ECM-Mimetic Nylon Nanofiber Scaffolds for Neurite Growth Guidance
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Nanofibrous Scaffolds and Films from Nylon
2.3. Morphological Studies of Nylon Nanofibers
2.4. Culture of Rat Hippocampal Neurons and Human Neuroblastoma Cells IMR-32
2.5. Cytotoxicity Test
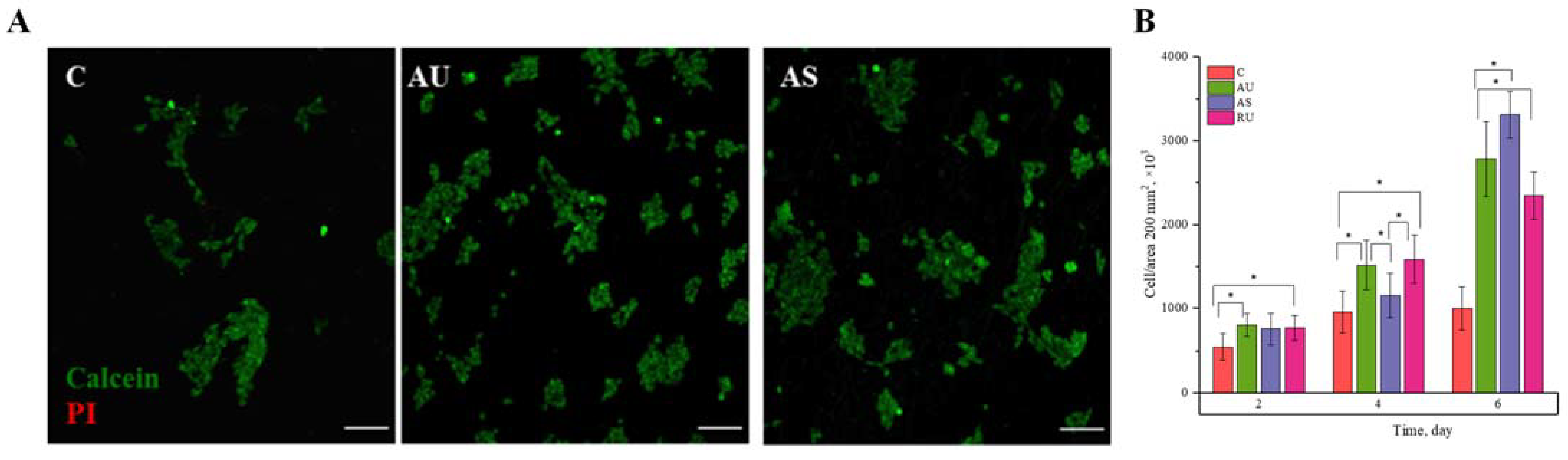
2.6. Evaluation of Cell Proliferation during Cultivation on a Nanofiber Scaffold
2.7. Scanning Electron Microscope Cell Imaging
2.8. Degradation of Nylon Nanofibers
2.9. Bright-Field Microscopy
2.10. Immunochemical Staining
2.11. Image Analysis
a. Analysis of neuron morphology
b. Synaptogenesis study
c. Focal adhesion analysis
2.12. Statistics
3. Results
3.1. Morphological Analysis of Electrospun Nylon Fibers
3.2. Viability and Proliferation of IMR-32 Cells
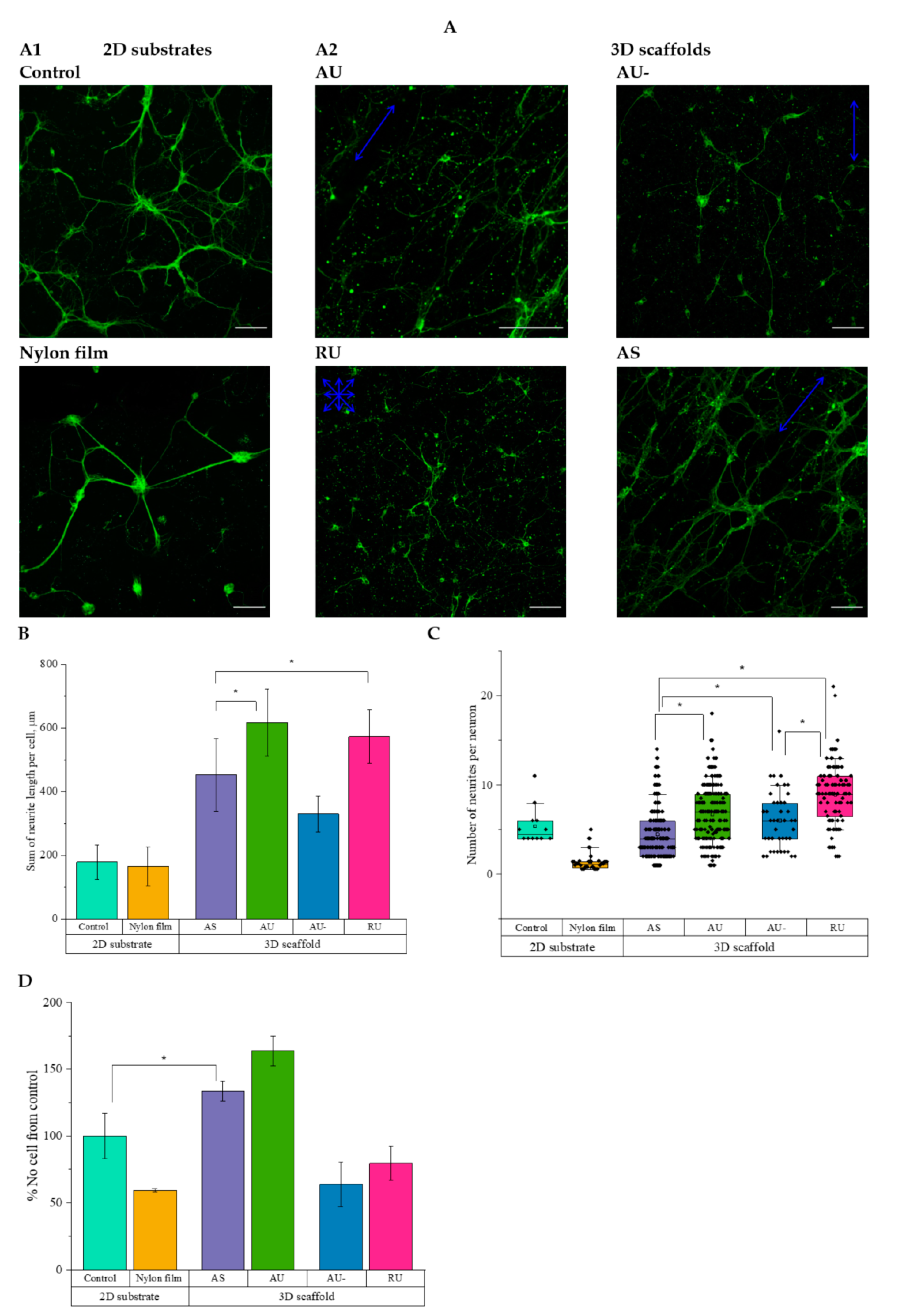
3.3. The Morphology of Rat Hippocampal Neurons Grown on Nanostructured Nylon Scaffolds
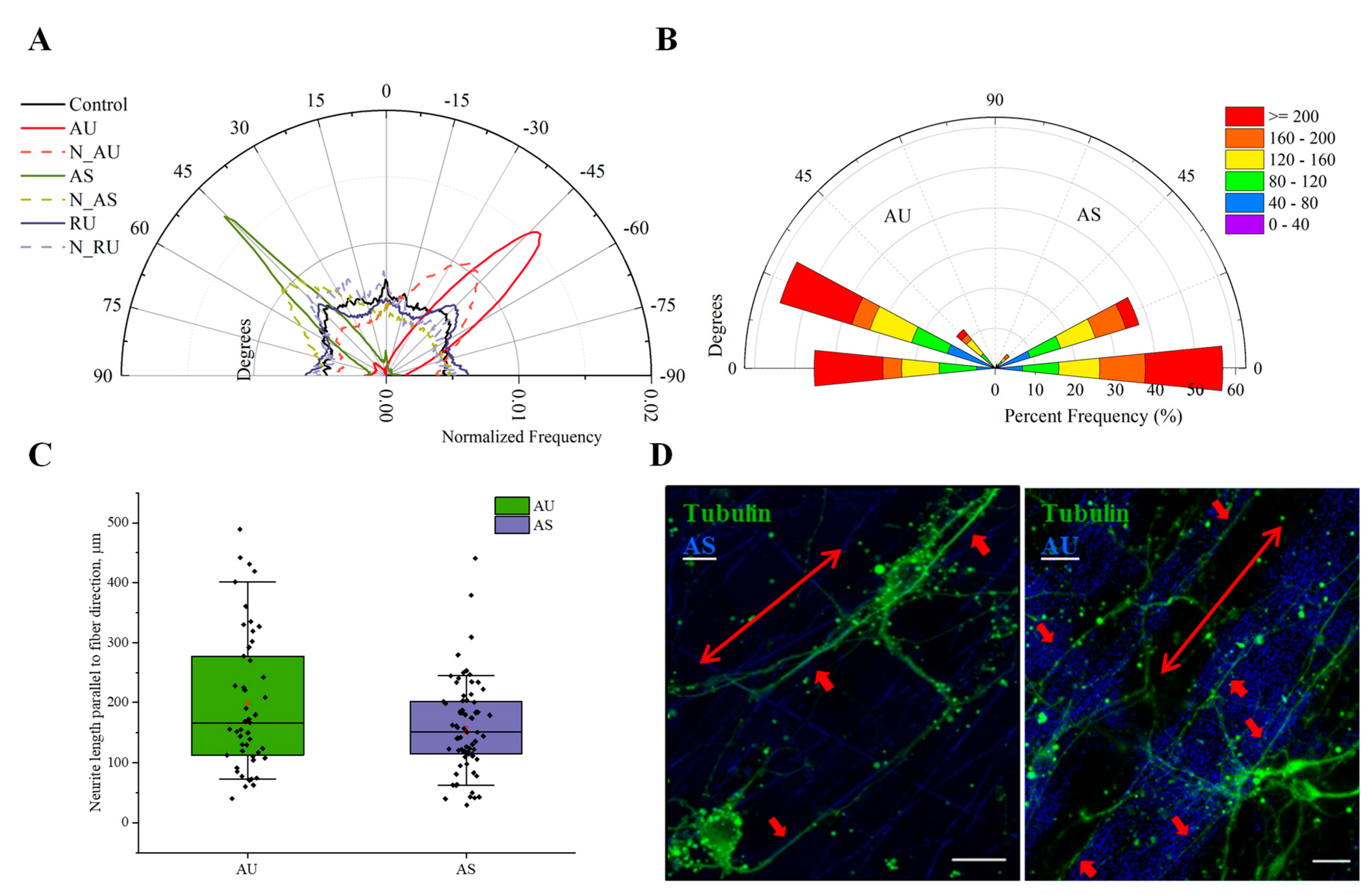
3.4. Ultrathin and Submicron Nylon Fibers Facilitate the Nanotopology-Mediated Directional Neurite Growth
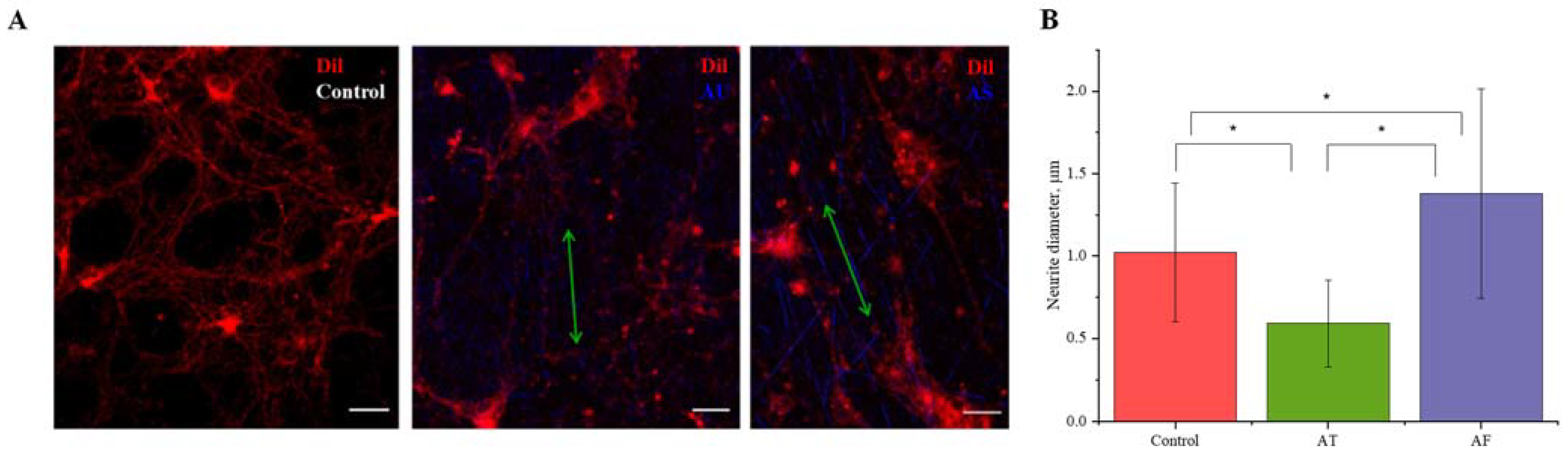
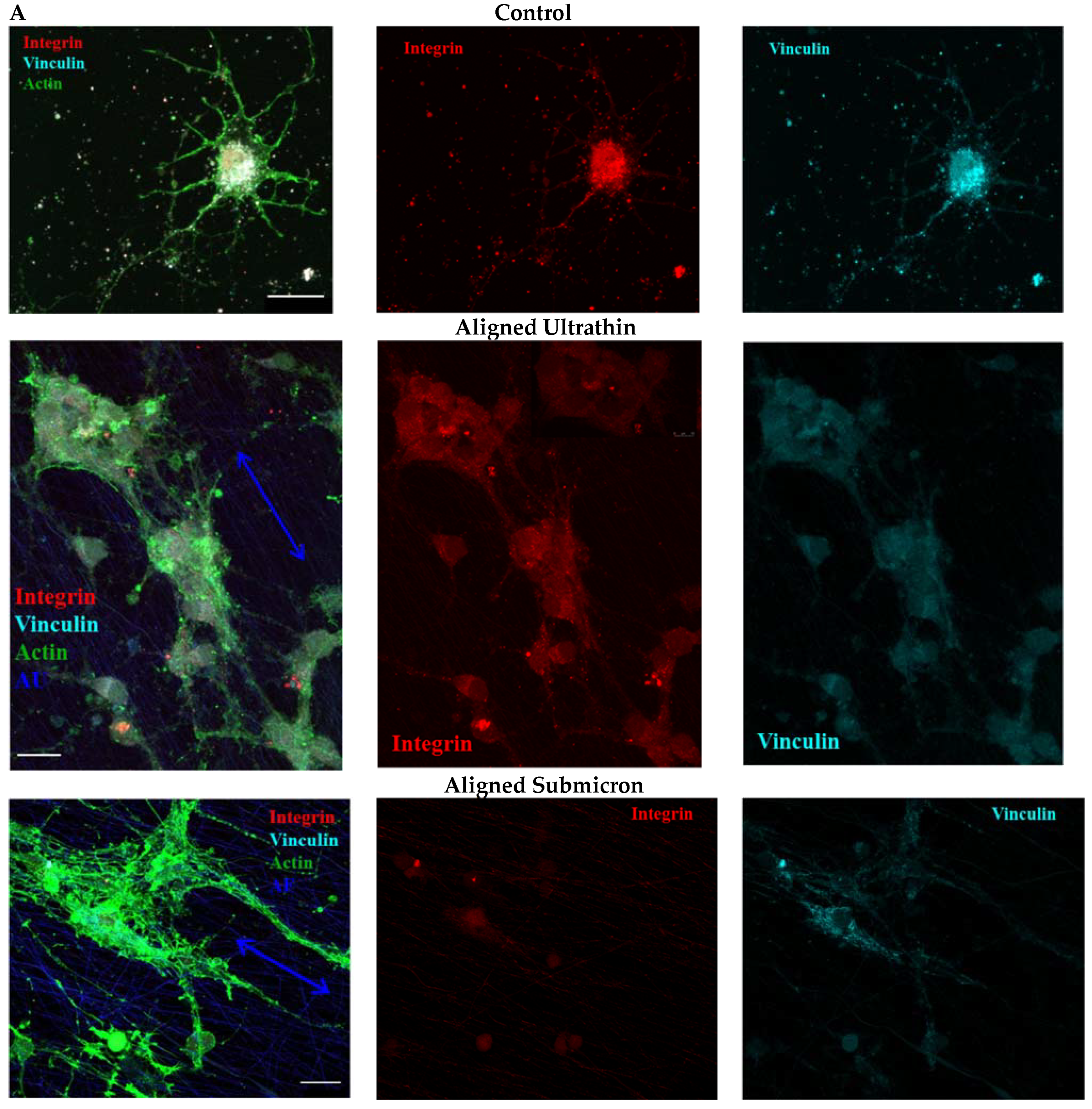
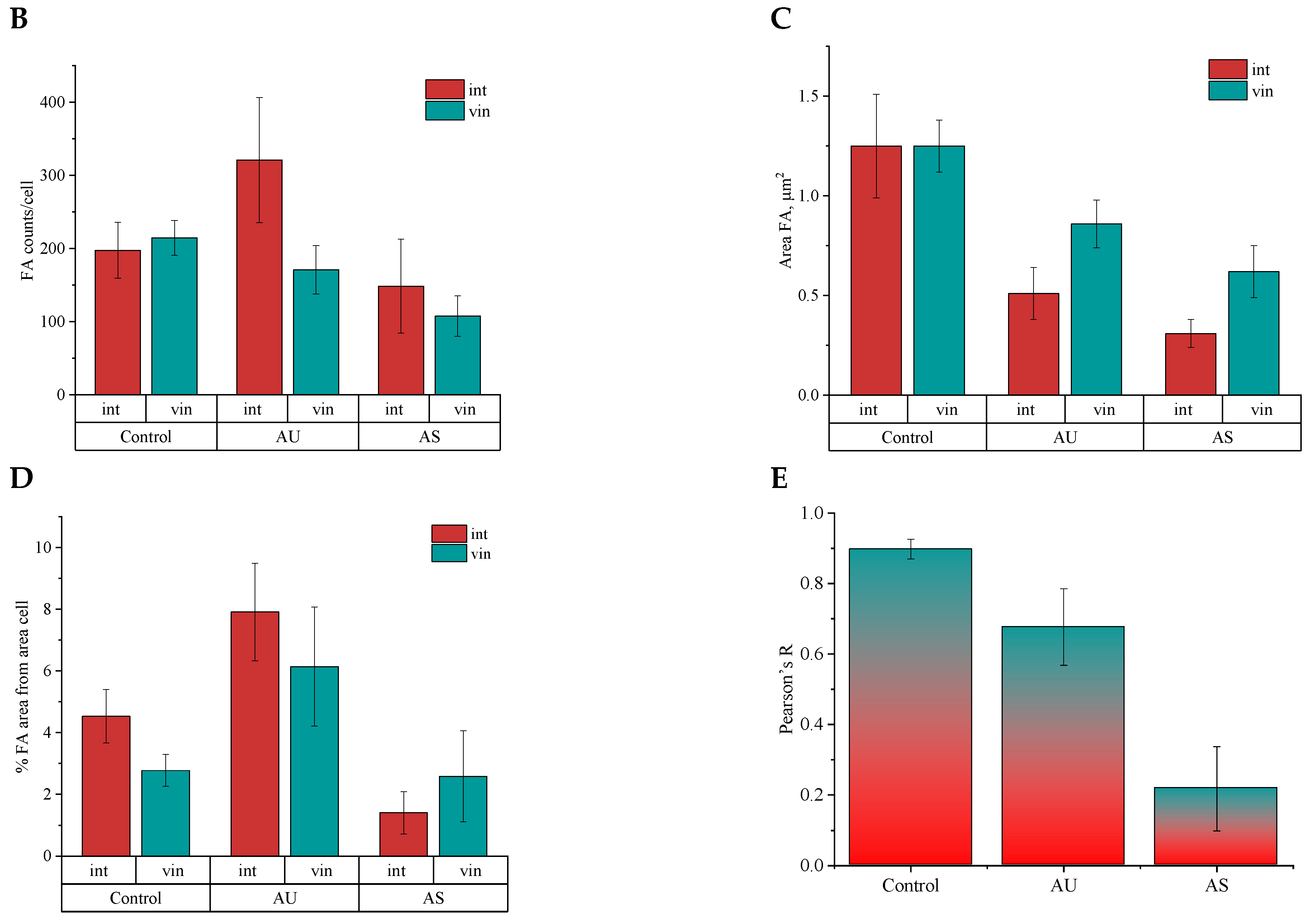
3.5. Influence of Matrix Ultrastructure on the Expression of Adhesion Receptors of Hippocampal Neurons
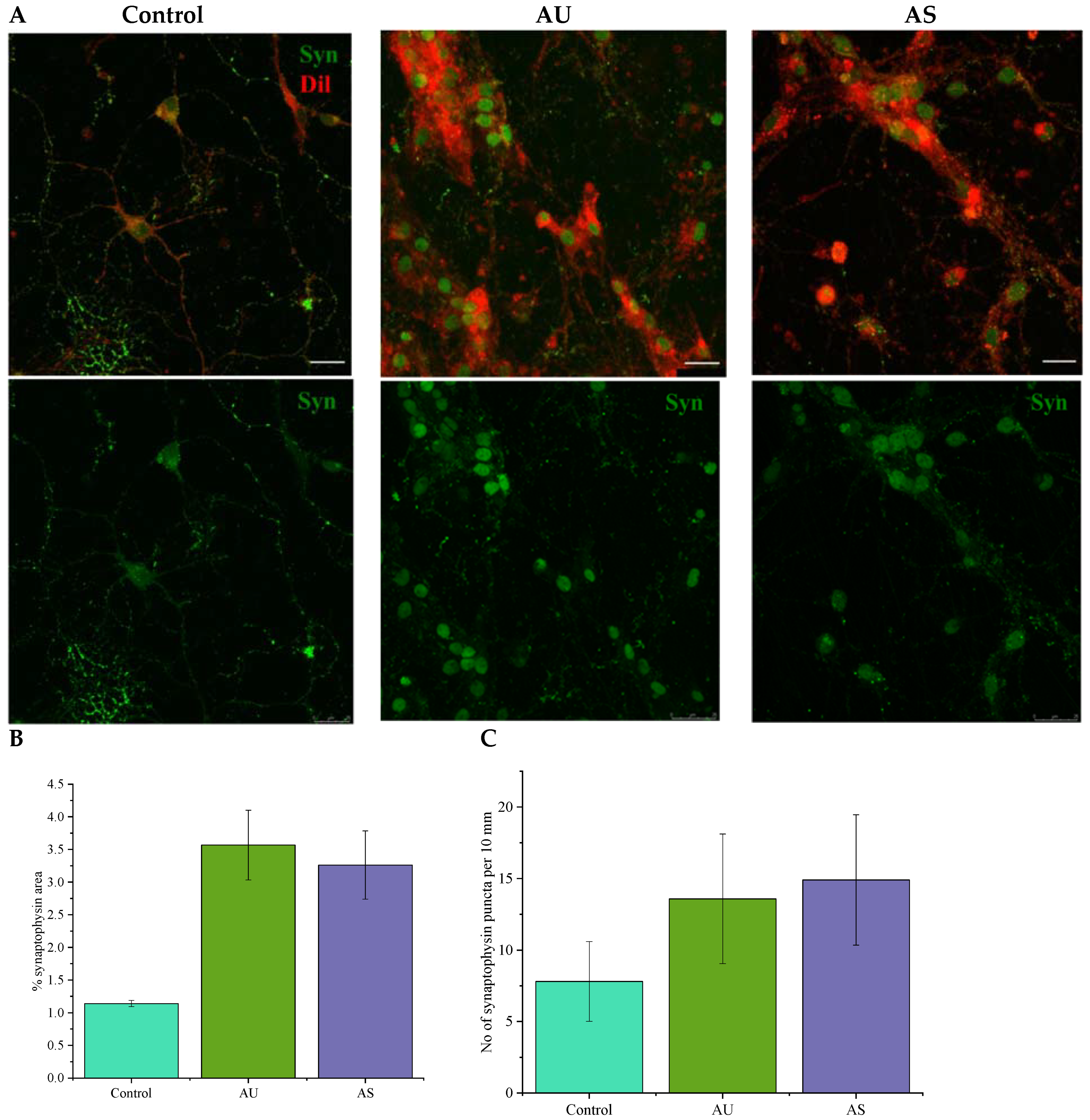
3.6. Nanofibers Increase the Synapse Number in Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; Aguilar, J.G.; et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef]
- Winnie, A.P.; Gupta, R. Surgical repair in humans after traumatic nerve injury provides limited functional neural regeneration in adults. Exp. Neurol. 2017, 290, 106–114. [Google Scholar] [CrossRef]
- Kuffler, D.P.; Foy, C. Restoration of Neurological Function Following Peripheral Nerve Trauma. Int. J. Mol. Sci. 2020, 21, 1808. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 2, 90. [Google Scholar] [CrossRef]
- Jain, D.; Mattiassi, S.; Goh, E.L.; Yim, E.K.F. Extracellular matrix and biomimetic engineering microenvironment for neuronal differentiation. Neural Regen. Res. 2020, 15, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Hong, Y.; Zhao, Y.; Qiu, S.; Wang, C.; Wei, Y. Influence of solvents on the formation of ultrathin uniform poly(vinyl pyrrolidone) nanofibers with electrospinning. Polym. Sci. Part B: Polym. Phys. 2004, 42, 3721–3726. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(epsilon-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Lim, S.H.; Liu, X.Y.; Song, H.; Yarema, K.J.; Mao, H.Q. The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials 2010, 31, 9031–9039. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hwang, T.; Aguilar, L.; Park, C.H.; Kim, C.S. A controlled design of aligned and random nanofibers for 3d bi-functionalized nerve conduits fabricated via a novel electrospinning set-up. Sci. Rep. 2016, 6, 23761. [Google Scholar] [CrossRef]
- Lü, X.; Yang, F.; Huang, Y.; Yu, Y. Role of integrin in influencing differentiation of PC12 cell grown on PLLA-aligned nanofiber: A mRNA–microRNA–protein integrative study. Regen. Biomater. 2017, 4, 89–96. [Google Scholar] [CrossRef]
- Johnson, C.D.L.; Zuidema, J.M.; Kearns, K.R.; Maguire, A.B.; Desmond, G.P.; Thompson, D.M.; Gilbert, R.J. The effect of electrospun fiber diameter on astrocyte-mediated neurite guidance and protection. ACS Appl. Bio Mater. 2019, 2, 104–117. [Google Scholar] [CrossRef]
- D’Amato, A.R.; Puhl, D.L.; Ziemba, A.M.; Johnson, C.D.L.; Doedee, J.; Bao, J.; Gilbert, R.J. Exploring the effects of electrospun fiber surface nanotopography on neurite outgrowth and branching in neuron cultures. PLoS ONE 2019, 14, e0211731. [Google Scholar] [CrossRef]
- Schaub, N.J.; Le Beux, C.; Miao, J.; Linhardt, R.J.; Alauzun, J.G.; Laurencin, D.; Gilbert, R.J. The effect of surface modification of aligned poly-l-lactic acid electrospun fibers on fiber degradation and neurite extension. PLoS ONE 2015, 10, e0136780. [Google Scholar] [CrossRef]
- Daud, M.F.; Pawar, K.C.; Claeyssens, F.; Ryan, A.J.; Haycock, J.W. An aligned 3D neuronal-glial co-culture model for peripheral nerve studies. Biomaterials 2012, 33, 5901–5913. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; McCarthy, C.W.; Gilbert, R.J. Varying the diameter of aligned electrospun fibers alters neurite outgrowth and Schwann cell migration. Acta Biomater. 2010, 6, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.; Bianchi, F.; Sleigh, J.N.; George, J.H.; Cader, M.Z.; Cui, Z.; Ye, H. Aligned electrospun fibers for neural patterning. Biotechnol. Lett. 2018, 40, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, N.; Ramakrishna, S.; Tian, L.; Mo, X. The effect of plasma treated plga/mwcnts-cooh composite nanofibers on nerve cell behavior. Polymers 2017, 9, 713. [Google Scholar] [CrossRef]
- Binder, C.; Milleret, V.; Hall, H.; Eberli, D.; Lühmann, T. Influence of micro and submicro poly(lactic-glycolic acid) fibers on sensory neural cell locomotion and neurite growth. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2013, 101, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Qin, J.; Huang, Z.; Yin, G.; Pu, X.; He, D. Fabrication of aligned conducting ppy-plla fiber films and their electrically controlled guidance and orientation for neuritis. ACS Appl. Mater. Interfaces 2016, 8, 12576–12582. [Google Scholar] [CrossRef]
- Qing, H.; Jin, G.; Zhao, G.; Ma, Y.; Zhang, X.; Sha, B.; Luo, Z.; Lu, T.J.; Xu, F. Heterostructured silk-nanofiber-reduced graphene oxide composite scaffold for sh-sy5y cell alignment and differentiation. ACS Appl. Mater. Interfaces 2018, 10, 39228–39237. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Lai, C.; Reneker, D.H.; Qiu, H.; Ye, Y.; Hou, H. Electrospun polymer nanofibres with small diameters. Nanotechnology 2006, 17, 1558. [Google Scholar] [CrossRef]
- Mikheev, A.Y.; Shlyapnikov, Y.M.; Kanev, I.L.; Avseenko, A.V.; Morozov, V.N. Filtering and optical properties of free standing electrospun nanomats from nylon-4,6. Eur. Polym. J. 2016, 75, 317–328. [Google Scholar] [CrossRef]
- Pant, H.R.; Risal, P.; Park, C.H.; Tijing, L.D.; Jeong, Y.J.; Kim, C.S. Synthesis, characterization, and mineralization of polyamide-6/calcium lactate composite nanofibers for bone tissue engineering. Colloids. Surf. B Biointerfaces 2013, 102, 152–157. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Mousa, H.M.; Tiwari, A.P.; Ko, S.W.; Park, C.H.; Kim, C.S. Development of polyamide-6,6/chitosan electrospun hybrid nanofibrous scaffolds for tissue engineering application. Carbohydr. Polym. 2016, 148, 107–114. [Google Scholar] [CrossRef]
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; De Geyter, N. Fabrication and plasma modification of nanofibrous tissue engineering scaffolds. Nanomaterials 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Xu, L.; Gu, X. Scaffolds for peripheral nerve repair and reconstruction. Exp. Neurol. 2019, 319, 112761. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.N.; Mikheev, A.Y. Water-soluble polyvinylpyrrolidone nanofilters manufactured by electrospray-neutralization technique. J. Membr. Sci. 2012, 403–404, 110–120. [Google Scholar] [CrossRef]
- Salazar, I.L.; Mele, M.; Caldeira, M.; Costa, R.O.; Correia, B.; Frisari, S.; Duarte, C.B. Preparation of primary cultures of embryonic rat hippocampal and cerebrocortical neurons. Bio-Protocol 2017, 7, e2551. [Google Scholar] [CrossRef]
- Horzum, U.; Ozdil, B.; Pesen-Okvur, D. Step-by-step quantitative analysis of focal adhesions. MethodsX 2014, 1, 56–59. [Google Scholar] [CrossRef]
- Katta, P.; Alessandro, M.; Ramsier, R.D.; Chase, G.G. Continuous electrospinning of aligned polymer nanofibers onto a wire drum collector. Nano Lett. 2004, 4, 2215–2218. [Google Scholar] [CrossRef]
- Recek, N. Biocompatibility of plasma-treated polymeric implants. Materials 2019, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Medda, R.; Liu, Z.; Galior, K.; Yehl, K.; Spatz, J.P.; Cavalcanti-Adam, E.A.; Salaita, K. Nanoparticle tension probes patterned at the nanoscale: Impact of integrin clustering on force transmission. Nano Lett. 2014, 14, 5539–5546. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.D.; Yamada, K.M. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp. Cell Res. 2016, 343, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Balasubramanian, S.; Hughes, D.; Vargo, S.; Powell, E.M.; Leach, J.B. β1-Integrin cytoskeletal signaling regulates sensory neuron response to matrix dimensionality. Neuroscience 2013, 248, 67–78. [Google Scholar] [CrossRef]
- Kulangara, K.; Yang, Y.; Yang, J.; Leong, K.W. Nanotopography as modulator of human mesenchymal stem cell function. Biomaterials 2012, 33, 4998–5003. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, T.; Chew, S.Y. The application of nanofibrous scaffolds in neural tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1055–1064. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. J. Biomed. Mater. 2011, 6, 025004. [Google Scholar] [CrossRef]
- Hajiali, H.; Contestabile, A.; Mele, E.; Athanassiou, A. Influence of topography of nanofibrous scaffolds on functionality of engineered neural tissue. J. Mater. Chem. B. 2018, 6, 930–939. [Google Scholar] [CrossRef]
- He, L.; Liao, S.; Quan, D. Synergistic effects of electrospun PLLA fiber dimension and pattern on neonatal mouse cerebellum C17.2 stem cells. Acta Biomater. 2010, 6, 2960–2969. [Google Scholar] [CrossRef]
- Chen, W.S.; Guo, L.Y.; Tang, C.C.; Tsai, C.K.; Huang, H.H.; Chin, T.Y.; Yang, M.L.; Chen-Yang, Y.W. The Effect of Laminin Surface Modification of Electrospun Silica Nanofiber Substrate on Neuronal Tissue Engineering. Nanomaterials 2018, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, G.T.; Song, H.; Mao, H.Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Merryweather, D.; Roach, P. The need for advanced three-dimensional neural models and developing enabling technologies. MRS Commun. 2017, 7, 309–319. [Google Scholar] [CrossRef]
- Tomba, C.; Braïni, C.; Bugnicourt, G.; Cohen, F.; Friedrich, B.M.; Gov, N.S.; Villard, C. Geometrical determinants of neuronal actin waves. Front. Cell. Neurosci. 2017, 11, 86. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Li, Z. The effects of target skeletal muscle cells on dorsal root ganglion neuronal outgrowth and migration in vitro. PLoS ONE 2013, 8, e52849. [Google Scholar] [CrossRef]
- Marcus, M. Interactions of Neurons with Physical Environments. Adv. Healthc. Mater. 2017, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kim, E.; Jang, H.; Kim, Y.K.; Kang, K. Neuron–material nanointerfaces: Surface nanotopography governs neuronal differentiation and development. ChemNanoMat 2017, 3, 278–287. [Google Scholar] [CrossRef]
- Baranes, K.; Chejanovsky, N.; Alon, N.; Sharoni, A.; Shefi, O. Topographic cues of nano-scale height direct neuronal growth pattern. Biotechnol. Bioeng. 2012, 109, 1791–1797. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Haenzi, B.; Andrews, M.R.; Verhaagen, J.; Fawcett, J.W. Integrins promote axonal regeneration after injury of the nervous system. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1339–1362. [Google Scholar] [CrossRef] [PubMed]
- Changede, R.; Sheetz, M. Integrin and cadherin clusters: A robust way to organize adhesions for cell mechanics. BioEssays. 2017, 39, 1–12. [Google Scholar] [CrossRef]
- Changede, R.; Cai, H.; Wind, S.J.; Sheetz, M.P. Integrin nanoclusters can bridge thin matrix fibres to form cell-matrix adhesions. Nat. Mater. 2019, 18, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Gautrot, J.E.; Malmström, J.; Sundh, M.; Margadant, C.; Sonnenberg, A.; Sutherland, D.S. The nanoscale geometrical maturation of focal adhesions controls stem cell differentiation and mechanotransduction. Nano Lett. 2014, 14, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Di Cio, S.; Iskratsch, T.; Connelly, J.T.; Gautrot, J.E. Contractile myosin rings and cofilin-mediated actin disassembly orchestrate ECM nanotopography sensing. Biomaterials 2020, 232, 119683. [Google Scholar] [CrossRef]
- Slater, J.H.; Boyce, P.J.; Jancaitis, M.P.; Gaubert, H.E.; Chang, A.L.; Markey, M.K.; Frey, W. Modulation of endothelial cell migration via manipulation of adhesion site growth using nanopatterned surfaces. ACS Appl. Mater. Interfaces 2015, 7, 4390–4400. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Gu, X.; Leong, K.W. Biophysical regulation of cell behavior-cross talk between substrate stiffness and nanotopography. Engineering 2017, 3, 36–54. [Google Scholar] [CrossRef]
- Schulte, C.; Rodighiero, S.; Cappelluti, M.A.; Puricelli, L.; Maffioli, E.; Borghi, F.; Negri, A.; Sogne, E.; Galluzzi, M.; Piazzoni, C.; et al. Conversion of nanoscale topographical information of cluster-assembled zirconia surfaces into mechanotransductive events promotes neuronal differentiation. J. Nanobiotechnol. 2016, 14, 18. [Google Scholar] [CrossRef]
- Schaufler, V.; Czichos-Medda, H.; Hirschfeld-Warnecken, V.; Neubauer, S.; Rechenmacher, F.; Medda, R.; Kessler, H.; Geiger, B.; Spatz, J.P.; Cavalcanti-Adam, E.A. Selective binding and lateral clustering of α5β1 and αvβ3 integrins: Unraveling the spatial requirements for cell spreading and focal adhesion assembly. Cell Adhes. Migr. 2016, 10, 505–515. [Google Scholar] [CrossRef]
- Rossier, O.; Octeau, V.; Sibarita, J.B.; Leduc, C.; Tessier, B.; Nair, D.; Gatterdam, V.; Destaing, O.; Albigès-Rizo, C.; Tampé, R.; et al. Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat. Cell Biol. 2012, 14, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Roca-Cusachs, P.; Gauthier, N.C.; Del Rio, A.; Sheetz, M.P. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc. Natl. Acad. Sci. USA 2009, 106. [Google Scholar] [CrossRef] [PubMed]
- Bidone, T.C.; Skeeters, A.V.; Oakes, P.W.; Voth, G.A. Multiscale model of integrin adhesion assembly. PLoS Comput. Biol. 2019, 15, 1007077. [Google Scholar] [CrossRef]
- Di Cio, S.; Bøggild, T.M.L.; Connelly, J.; Sutherland, D.S.; Gautrot, J.E. Differential integrin expression regulates cell sensing of the matrix nanoscale geometry. Acta Biomater. 2017, 50, 280–292. [Google Scholar] [CrossRef] [PubMed]
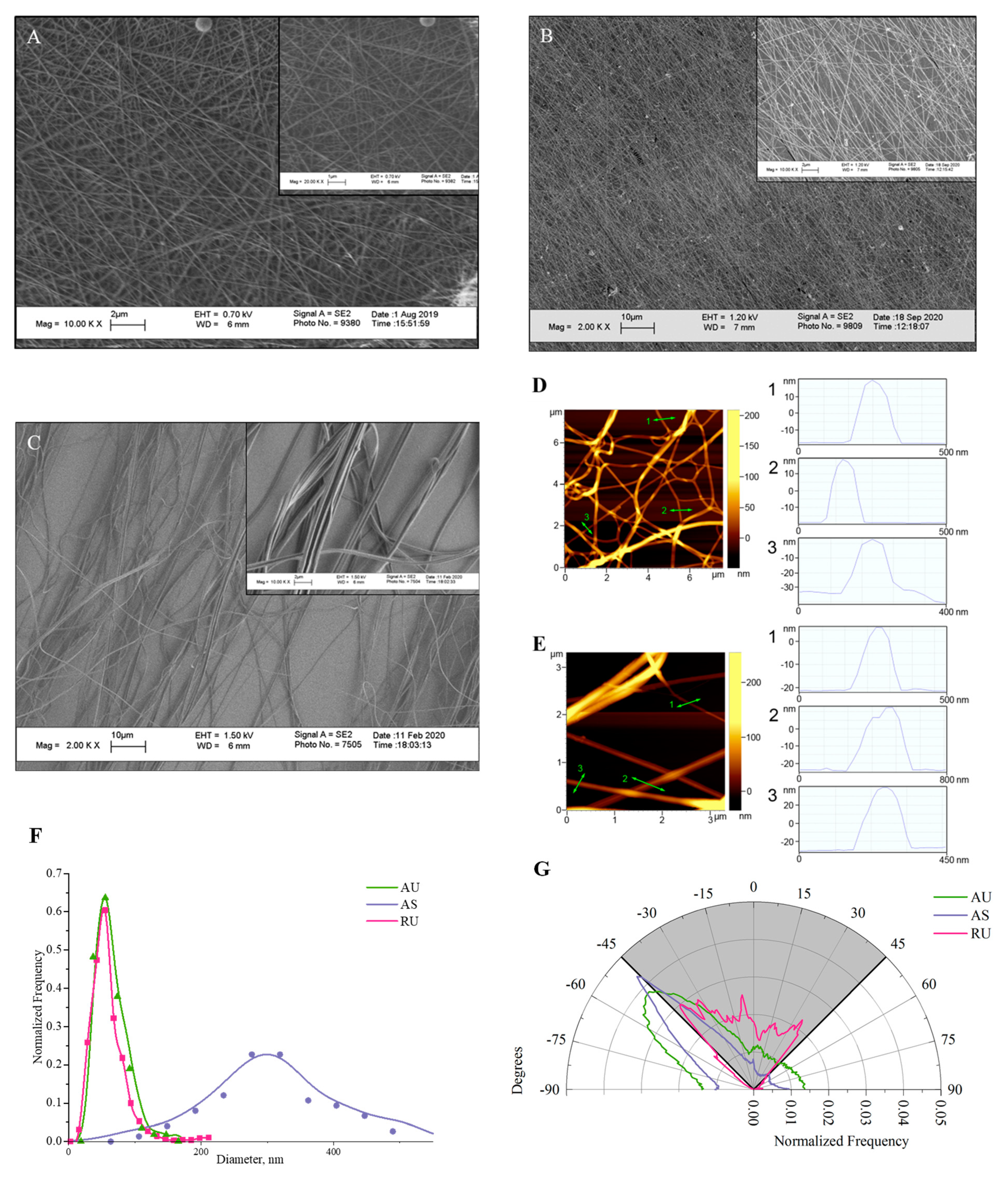

| Scaffold Material/Cell Model | Diameter Fiber, nm | Cell Response/Behavior | Reference |
|---|---|---|---|
| Fiber diameter < 500 nm | |||
| PCL, PCL/gelatin + serum protein // C17.2 | 113 ± 33, 189 ± 56, 431 ± 118 | Oriented growth of neurites on 189 ± 56 nm fibers | Ghasemi-Mobarakeh et al., 2008 [8] |
| PCL + PLO and laminin // ANSCs | 260, 480 and 930 | Cell elongation and enhanced differentiation on 480 nm aligned fibers | Lim et al., 2010 [9] |
| PLGA without treatment / PC12 | 400–500 | ANL - 160 μm in 5 days | Kim et al., 2016 [10]. |
| PLLA/PC12 | 269.30 ± 4.27 | ANL - 21.29 μm in 24 h | Lü et al., 2017 [11] |
| PLLA + fibronectin // Astrocytes and neurons from DRG | 386/808 | No directional alignment along 386 nm fibers. ANL parallel to 386 nm fibers - 220 µm, 808 nm - 320 µm in 4 days | Johnson et al., 2019 [12] |
| Fiber diameter > 500 nm | |||
| Diameter fiber, μm | |||
| PLLA + laminin // neurons from DRG | 2.19 ± 0.19 2.31 ± 0.06 2.44 ± 0.07 | MNL - 476.3 - 501.7 μm in 4 days | D’Amato et al., 2019 [13] |
| PLLA + DETA, AEE, GRGDS and treatment O2 plasma // chicken DRG | 0.9–1.3 | MNL increases from 844 to 1651 μm in 5 days, depending on the treatment of fibers | Schaub et al., 2015 [14] |
| PCL // NG108-15 | 1.02 ± 0.05 5.08 ± 0.130 8.07 ± 0.07 | MNL decreases from 142.36 to 61.83 μm in 4 days with decreasing fiber diameter. Oriented growth of cells | Daud et al., 2012 [15] |
| PLLA + serum protein // DRG | 1.325 ± 0.383 0.759 ± 0.179 0.293 ± 0.65 | MNL decreases from 1400 to 1000 μm in 5 days with decreasing fiber diameter. No oriented growth of cells on 293 nm fibers | Wang et al., 2010 [16] |
| Gelatin/PCL (70:30) // NG108-15 | 0.63 ± 0.13 | ANL ~ 120 µm in 5 days. Oriented growth of cells | Soliman et al., 2018 [17] |
| PLGA and PLGA-MWCNTs-COOH + plasma treatment // DRG | 0.913 ± 0.185 0.768 ± 0.127 0.708 ± 0.142 0.631 ± 0.94 | ANL increases from 19.23 to 78.27 μm in 3 days with increasing CNTs content | Wang et al., 2017 [18] |
| PLGA + laminin // ND7/23 | 5.73 ± 0.57 0.74 ± 0.18 | ANL on 5 μm fibers ~ 175 μm (160% of control), on 0.74 μm fibers ~ 110 μm (105%). Oriented growth of cells | Binder et al., 2013 [19] |
| PPy-PLLA + laminin and collagen // PC12 | ~0.8 | ANL on random and aligned fibers - 65.44 and 114.73 μm in 3 days. Oriented growth of cells | Zou et al., 2016 [20] |
| Silk + reduced graphene paper // SH-SY5Y | 0.5–0.55 | ANL on random and aligned fibers - ~350 and 1200 μm in 10 days. Oriented growth of cells | Qing et al., 2018 [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonova, O.Y.; Kochetkova, O.Y.; Shlyapnikov, Y.M. ECM-Mimetic Nylon Nanofiber Scaffolds for Neurite Growth Guidance. Nanomaterials 2021, 11, 516. https://doi.org/10.3390/nano11020516
Antonova OY, Kochetkova OY, Shlyapnikov YM. ECM-Mimetic Nylon Nanofiber Scaffolds for Neurite Growth Guidance. Nanomaterials. 2021; 11(2):516. https://doi.org/10.3390/nano11020516
Chicago/Turabian StyleAntonova, Olga Y., Olga Y. Kochetkova, and Yuri M. Shlyapnikov. 2021. "ECM-Mimetic Nylon Nanofiber Scaffolds for Neurite Growth Guidance" Nanomaterials 11, no. 2: 516. https://doi.org/10.3390/nano11020516
APA StyleAntonova, O. Y., Kochetkova, O. Y., & Shlyapnikov, Y. M. (2021). ECM-Mimetic Nylon Nanofiber Scaffolds for Neurite Growth Guidance. Nanomaterials, 11(2), 516. https://doi.org/10.3390/nano11020516







