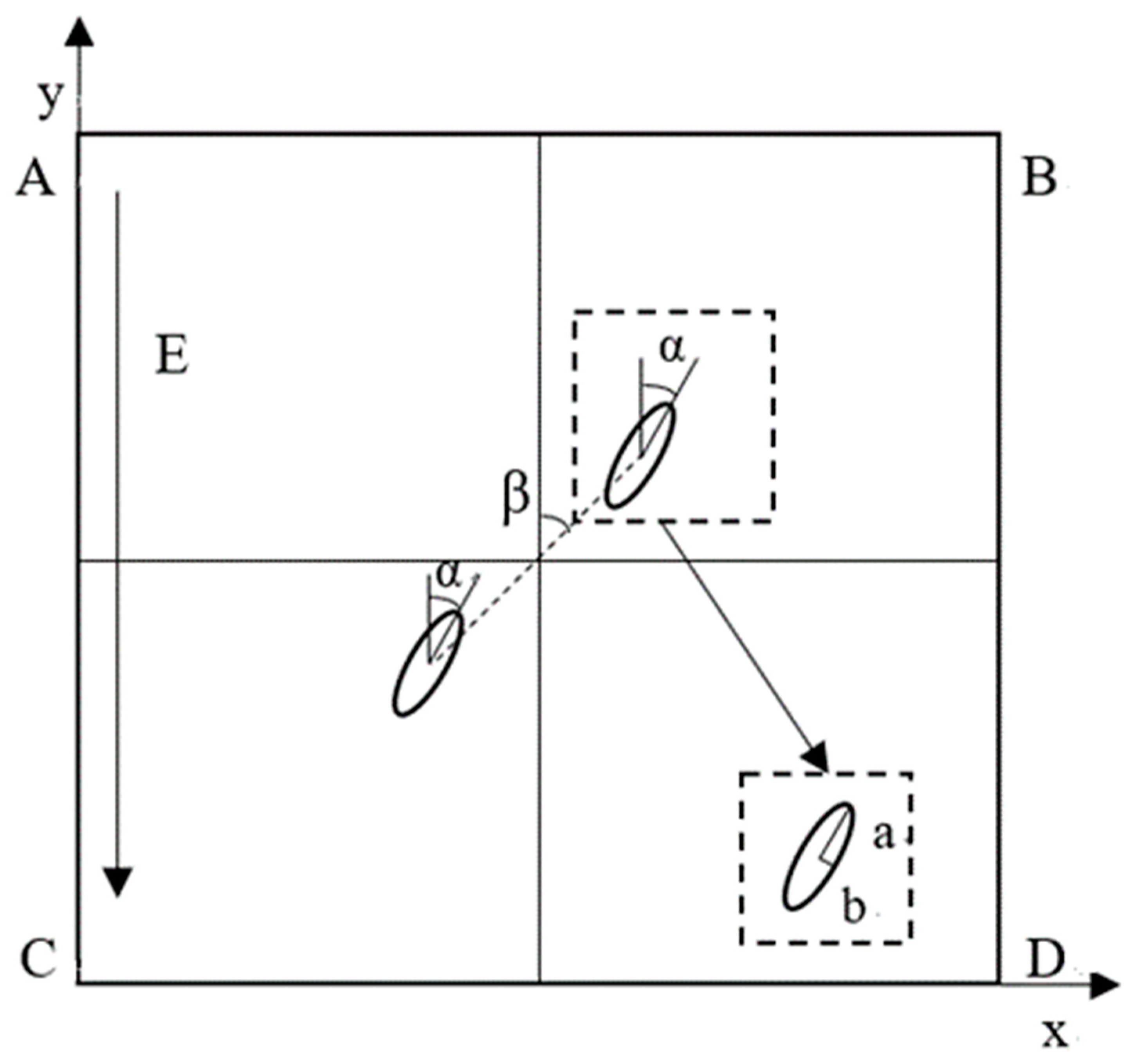
2.2. Mathematical Model
3. Simulation Results and Discussion
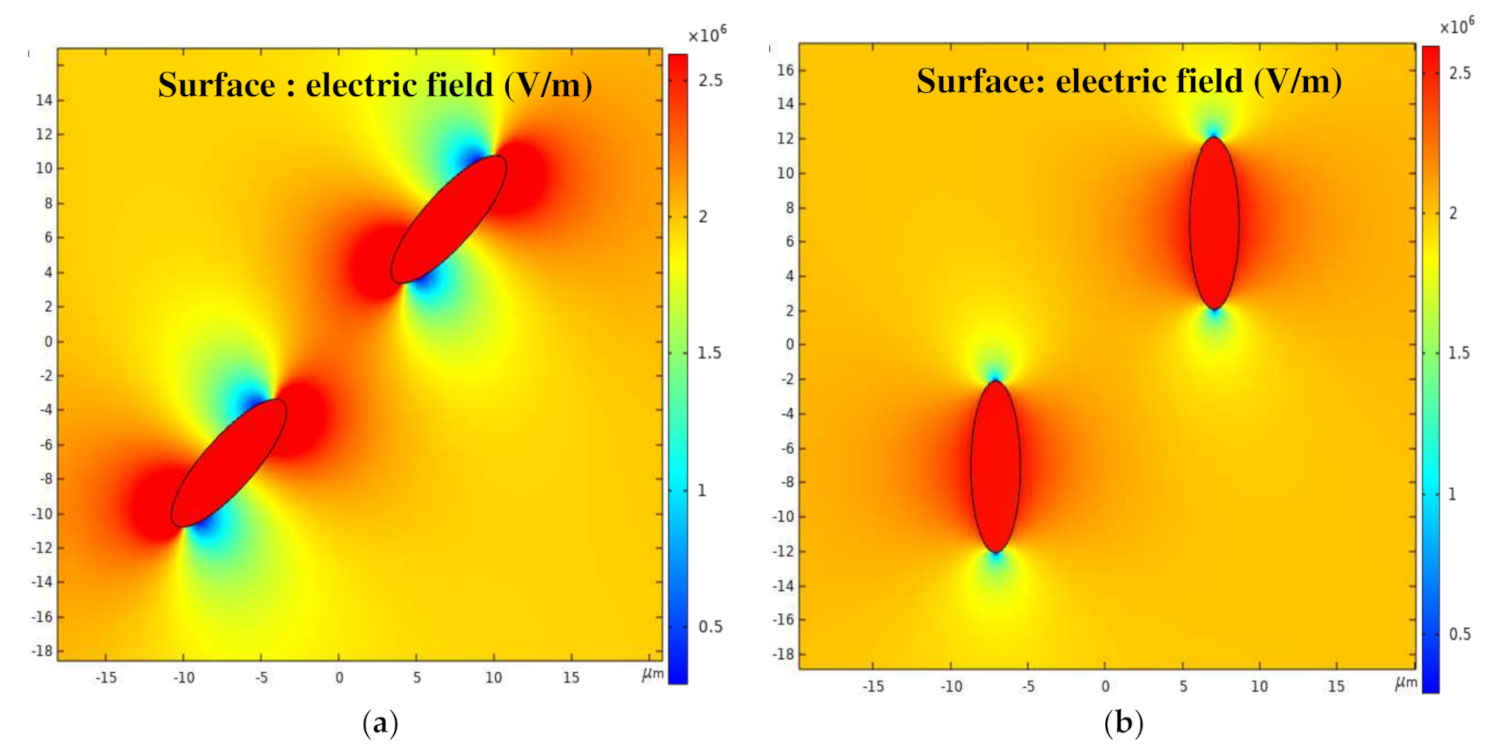
3.1. Distribution of the Electric Field Intensity
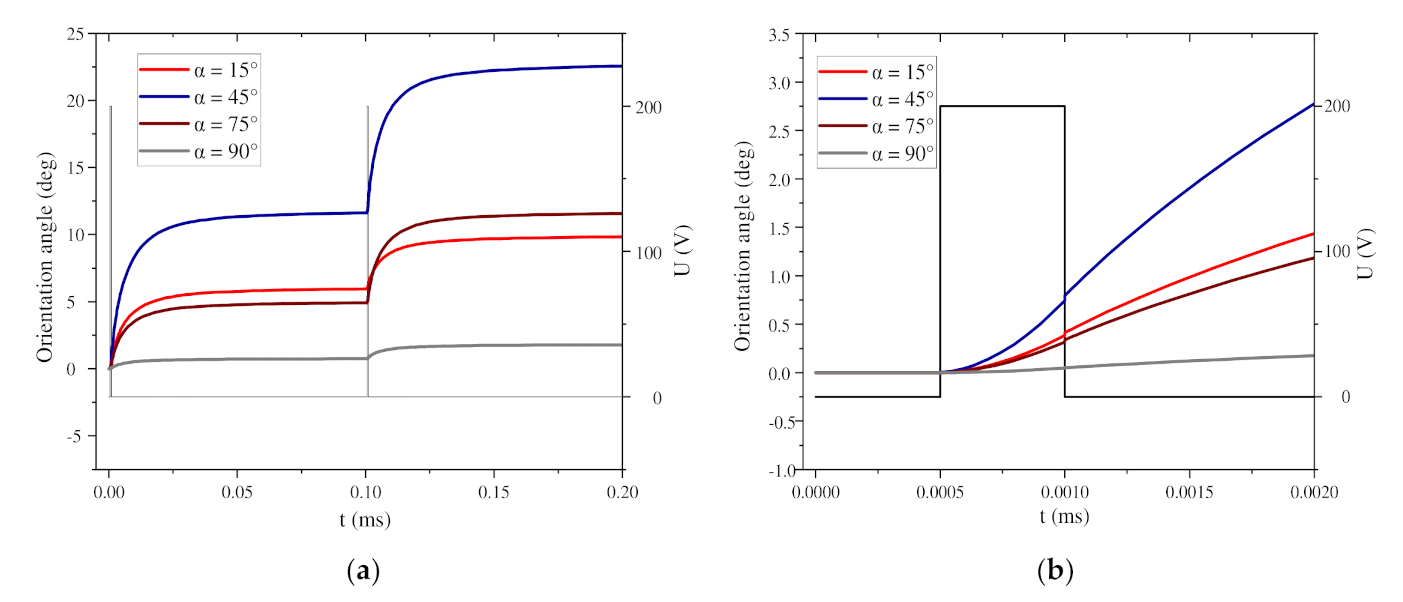
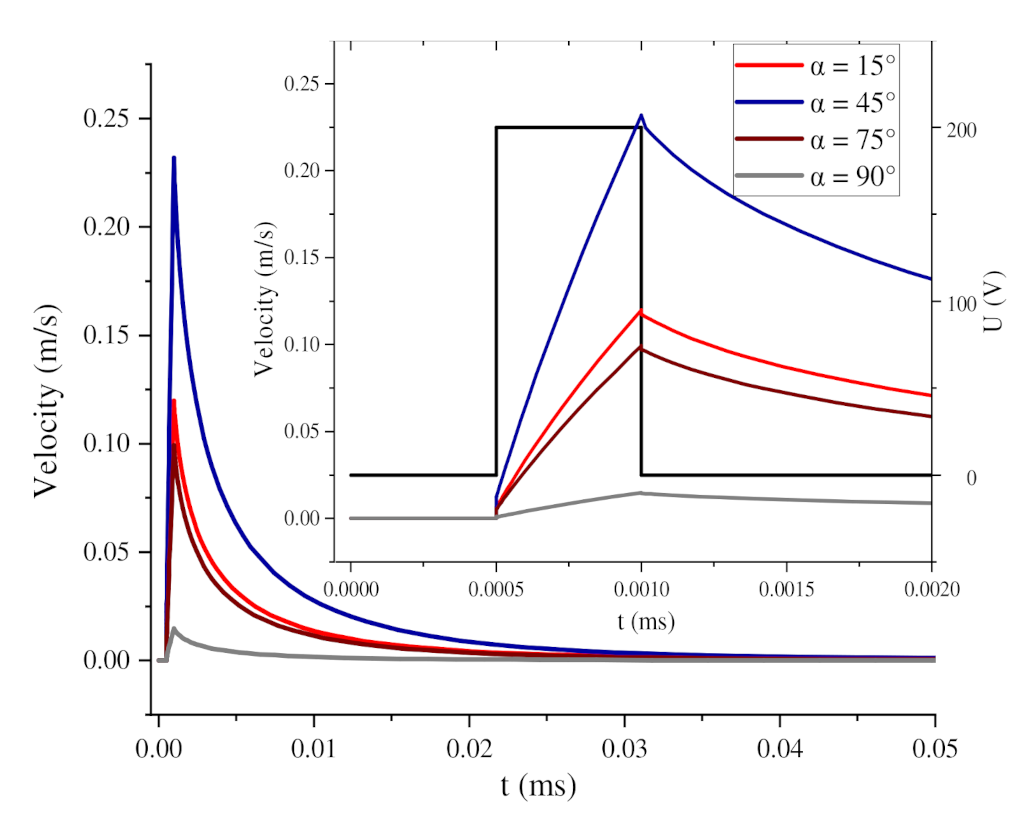
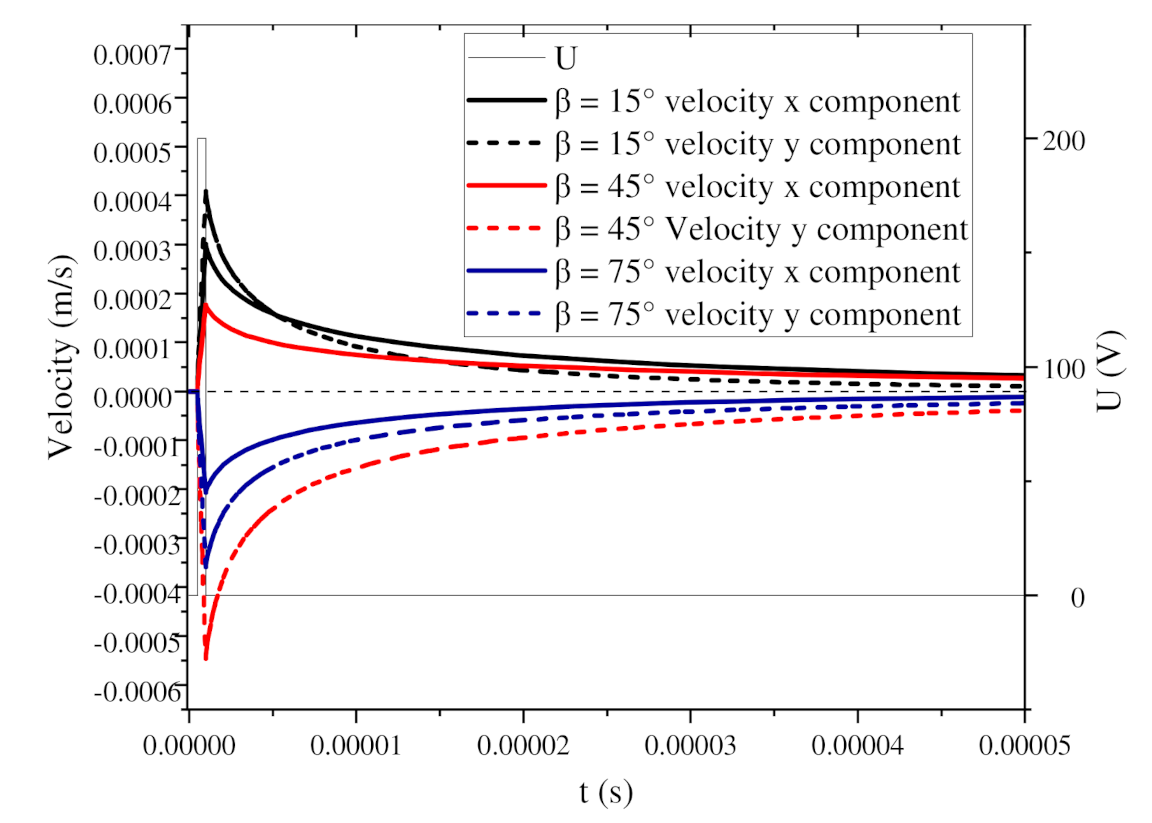
3.2. Influence of Self-Angle α on Local Orientation
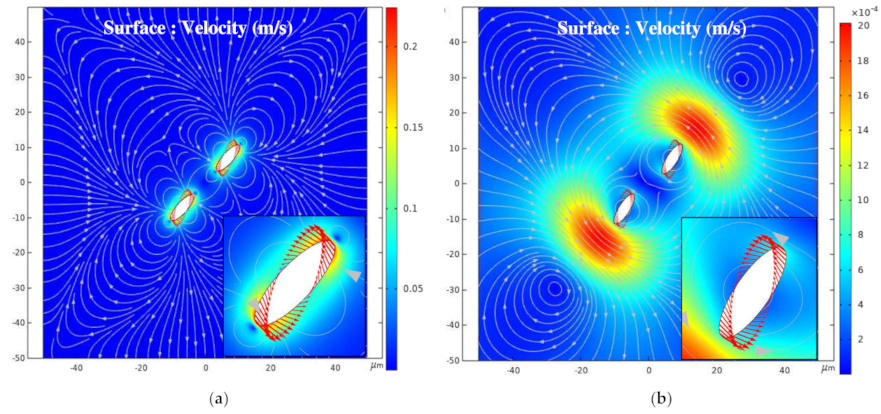
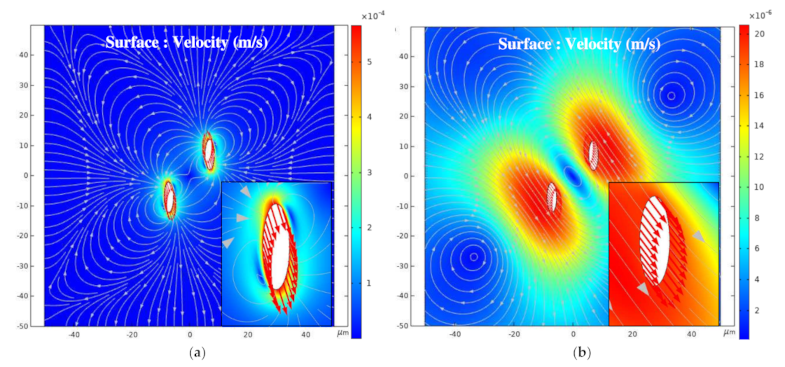
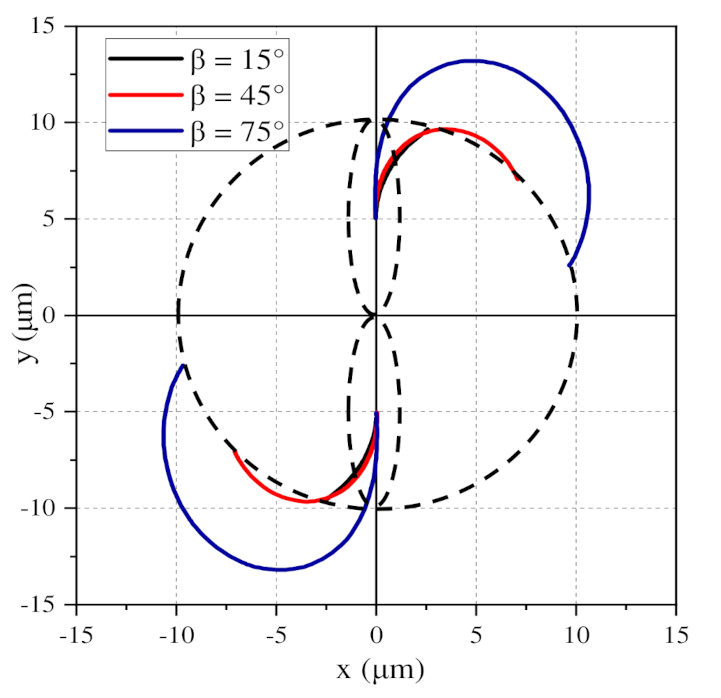
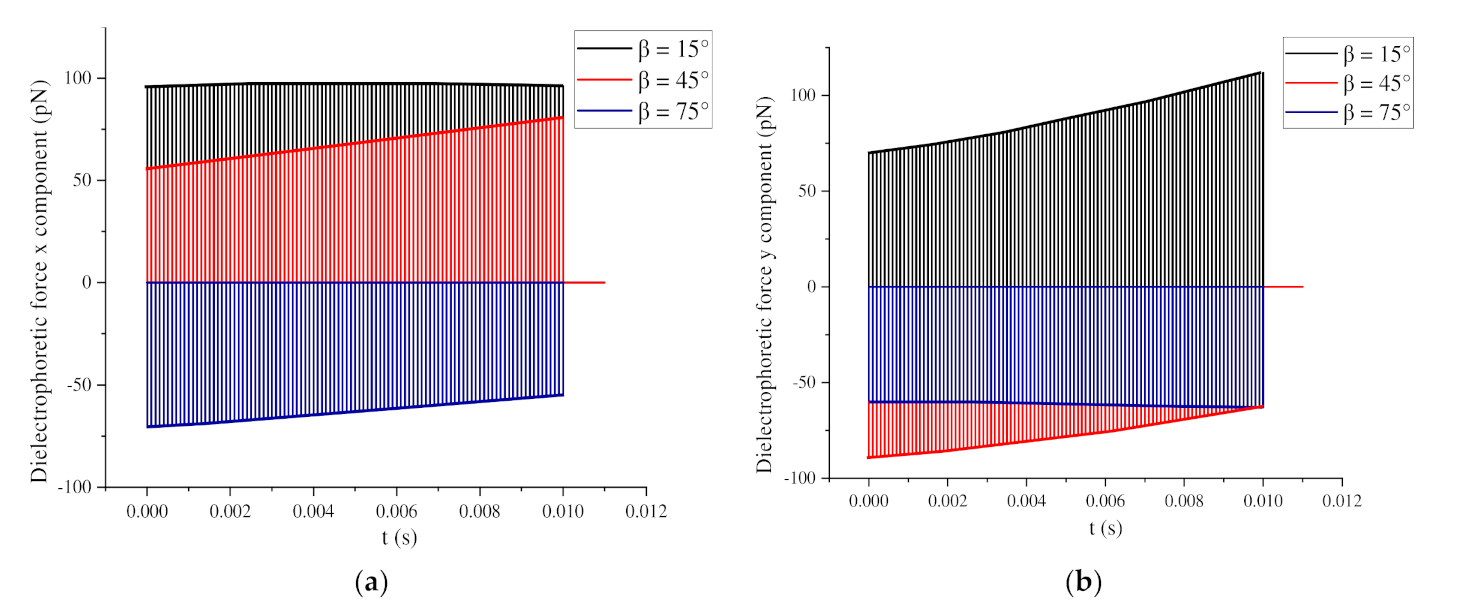
3.3. Influence of the Relative Angle β on the Global Arrangement
3.3.1. 0° < β < 90°
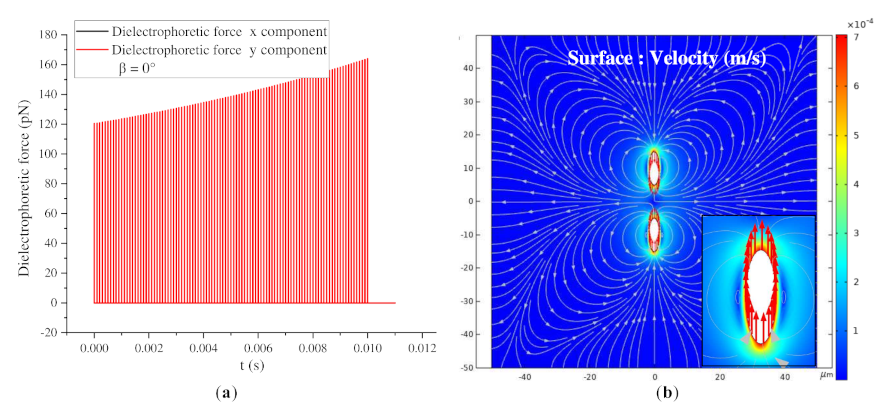
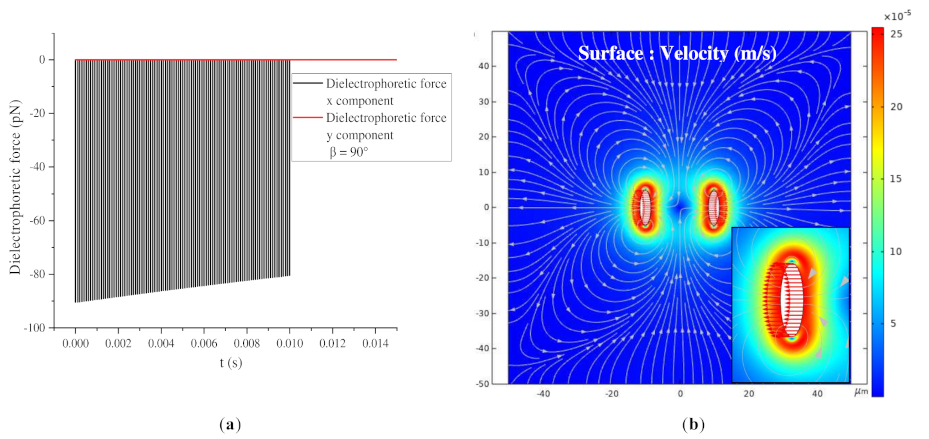
3.3.2. β = 0° and β = 90°
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Huang, X.; Sun, B.; Jiang, P. Highly thermally conductive yet electrically insulating polymer/boron nitride nanosheets nanocomposite films for improved thermal management capability. ACS Nano 2019, 13, 337–345. [Google Scholar] [CrossRef]
- Martin, C.; Sandler, J.; Windle, A.; Schwarz, M.-K.; Bauhofer, W.; Schulte, K.; Shaffer, M. Electric field-induced aligned multi-wall carbon nanotube networks in epoxy composites. Polymers 2005, 46, 877–886. [Google Scholar] [CrossRef]
- Cho, H.-B.; Nakayama, T.; Huynh, M.T.T.; Nguyen, S.T.; Jiang, W.; Suzuki, T.; Suematsu, H.; Niihara, K.; Shin, J.H.; Choa, Y.-H. Texture-controlled hybrid materials fabricated using nanosecond technology. J. Ceram. Soc. Jpn. 2016, 124, 197–202. [Google Scholar] [CrossRef]
- Mi, Y.; Liu, L.; Gui, L.; Ge, X. Effect of frequency of microsecond pulsed electric field on orientation of boron nitride nanosheets and thermal conductivity of epoxy resin-based composites. J. Appl. Phys. 2019, 126, 205105. [Google Scholar] [CrossRef]
- Cho, H.-B.; Tu, N.C.; Fujihara, T.; Endo, S.; Suzuki, T.; Tanaka, S.; Jiang, W.; Suematsu, H.; Niihara, K.; Nakayama, T. Epoxy resin-based nanocomposite films with highly oriented BN nanosheets prepared using a nanosecond-pulse electric field. Mater. Lett. 2011, 65, 2426–2428. [Google Scholar] [CrossRef]
- Cho, H.-B.; Nakayama, T.; Suzuki, T.; Tanaka, S.; Jiang, W.; Suematsu, H.; Lee, J.-W.; Kim, H.-D.; Niihara, K. Electric-field-assisted fabrication of linearly stretched bundles of microdiamonds in polysiloxane-based composite material. Diam. Relat. Mater. 2012, 26, 7–14. [Google Scholar] [CrossRef]
- Huynh, M.T.T.; Cho, H.-B.; Suzuki, T.; Suematsu, H.; Nguyen, S.T.; Niihara, K.; Nakayama, T. Electrical property enhancement by controlled percolation structure of carbon black in polymer-based nanocomposites via nanosecond pulsed electric field. Compos. Sci. Technol. 2018, 154, 165–174. [Google Scholar] [CrossRef]
- Li, M.; Qu, Y.; Dong, Z.; Wang, Y.; Li, W.J. Limitations of au particle nanoassembly using dielectrophoretic force—A Parametric experimental and theoretical study. IEEE Trans. Nanotechnol. 2008, 7, 477–479. [Google Scholar] [CrossRef]
- Pohl, H.A. Dielectrophoresis; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Aubry, N.; Singh, P. Control of electrostatic particle-particle interactions in dielectrophoresis. Europhys. Lett. 2006, 74, 623–629. [Google Scholar] [CrossRef]
- Ai, Y.; Joo, S.W.; Jiang, Y.; Xuan, X.; Qian, S. Transient electrophoretic motion of a charged particle through a converging-diverging microchannel: Effect of direct current-dielectrophoretic force. Electrophoresis 2009, 30, 2499–2506. [Google Scholar] [CrossRef]
- Ai, Y.; Beskok, A.; Gauthier, D.T.; Joo, S.W.; Qian, S. DC electrokinetic transport of cylindrical cells in straight microchannels. Biomicrofluidics 2009, 3, 044110. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Park, S.; Zhu, J.; Xuan, X.; Beskok, A.; Qian, S. DC electrokinetic particle transport in an L-shaped microchannel. Langmuir 2010, 26, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Qian, S. DC dielectrophoretic particle–particle interactions and their relative motions. J. Colloid Interface Sci. 2010, 346, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Hesketh, P.J. Interpretation of ac dielectrophoretic behavior of tin oxide nanobelts using Maxwell stress tensor approach modeling. Sens. Actuators B Chem. 2012, 161, 1198–1208. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.-B.; Gascoyne, P.R. General expressions for dielectrophoretic force and electrorotational torque derived using the Maxwell stress tensor method. J. Electrost. 1997, 39, 277–295. [Google Scholar] [CrossRef]
- Rosales, C.; Lim, K.M. Numerical comparison between Maxwell stress method and equivalent multipole approach for calculation of the dielectrophoretic force in single-cell traps. Electrophoresis 2005, 26, 2057–2065. [Google Scholar] [CrossRef]
- Al-Jarro, A.; Paul, J.; Thomas, D.W.P.; Crowe, J.; Sawyer, N.; Rose, F.R.A.; Shakesheff, K.M. Direct calculation of Maxwell stress tensor for accurate trajectory prediction during DEP for 2D and 3D structures. J. Phys. D Appl. Phys. 2006, 40, 71–77. [Google Scholar] [CrossRef]
- Ai, Y.; Zeng, Z.; Qian, S. Direct numerical simulation of AC dielectrophoretic particle–particle interactive motions. J. Colloid Interface Sci. 2014, 417, 72–79. [Google Scholar] [CrossRef]
- Ellison, W.; Lamkaouchi, K.; Moreau, J.-M. Water: A dielectric reference. J. Mol. Liq. 1996, 68, 171–279. [Google Scholar] [CrossRef]
- Vijayaraghavan, V.; Zhang, L. Effective mechanical properties and thickness determination of boron nitride nanosheets using molecular dynamics simulation. Nanomaterials 2018, 8, 546. [Google Scholar] [CrossRef] [PubMed]
- Alem, N.; Erni, R.; Kisielowski, C.; Rossell, M.D.; Gannett, W.; Zettl, A. Atomically thin hexagonal boron nitride probed by ultrahigh-resolution transmission electron microscopy. Phys. Rev. B 2009, 80, 155425. [Google Scholar] [CrossRef]
- Varadan, V.; Hollinger, R.; Ghodgaonkar, D.; Varadan, V. Free-space, broadband measurements of high-temperature, complex dielectric properties at microwave frequencies. IEEE Trans. Instrum. Meas. 1991, 40, 842–846. [Google Scholar] [CrossRef]
- Çetin, B.; Li, D. Dielectrophoresis in microfluidics technology. Electrophoresis 2011, 32, 2410–2427. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, R.; Wang, C. Dynamics of a pair of ellipsoidal microparticles under a uniform magnetic field. J. Micromech. Microeng. 2019, 29, 104002. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, Y.; Ge, X.; Dai, J.; Chen, Y.; Zhu, Y. Simulation of BNNSs Dielectrophoretic Motion under a Nanosecond Pulsed Electric Field. Nanomaterials 2021, 11, 682. https://doi.org/10.3390/nano11030682
Mi Y, Ge X, Dai J, Chen Y, Zhu Y. Simulation of BNNSs Dielectrophoretic Motion under a Nanosecond Pulsed Electric Field. Nanomaterials. 2021; 11(3):682. https://doi.org/10.3390/nano11030682
Chicago/Turabian StyleMi, Yan, Xin Ge, Jinyan Dai, Yong Chen, and Yakui Zhu. 2021. "Simulation of BNNSs Dielectrophoretic Motion under a Nanosecond Pulsed Electric Field" Nanomaterials 11, no. 3: 682. https://doi.org/10.3390/nano11030682
APA StyleMi, Y., Ge, X., Dai, J., Chen, Y., & Zhu, Y. (2021). Simulation of BNNSs Dielectrophoretic Motion under a Nanosecond Pulsed Electric Field. Nanomaterials, 11(3), 682. https://doi.org/10.3390/nano11030682





