Local and Systemic In Vivo Responses to Osseointegrative Titanium Nanotube Surfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Implant Fabrication
2.2. In Vitro Experimentation to Assess Cell Attachment and Morphology
2.3. In Vivo Experimentation to Assess Biologic Response to Nanotube Surfaces
2.4. Surgical Procedure for Unilateral Intramedullary Implantation
2.5. Postoperative Care and Health Assessments
2.6. Hematologic Analysis
2.7. Metal Ion Analysis of Whole Blood and Remote Organs
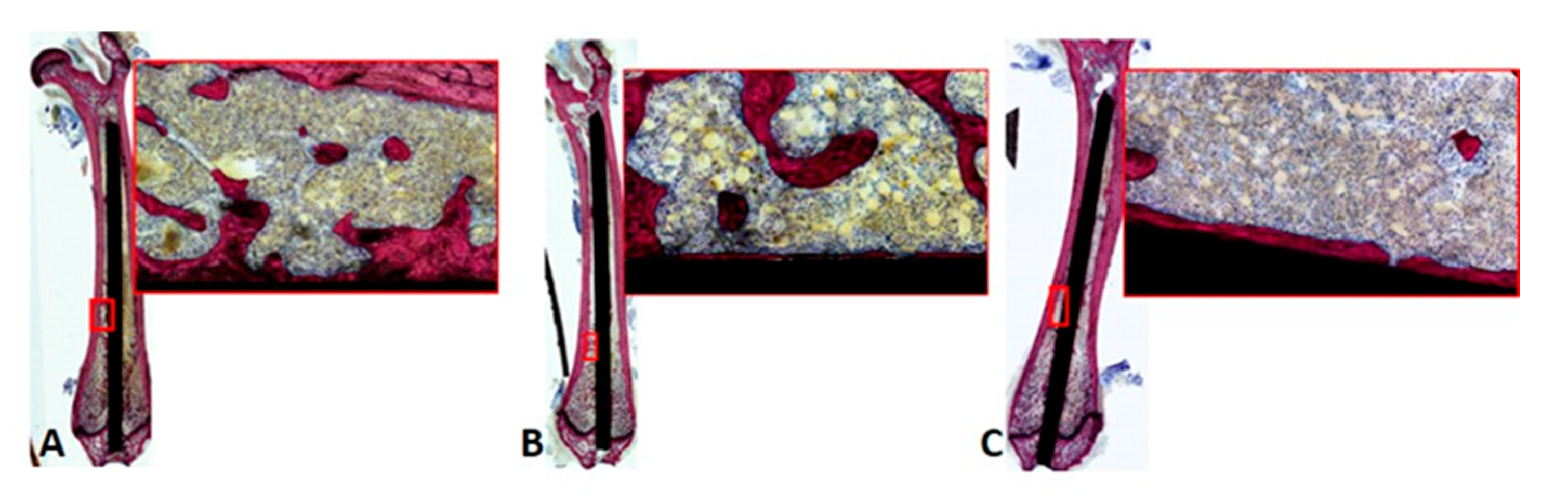
2.8. Nondecalcified Histologic Analysis
3. Results
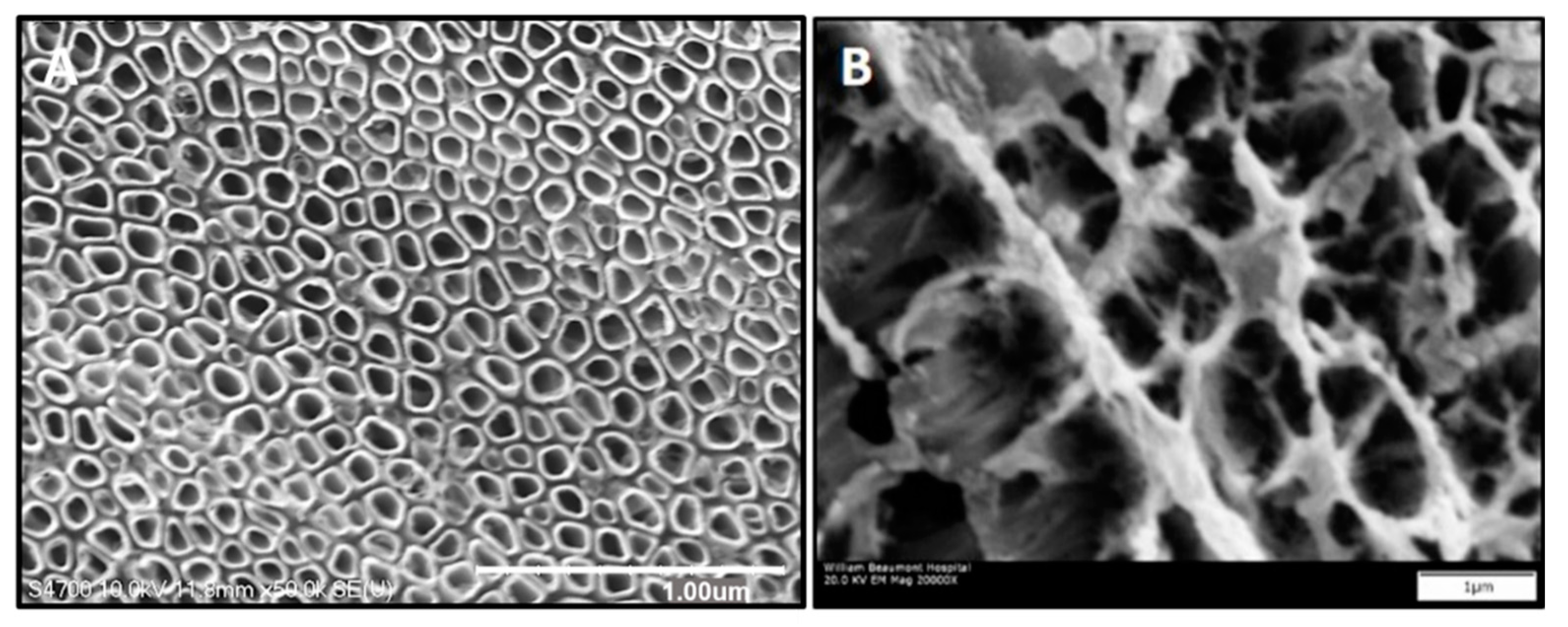
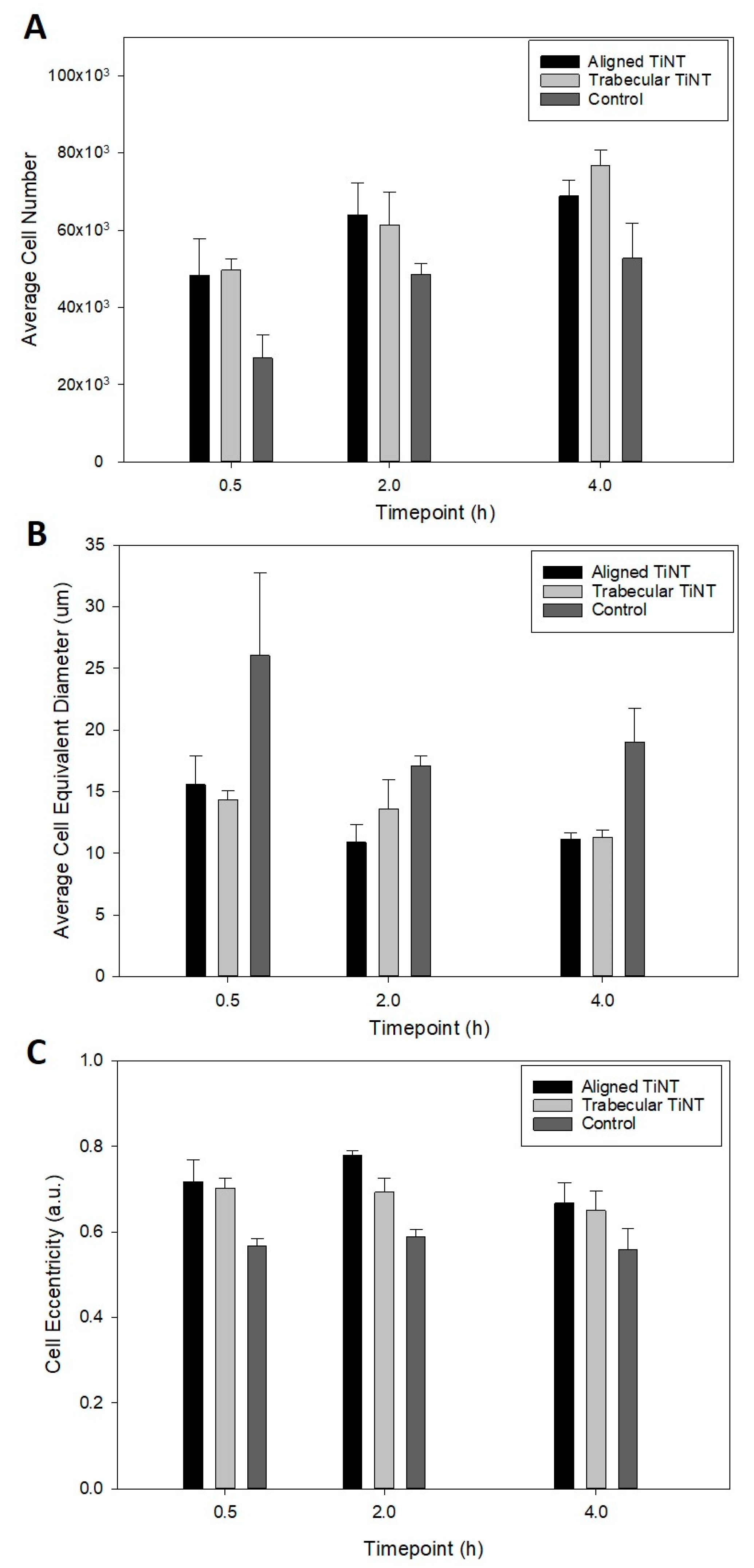
3.1. In Vitro Experimentation to Assess Cell Attachment and Morphology
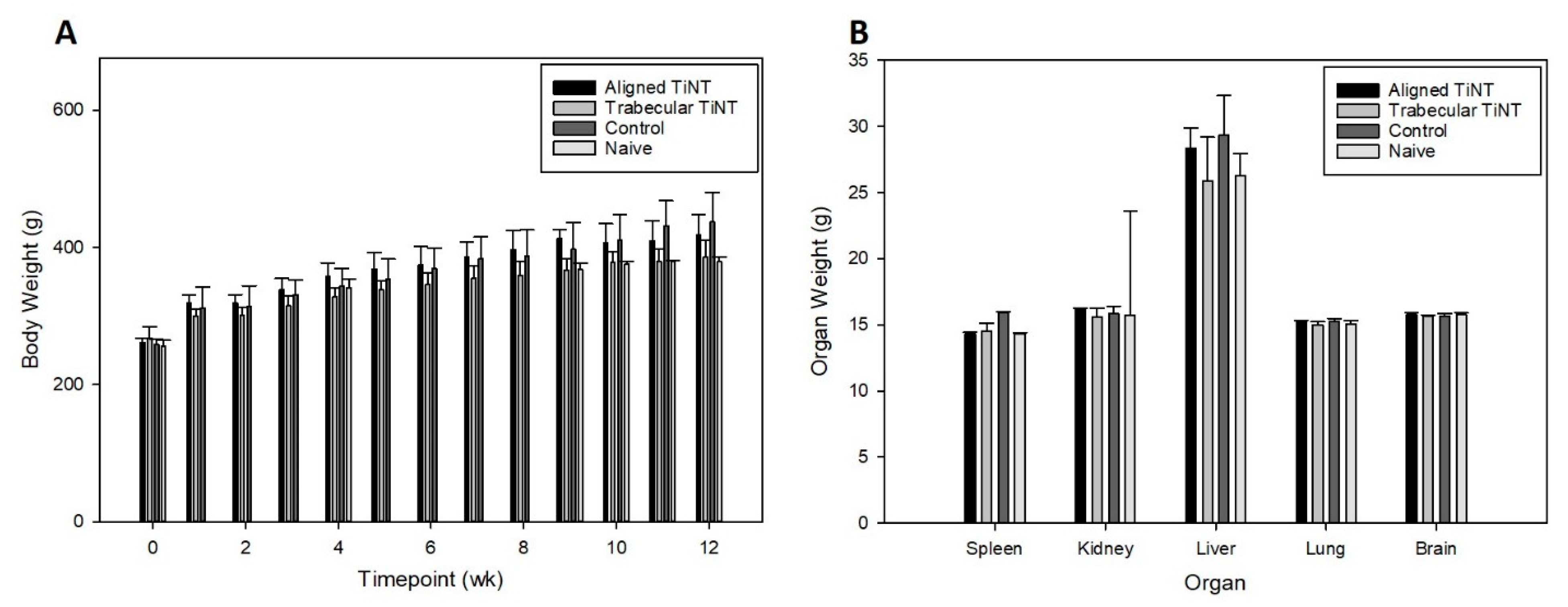
3.2. In Vivo Experimentation to Assess Biologic Response to Nanotube Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Supplemental Methods and Results
Appendix A.1. Titanium Nanotube-Etched Specimen Fabrication

Appendix A.2. In Vitro Experimentation to Assess Adherent Cell Count, Cell Equivalent Diameter, and Cell Eccentricity

Appendix A.3. Metal Ion Analysis of Remote Organs and Whole Blood
Appendix A.4. Histologic Analysis of Bone-Implant Interface
| ROI | Group | Foreign Body Giant/Multinucleated | Granulocyte | Neutrophil | Monocyte | Lymphocyte |
|---|---|---|---|---|---|---|
| Distal | Trabecular TiNT | 0.8 (1.0) | 0.1 (0.0) | 0.7 (1.0) | 0.8 (1.0) | 1.1 (1.0) |
| Aligned TiNT | 0.7 (1.0) | 0.2 (0.0) | 1.1 (1.0) | 0.8 (1.0) | 1.1 (1.0) | |
| Control | 0.6 (0.5) | 0.3 (0.0) | 1.0 (1.0) | 0.7 (1.0) | 1.2 (1.0) | |
| Midshaft | Trabecular TiNT | 0.5 (0.5) | 0.6 (1.0) | 1.0 (1.0) | 0.6 (1.0) | 1.2 (1.0) |
| Aligned TiNT | 0.4 (0.0) | 0.8 (1.0) | 0.7 (1.0) | 0.7 (1.0) | 1.1 (1.0) | |
| Control | 0.8 (1.0) | 0.7 (1.0) | 1.0 (1.0) | 0.7 (1.0) | 1.2 (1.0) | |
| Proximal | Trabecular TiNT | 0.8 (1.0) | 0.6 (1.0) | 0.9 (1.0) | 0.8 (1.0) | 1.4 (1.0) |
| Aligned TiNT | 0.7 (1.0) | 0.4 (0.0) | 1.1 (1.0) | 0.9 (1.0) | 1.3 (1.0) | |
| Control | 0.7 (1.0) | 0.7 (1.0) | 1.0 (1.0) | 0.8 (1.0) | 1.4 (1.0) |
References
- Branemark, P.I.; Adell, R.; Albrektsson, T. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials 1983, 4, 25–28. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated Titanium Implants:Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, F.; Reclaru, L.; Ardelean, L.C.; Blatter, A. Comparative Analysis of the Corrosion Resistance of Titanium Alloys Intended to Come into Direct or Prolonged Contact with Live Tissues. Materials 2019, 12, 2841. [Google Scholar] [CrossRef]
- Ratner, B.D.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Elsevier Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Goodman, S.B.; Gallo, J. Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. J. Clin. Med. 2019, 8, 2091. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Gallo, J.; Gibon, E.F.; Takagi, M. Diagnosis and management of implant debris-associated inflammation. Expert Rev. Med. Devices 2019, 17, 41–56. [Google Scholar] [CrossRef]
- Hergel, C.A.; Acar, Y.B.; Ates, M.; Kucukkeles, N. In-vitro evaluation of the effects of insertion and sterilization procedures on the mechanical and surface characteristics of mini screws. Eur. Oral Res. 2019, 53, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Fan, Y. Adverse Biological Effect of TiO₂ and Hydroxyapatite Nanoparticles Used in Bone Repair and Replacement. Int. J. Mol. Sci. 2016, 17, 798. [Google Scholar] [CrossRef]
- Hallab, N.J.; Jacobs, J.J. Biologic effects of implant debris. Bull. NYU Hosp. Jt. Dis. 2009, 67, 182–188. [Google Scholar] [PubMed]
- Hallab, N.J. Biologic Responses to Orthopedic Implants: Innate and Adaptive Immune Responses to Implant Debris. Spine 2016, 41, S30–S31. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Caicedo, M.S.; Samelko, L.; Jacobs, J.J.; Hallab, N.J. Implant debris particle size affects serum protein adsorption which may contribute to particle size-based bioreactivity differences. J. Long Term Eff. Med. 2014, 24, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Bjursten, L.M.; Rasmusson, L.; Oh, S.; Smith, G.C.; Brammer, K.S.; Jin, S. Titanium dioxide nanotubes enhance bone bondingin vivo. J. Biomed. Mater. Res. Part A 2009, 92, 1218–1224. [Google Scholar] [CrossRef]
- Von Wilmowsky, C.; Bauer, S.; Roedl, S.; Neukam, F.W.; Schmuki, P.; Schlegel, K.A. The diameter of anodic TiO2 nanotubes affects bone formation and correlates with the bone morphogenetic protein-2 expression in vivo. Clin. Oral Implant. Res. 2012, 23, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Komasa, S.; Taguchi, Y.; Sekino, T.; Okazaki, J. Osteogenic activity of titanium surfaces with nanonetwork structures. Int. J. Nanomed. 2014, 9, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.D.; Vinoth-Kumar, L.; Anitha, V.; Manzoor, K.; Deepthy, M.; Shantikumar, V.N. Osteointegration of titanium implant is sensitive to specific nanostructure morphology. Acta Biomater. 2012, 8, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.A.; Vara, A.D.; Salisbury, M.R.; Fleischer, M.M.; Baker, K.C.; Fortin, P.T.; Roberts, R.V.; Friedrich, C.R. Titania nanotube morphologies for osseointegration via models of in vitro osseointegrative potential and in vivo intramedullary fixation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1483–1493. [Google Scholar] [CrossRef]
- Wang, J.; Meng, F.; Song, W.; Jin, J.; Ma, Q.; Fei, D.; Fang, L.; Chen, L.; Wang, Q.; Zhang, Y. Nanostructured titanium regulates osseointegration via influencing macrophage polarization in the osteogenic environment. Int. J. Nanomed. 2018, 13, 4029–4043. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, Y.; Du, Z.; Weng, Z.; Yao, W.; Zhang, X.; Huang, X.; Yao, X.; Crawford, R.; Hang, R.; et al. Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomater. 2018, 76, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Shokuhfar, T.; Hamlekhan, A.; Chang, J.-Y.; Choi, C.K.; Sukotjo, C.; Friedrich, C. Biophysical evaluation of cells on nanotubular surfaces: The effects of atomic ordering and chemistry. Int. J. Nanomed. 2014, 9, 3737–3748. [Google Scholar] [CrossRef]
- Shin, D.H.; Shokuhfar, T.; Choi, C.K.; Lee, S.-H.; Friedrich, C. Wettability changes of TiO2nanotube surfaces. Nanotechnology 2011, 22, 315704. [Google Scholar] [CrossRef]
- Friedrich, C.R.; Kolati, M.; Moser, T.; Sukotjo, C.; Shokuhfar, T. Survivability of TiO2nanotubes on the surface of bone screws. Surf. Innov. 2014, 2, 60–68. [Google Scholar] [CrossRef]
- Gupta, A.; Liberati, T.A.; Verhulst, S.J.; Main, B.J.; Roberts, M.H.; Potty, A.G.R.; Pylawka, T.K.; Iii, S.F.E.-A. Biocompatibility of single-walled carbon nanotube composites for bone regeneration. Bone Jt. Res. 2015, 4, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Back, D.A.; Pauly, S.; Rommel, L.; Haas, N.P.; Schmidmaier, G.; Wildemann, B.; Greiner, S.H. Effect of local zoledronate on implant osseointegration in a rat model. BMC Musculoskelet. Disord. 2012, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Komasa, S.; Sekino, T.; Nishizaki, H.; Okazaki, J. Nanostructured Ti6Al4V alloy fabricated using modified alkali-heat treatment: Characterization and cell adhesion. Mater. Sci. Eng. C 2016, 59, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Pauly, S.; Back, D.A.; Kaeppler, K.; Haas, N.P.; Schmidmaier, G.; Wildemann, B. Influence of Statins locally applied from orthopedic implants on osseous integration. BMC Musculoskelet. Disord. 2012, 13, 208. [Google Scholar] [CrossRef]
- Virdi, A.S.; Irish, J.; Sena, K.; Liu, M.; Ke, H.Z.; McNulty, M.A.; Sumner, D.R. Sclerostin Antibody Treatment Improves Implant Fixation in a Model of Severe Osteoporosis. J. Bone Jt. Surg. Am. Vol. 2015, 97, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Betoni, W.; Queiroz, T.P.; Luvizuto, E.R.; Valentini-Neto, R.; Garcia-Júnior, I.R.; Bernabé, P.F.E. Evaluation of Centrifuged Bone Marrow on Bone Regeneration Around Implants in Rabbit Tibia. Implant. Dent. 2012, 21, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.R.; Rossi, A.C.; Queiroz, T.P.; Gulinelli, J.L.; Souza, F.A.; Margonar, R.; Garcia-Júnior, I.R.; Hochuli-Vieira, E.; Okamoto, R. Histometric Analysis of Bone Repair in Bone-Implant Interface Using a Polylactic/Polyglycolic Acid Copolymer Associated With Implants in Rabbit Tibia. J. Oral Implant. 2012, 38, 449–457. [Google Scholar] [CrossRef]
- Nich, C.; Marchadier, A.; Sedel, L.; Petite, H.; Vidal, C.; Hamadouche, M. Decrease in particle-induced osteolysis in ovariectomized mice. J. Orthop. Res. 2009, 28, 178–183. [Google Scholar] [CrossRef]
- Popat, K.C.; Leoni, L.; Grimes, C.A.; Desai, T.A. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials 2007, 28, 3188–3197. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, X.; Li, S.; Lai, R.; Zhou, Z.; Zhang, Y.; Zhou, L. Effects of titania nanotubes with or without bovine serum albumin loaded on human gingival fibroblasts. Int. J. Nanomed. 2014, 9, 1185–1198. [Google Scholar] [CrossRef]
- Minagar, S.; Wang, J.; Berndt, C.C.; Ivanova, E.P.; Wen, C. Cell response of anodized nanotubes on titanium and titanium alloys. J. Biomed. Mater. Res. Part A 2013, 101, 2726–2739. [Google Scholar] [CrossRef]
- Rangamani, P.; Lipshtat, A.; Azeloglu, E.U.; Calizo, R.C.; Hu, M.; Ghassemi, S.; Hone, J.; Scarlata, S.; Neves, S.R.; Iyengar, R. Decoding Information in Cell Shape. Cell 2013, 154, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Fan, Y.-B.; Gao, Y.; Hu, Q.-H.; Wang, T.-C. TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection. Biomaterials 2009, 30, 4590–4600. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Feng, B.; Liu, Z.; Tan, J.; Zhi, W.; Lu, X.; Wang, J.; Weng, J. Fabrication of TiO2 nanotubes on porous titanium scaffold and biocompatibility evaluation in vitro and in vivo. J. Biomed. Mater. Res. Part A 2012, 100, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Bayat, N.; Lopes, V.R.; Schölermann, J.; Jensen, L.D.; Cristobal, S. Vascular toxicity of ultra-small TiO2 nanoparticles and single walled carbon nanotubes in vitro and in vivo. Biomaterials 2015, 63, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Lee, F.D.; Durston, W.E. An Improved Bacterial Test System for the Detection and Classification of Mutagens and Carcinogens. Proc. Natl. Acad. Sci. USA 1973, 70, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Naya, M.; Kobayashi, N.; Ema, M.; Kasamoto, S.; Fukumuro, M.; Takami, S.; Nakajima, M.; Hayashi, M.; Nakanishi, J. In vivo genotoxicity study of titanium dioxide nanoparticles using comet assay following intratracheal instillation in rats. Regul. Toxicol. Pharmacol. 2012, 62, 1–6. [Google Scholar] [CrossRef]
- Neacsu, P.; Mazare, A.; Cimpean, A.; Park, J.; Costache, M.; Schmuki, P.; Demetrescu, I. Reduced inflammatory activity of RAW 264.7 macrophages on titania nanotube modified Ti surface. Int. J. Biochem. Cell Biol. 2014, 55, 187–195. [Google Scholar] [CrossRef]
- Östberg, A.-K.; Dahlgren, U.; Sul, Y.-T.; Johansson, C.B. Inflammatory cytokine release is affected by surface morphology and chemistry of titanium implants. J. Mater. Sci. Mater. Electron. 2015, 26, 1–9. [Google Scholar] [CrossRef]
- Latha, T.S.; Reddy, M.C.; Durbaka, P.V.R.; Muthukonda, S.V.; Lomada, D. Immunomodulatory properties of titanium dioxide nanostructural materials. Indian J. Pharmacol. 2017, 49, 458–464. [Google Scholar] [CrossRef]
- Smith, B.S.; Yoriya, S.; Grissom, L.; Grimes, C.A.; Popat, K.C. Hemocompatibility of titania nanotube arrays. J. Biomed. Mater. Res. Part A 2010, 95, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Radziun, E.; Wilczyńska, J.D.; Książek, I.; Nowak, K.; Anuszewska, E.; Kunicki, A.; Olszyna, A.; Ząbkowski, T. Assessment of the cytotoxicity of aluminium oxide nanoparticles on selected mammalian cells. Toxicol. Vitr. 2011, 25, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A.; Subbarayan, P.V.; Ramesh, E.; Al-Hazzani, A.A.; AlSaif, M.A.; Alwarthan, A.A. Al2O3Nanoparticles Induce Mitochondria-Mediated Cell Death and Upregulate the Expression of Signaling Genes in Human Mesenchymal Stem Cells. J. Biochem. Mol. Toxicol. 2012, 26, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Swiatkowska, I.; Martin, N.G.; Henckel, J.; Apthorp, H.; Hamshere, J.; Hart, A.J. Blood and plasma titanium levels associated with well-functioning hip implants. J. Trace Elements Med. Biol. 2020, 57, 9–17. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Aluminum; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2008.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Vanadium; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012.
- Maerz, T.; Fleischer, M.; Newton, M.D.; Davidson, A.; Salisbury, M.; Altman, P.; Kurdziel, M.D.; Anderson, K.; Bedi, A.; Baker, K.C. Acute mobilization and migration of bone marrow-derived stem cells following anterior cruciate ligament rupture. Osteoarthritis Cartilage 2017, 25, 1335–1344. [Google Scholar] [CrossRef]

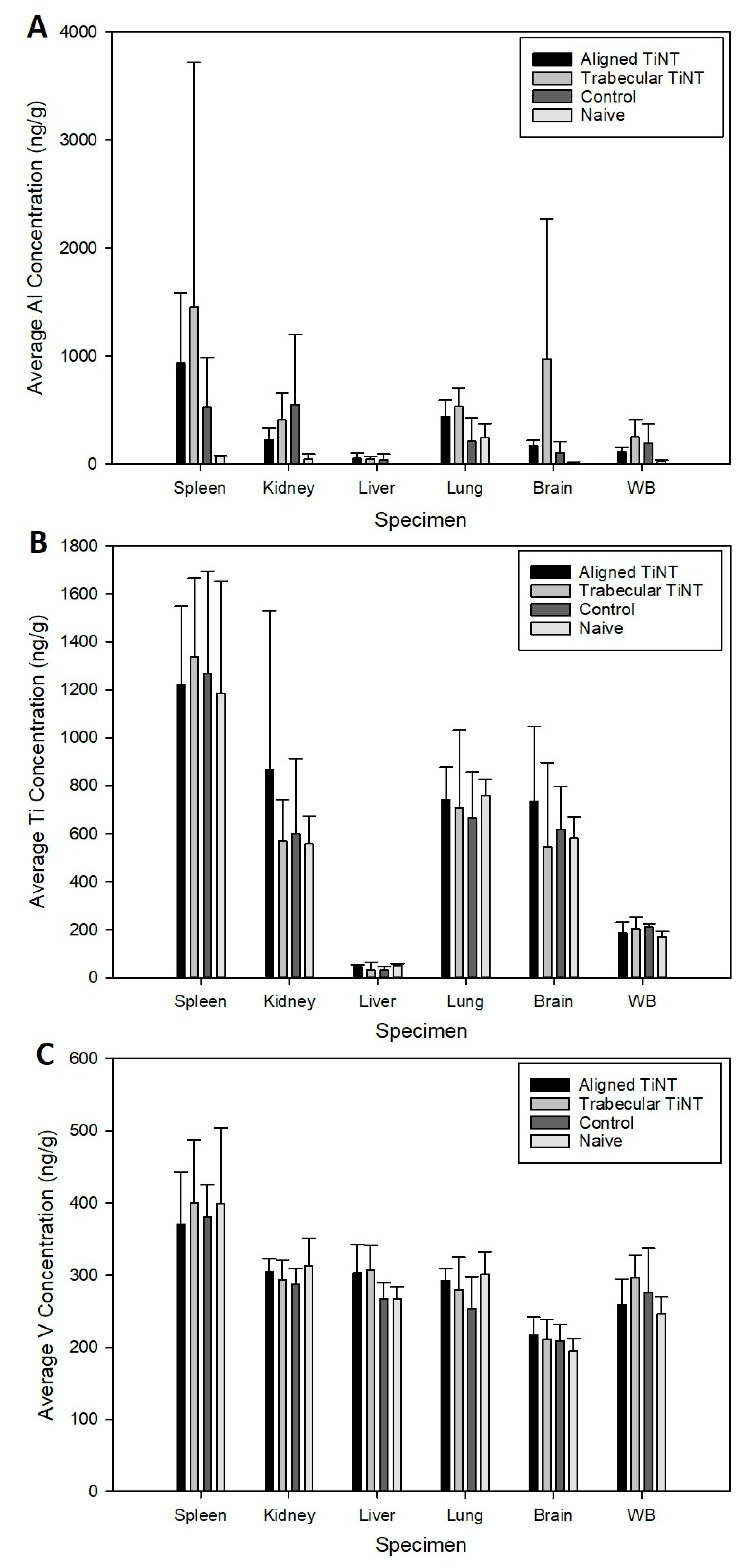
| Group | WBC | Lymph | Mono | Gran | Lymph% | Mono% | Gran% |
|---|---|---|---|---|---|---|---|
| Aligned TiNT | 9.5 (2.7) | 7.3 (1.8) | 0.4 (0.2) | 1.7 (0.7) | 77.9 (2.7) | 3.9 (1.3) | 18.2 (2.6) |
| Trabecular TiNT | 8.02 (3.5) | 6.2 (2.7) | 0.3 (0.1) | 1.5 (0.8) | 77.9 (3.9) | 3.3 (1.6) | 18.8 (2.9) |
| Control | 8.9 (2.2) | 7.0 (1.6) | 0.3 (0.1) | 1.5 (0.6) | 79.7 (2.5) | 3.1 (0.8) | 17.3 (2.2) |
| Naïve | 11.0 (6.1) | 8.4 (3.9) | 0.5 (0.4) | 2.1 (1.8) | 79.1 (6.1) | 3.1 (0.8) | 17.8 (5.3) |
| Group | HCT | MCV | RDWa | RDW% | Hgb | MCHC | MCH | RBC | PLT | MPV |
|---|---|---|---|---|---|---|---|---|---|---|
| Aligned TiNT | 40.1 (3.2) | 49.7 (1.1) | 32.8 (1.0) | 16.4 (0.5) | 15.3 (1.2) | 38.3 (0.5) | 19.0 (0.3) | 8.1 (0.6) | 318.7 (149.4) | 6.4 (0.8) |
| Trabecular TiNT | 38.3 (1.5) | 50.0 (0.9) | 32.9 (0.7) | 16.4 (0.6) | 14.6 (0.4) | 38.3 (0.6) | 19.1 (0.5) | 7.7 (0.4) | 275.8 (151.6) | 6.7 (0.6) |
| Control | 40.1 (3.5) | 50.9 (1.2) | 34.0 (1.6) | 16.4 (0.4) | 15.1 (1.2) | 37.7 (0.5) | 19.1 (0.4) | 7.9 (0.6) | 335.0 (89.7) | 6.4 (0.5) |
| Naïve | 41.4 (2.8) | 50.6 (0.8) | 33.7 (0.2) | 16.5 (0.4) | 15.6 (1.1) | 37.7 (0.2) | 19.1 (0.3) | 8.2 (0.5) | 305.7 (93.5) | 6.1 (0.8) |
| ROI | Group (vs. Control) | Foreign Body Giant/Multinucleated | Granulocyte | Neutrophil | Monocyte | Lymphocyte |
|---|---|---|---|---|---|---|
| Distal | Trabecular TiNT | 0.126 | 0.040 | 0.019 | 0.720 | 0.391 |
| Aligned TiNT | 0.287 | 0.476 | 0.345 | 0.720 | 0.260 | |
| Midshaft | Trabecular TiNT | 0.142 | 0.603 | 1.000 | 0.314 | 0.982 |
| Aligned TiNT | 0.039 | 0.682 | 0.019 | 0.736 | 0.260 | |
| Proximal | Trabecular TiNT | 0.527 | 0.646 | 0.345 | 0.652 | 0.871 |
| Aligned TiNT | 0.939 | 0.219 | 0.345 | 0.747 | 0.314 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, E.A.; Fleischer, M.M.; Vara, A.D.; Salisbury, M.R.; Baker, K.C.; Fortin, P.T.; Friedrich, C.R. Local and Systemic In Vivo Responses to Osseointegrative Titanium Nanotube Surfaces. Nanomaterials 2021, 11, 583. https://doi.org/10.3390/nano11030583
Baker EA, Fleischer MM, Vara AD, Salisbury MR, Baker KC, Fortin PT, Friedrich CR. Local and Systemic In Vivo Responses to Osseointegrative Titanium Nanotube Surfaces. Nanomaterials. 2021; 11(3):583. https://doi.org/10.3390/nano11030583
Chicago/Turabian StyleBaker, Erin A., Mackenzie M. Fleischer, Alexander D. Vara, Meagan R. Salisbury, Kevin C. Baker, Paul T. Fortin, and Craig R. Friedrich. 2021. "Local and Systemic In Vivo Responses to Osseointegrative Titanium Nanotube Surfaces" Nanomaterials 11, no. 3: 583. https://doi.org/10.3390/nano11030583
APA StyleBaker, E. A., Fleischer, M. M., Vara, A. D., Salisbury, M. R., Baker, K. C., Fortin, P. T., & Friedrich, C. R. (2021). Local and Systemic In Vivo Responses to Osseointegrative Titanium Nanotube Surfaces. Nanomaterials, 11(3), 583. https://doi.org/10.3390/nano11030583






