Grafting TRAIL through Either Amino or Carboxylic Groups onto Maghemite Nanoparticles: Influence on Pro-Apoptotic Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Maghemite Nanoparticle (NP) Synthesis
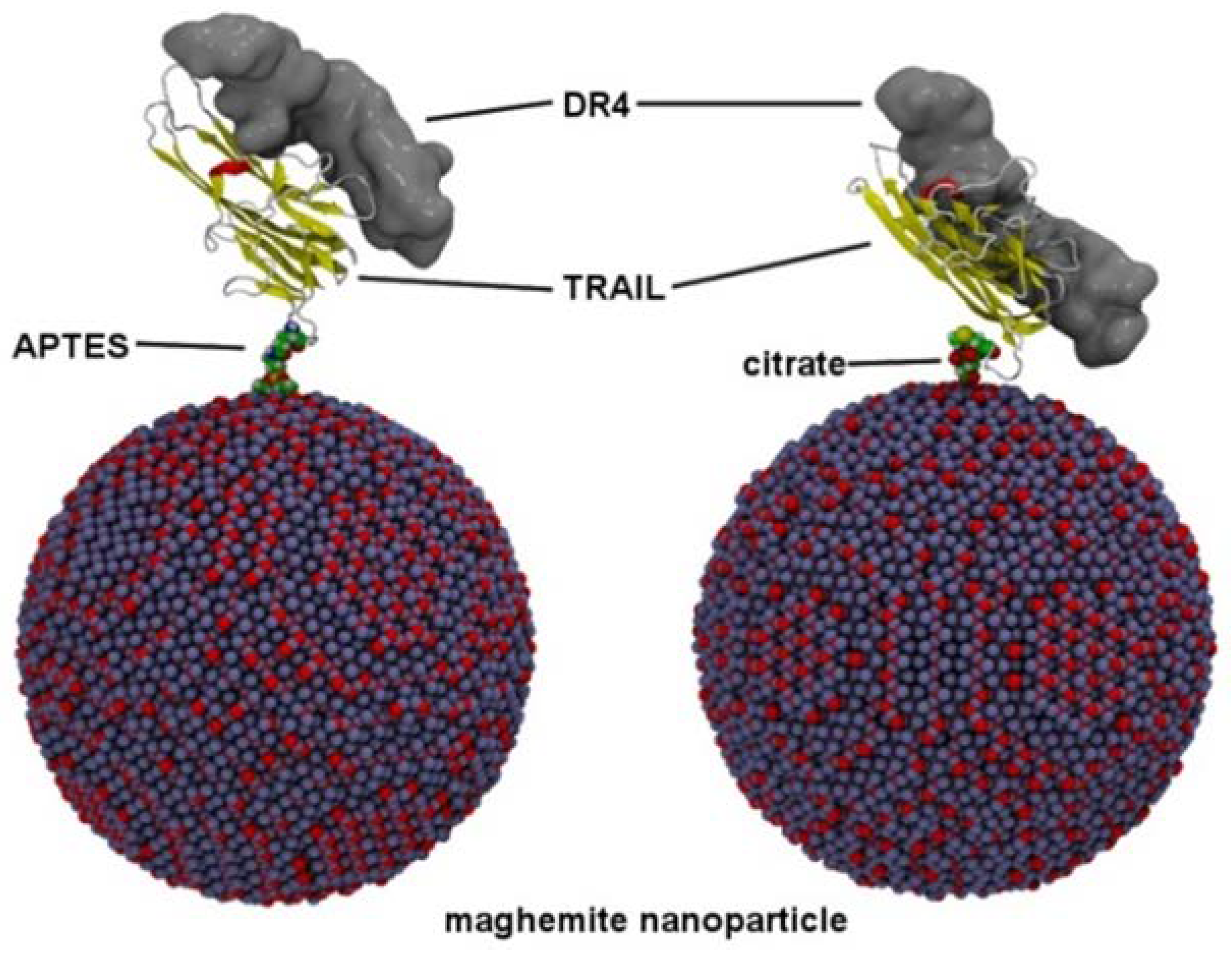
2.3. Elaboration of Magnetic Nanohybrids
2.4. Elaboration of Nanovectors
2.5. Characterization Techniques
2.6. Cell Lines and Cell Culture: Viability Assay and Apoptosis Survey
2.7. Computational Study
3. Results and Discussion
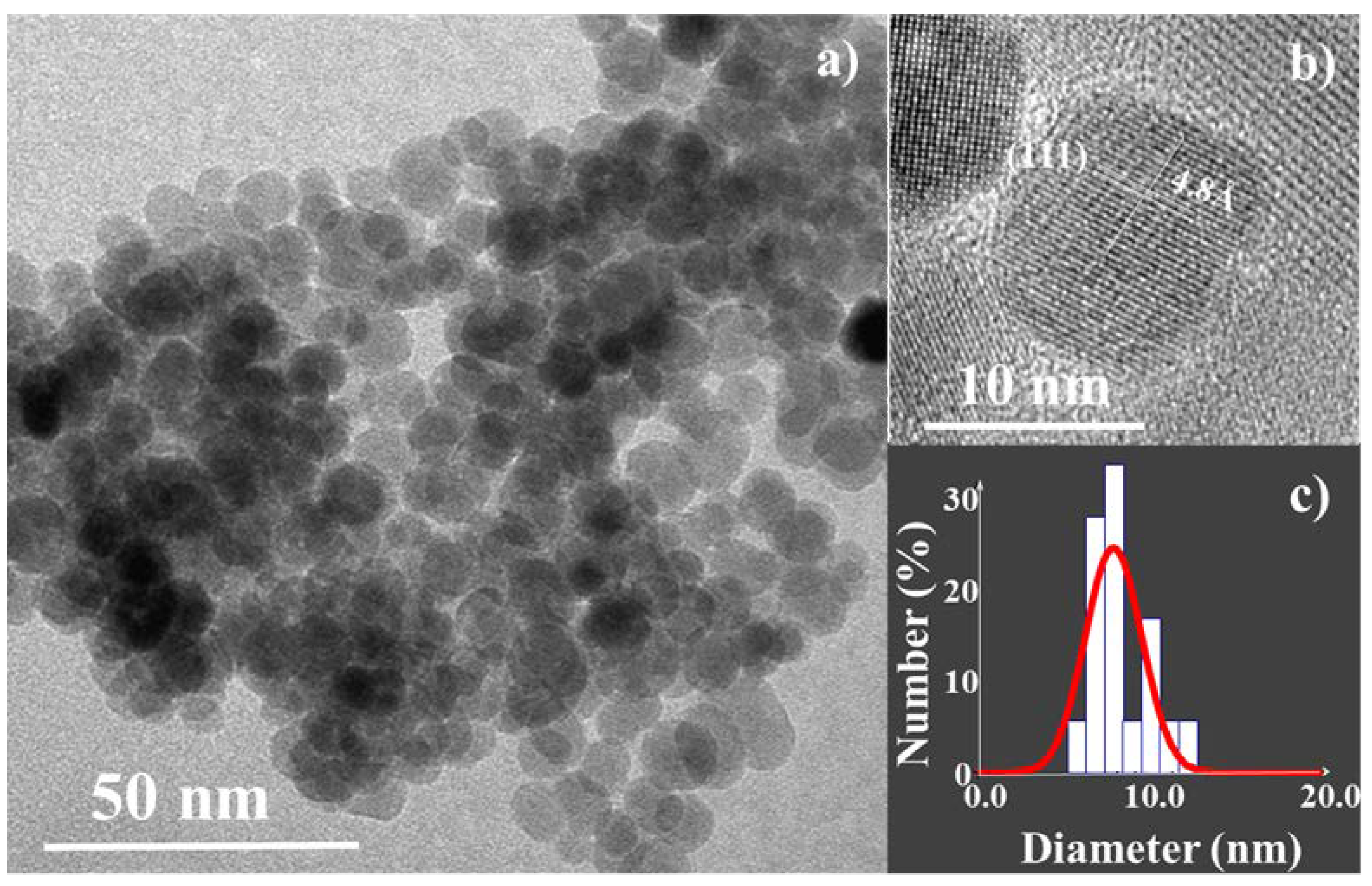
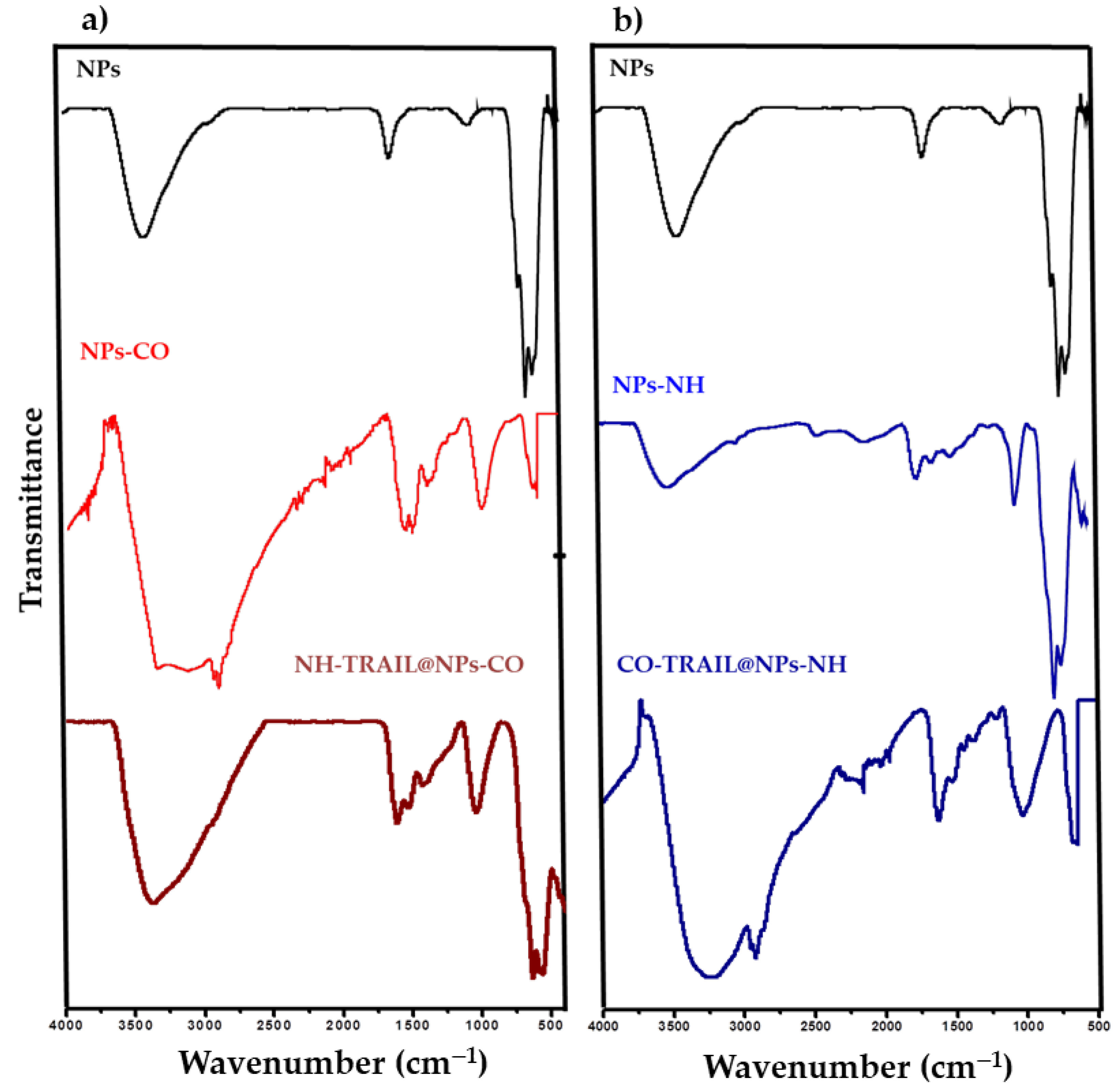
3.1. Structural and Microstructural Properties
3.2. Magnetic Properties
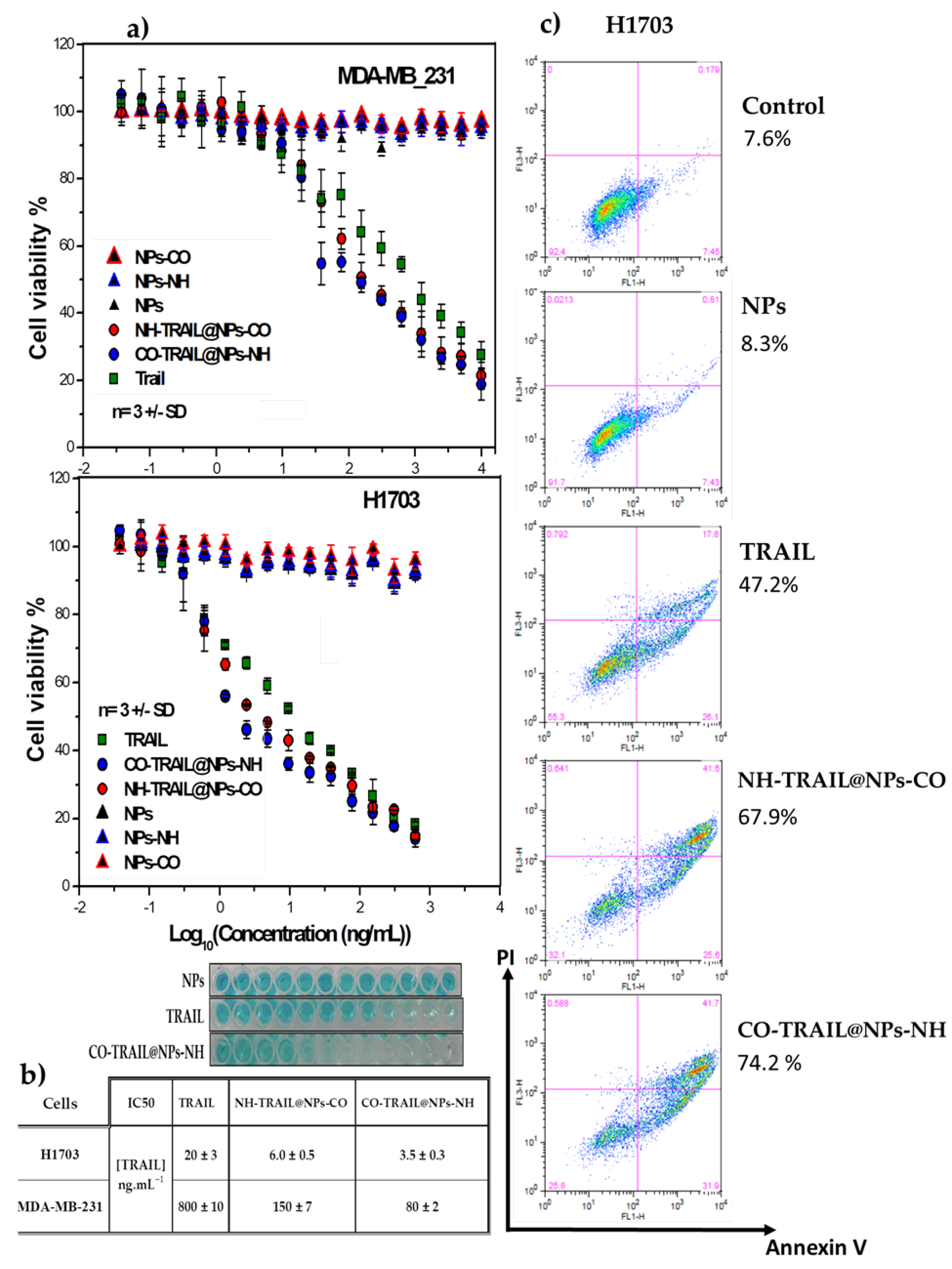
3.3. Antitumoral Properties and Capabilities of the Nanovectors in Cancer Therapy
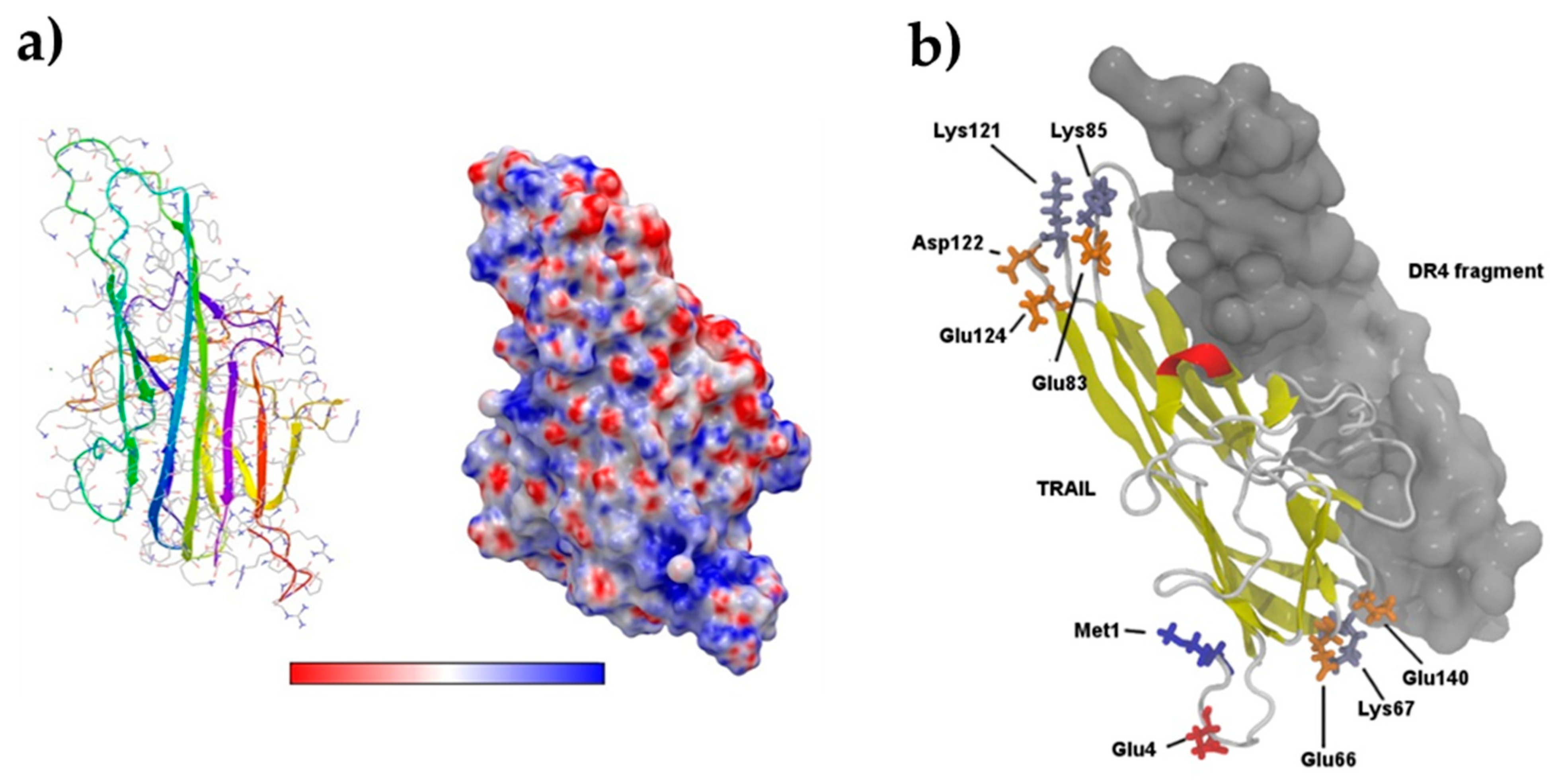
3.4. Computational Study on TRAIL–DR4 Recognition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Merino, D.; Lalaoui, N.; Morizot, A.; Schneider, P.; Solary, E.; Micheau, O. Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol. Cell. Biol. 2006, 26, 7046–7055. [Google Scholar] [CrossRef]
- Zakaria, A.B.; Picaud, F.; Rattier, T.; Pudlo, M.; Dufour, F.; Saviot, L.; Chassagnon, R.; Lherminier, J.; Gharbi, T.; Micheau, O.; et al. Nanovectorization of TRAIL with single wall carbon nanotubes enhances tumor cell killing. Nano Lett. 2015, 15, 891–895. [Google Scholar] [CrossRef]
- Belkahla, H.; Mazario, E.; Sangnier, A.P.; Lomas, J.S.; Gharbi, T.; Ammar, S.; Micheau, O.; Wilhelm, C.; Hemadi, M. TRAIL acts synergistically with iron oxide nanocluster-mediated magneto- and photothermia. Theranostics 2019, 9, 5924–5936. [Google Scholar] [CrossRef] [PubMed]
- Belkahla, H.; Herlem, G.; Picaud, F.; Gharbi, T.; Hémadi, M.; Ammar, S.; Micheau, O. TRAIL-NP hybrids for cancer therapy: A review. Nanoscale 2017, 9, 5755–5768. [Google Scholar] [CrossRef] [PubMed]
- Belkahla, H.; Haque, A.; Revzin, A.; Gharbi, T.; Constantinescu, A.A.; Micheau, O.; Hémadi, M.; Ammar, S. Coupling TRAIL to iron oxide nanoparticles increases its apoptotic activity on HCT116 and HepG2 malignant cells: Effect of magnetic core size. J. Interdiscip. Nanomed. 2019, 4, 34–50. [Google Scholar] [CrossRef]
- Choi, S.H.; Byeon, H.J.; Choi, J.S.; Thao, L.; Kim, I.; Lee, E.S.; Shin, B.S.; Lee, K.C.; Youn, Y.S. Inhalable self-assembled albumin nanoparticles for treating drug-resistant lung cancer. J. Control Release 2015, 197, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.E. Amine coupling through EDC/NHS: A practical approach. Methods Mol. Biol. 2010, 627, 55–73. [Google Scholar] [CrossRef]
- Piraux, H.; Hai, J.; Verbeke, P.; Serradji, N.; Ammar, S.; Losno, R.; Ha-Duong, N.T.; Hemadi, M.; El Hage Chahine, J.M. Transferrin receptor-1 iron-acquisition pathway—synthesis, kinetics, thermodynamics and rapid cellular internalization of a holotransferrin-maghemite nanoparticle construct. Biochim. Biophys. Acta 2013, 1830, 4254–4264. [Google Scholar] [CrossRef] [PubMed]
- Piraux, H.; Hai, J.; Gaudisson, T.; Ammar, S.; Gazeau, F.; El Hage Chahine, J.; Hémadi, M. Transferrin-bearing maghemite nano-constructs for biomedical applications. J. Appl. Phys. 2015, 17, 17A336. [Google Scholar] [CrossRef]
- Hai, J.; Piraux, H.; Mazario, E.; Volatron, J.; Ha-Duong, N.T.; Decorse, P.; Lomas, J.S.; Verbeke, P.; Ammar, S.; Wilhelm, C.; et al. Maghemite nanoparticles coated with human serum albumin: Combining targeting by the iron-acquisition pathway and potential in photothermal therapies. J. Mater. Chemsitry B 2017, 5, 3154–3162. [Google Scholar] [CrossRef]
- Bae, S.; Ma, K.; Kim, T.H.; Lee, E.S.; Oh, K.T.; Park, E.S.; Lee, K.C.; Youn, Y.S. Doxorubicin-loaded human serum albumin nanoparticles surface-modified with TNF-related apoptosis-inducing ligand and transferrin for targeting multiple tumor types. Biomaterials 2012, 33, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Poul, L.; Ammar, S.; Jouini, N.; Fiévet, F.; Villain, F. Synthesis of inorganic compounds (metal, oxide and hydroxide) in polyol medium: A versatile route related to the sol-gel process. J. Sol-Gel Sci. Technol. 2003, 26, 261–265. [Google Scholar] [CrossRef]
- Fievet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Hanini, A.; Schmitt, A.; Kacem, K.; Chau, F.; Ammar, S.; Gavard, J. Evaluation of iron oxide nanoparticle biocompatibility. Int. J. Nanomed. 2011, 6, 787–794. [Google Scholar]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2009. [Google Scholar]
- Basti, H.; Ben Tahar, L.; Smiri, L.S.; Herbst, F.; Vaulay, M.J.; Chau, F.; Ammar, S.; Benderbous, S. Catechol derivatives-coated Fe3O4 and gamma-Fe2O3 nanoparticles as potential MRI contrast agents. J. Colloid Interface Sci. 2010, 341, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Mazario, M.; Forget, A.; Belkahla, H.; Lomas, J.S.; Decorse, P.; Chevillot-Biraud, A.; Verbeke, P.; Wilhelm, C.; Ammar, S.; El Hage Chahine, J.M.; et al. Design and Characterization of Iron Oxide Nanoparticles Functionalized with HSA Protein for Thermal Therapy. IEEE Trans. Magn. 2017, 53, 1–6. [Google Scholar] [CrossRef]
- Morle, A.; Garrido, C.; Micheau, O. Hyperthermia restores apoptosis induced by death receptors through aggregation-induced c-FLIP cytosolic depletion. Cell Death Dis. 2015, 6, e1633. [Google Scholar] [CrossRef] [PubMed]
- Volatron, J.; Carn, F.; Kolosnjaj-Tabi, J.; Javed, Y.; Vuong, Q.L.; Gossuin, Y.; Ménager, C.; Luciani, N.; Charron, G.; Hémadi, M.; et al. Ferritin Protein Regulates the Degradation of Iron Oxide Nanoparticles. Small 2016, 13, 1602030. [Google Scholar] [CrossRef]
- Hanini, A.; Lartigue, L.; Gavard, J.; Schmitt, A.; Kacem, K.; Wilhelm, C.; Gazeau, F.; Ammar, S. Thermosensitivity profile of malignant glioma U87-MG cells and human endothelial cells following g-Fe2O3 NPs internalization and magnetic field application. RSC Adv. 2016, 6, 15415. [Google Scholar] [CrossRef]
- Belin, T.; Guigue-Millot, N.; Caillot, T.; Aymes, D.; Niepce, J.C. Influence of Grain Size, Oxygen Stoichiometry, and Synthesis Conditions on the -Fe2O3 Vacancies Ordering and Lattice Parameters. J. Solid State Chem. 2002, 163, 459–465. [Google Scholar] [CrossRef]
- Schneider, P. Production of recombinant TRAIL and TRAIL receptor: Fc chimeric proteins. Methods Enzymol. 2000, 322, 325–345. [Google Scholar] [CrossRef]
- Hemadi, M.; Kahn, P.H.; Miquel, G.; El Hage Chahine, J.M. Transferrin’s mechanism of interaction with receptor 1. Biochemistry 2004, 43, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, V.; Yamniuk, A.P.; Lawrence, E.J.; Yong, W.; Schneeweis, L.A.; Cheng, L.; Murdock, M.; Corbett, M.J.; Doyle, M.L.; Sheriff, S. The structure of the death receptor 4-TNF-related apoptosis-inducing ligand (DR4-TRAIL) complex. Acta Crystallographica. Sect. Fstructural Biol. Commun. 2015, 71, 1273–1281. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef]
- Anandakrishnan, R.; Aguilar, B.; Onufriev, A.V. H++ 3.0: Automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res. 2012, 40, W537–W541. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E.; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Homeyer, N.; et al. Amber 2016 Reference Manual; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Hu, R.; Barbault, F.; Maurel, F.; Delamar, M.; Zhang, R. Molecular dynamics simulations of 2-amino-6-arylsulphonylbenzonitriles analogues as HIV inhibitors: Interaction modes and binding free energies. Chem. Biol. Drug Des. 2010, 76, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., 3rd. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Waldron, R.D. Infra red spectra of ferrites. Phys. Rev. 1955, 99, 1727–1735. [Google Scholar] [CrossRef]
- Naval, J.; de Miguel, D.; Gallego-Lleyda, A.; Anel, A.; Martinez-Lostao, L. Importance of TRAIL Molecular Anatomy in Receptor Oligomerization and Signaling. Implications for Cancer Therapy. Cancers 2019, 11, 444. [Google Scholar] [CrossRef]
- Yoshida, W.; Castro, R.P.; Jou, J.D.; Cohen, Y. Multilayer alkoxysilane silylation of oxide surfaces. Langmuir ACS J. Surf. Colloids 2001, 17, 5882–5888. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A. In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef]
- Aronniemi, M.; Lahtinen, J.; Hautojarvi, P. Characterization of iron oxide thin films. Surf. Interface Anal. 2004, 36, 1004–1006. [Google Scholar] [CrossRef]
- Barazzouk, S.; Daneault, C. Amino Acid and Peptide Immobilization on Oxidized Nanocellulose: Spectroscopic Characterization. Nanomaterials 2012, 2, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Grasset, F.; Labhsetwar, N.; Li, D.; Park, D.C.; Saito, N.; Haneda, H.; Cador, O.; Roisnel, T.; Mornet, S.; Duguet, E.; et al. Synthesis and magnetic characterization of zinc ferrite nanoparticles with different environments: Powder, colloidal solution, and zinc ferrite-silica core-shell nanoparticles. Langmuir ACS J. Surf. Colloids 2002, 18, 8209–8216. [Google Scholar] [CrossRef]
- Das, S.K.; Dickinson, C.; Lafir, F.; Brougham, D.F.; Marsili, E. Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopus oryzae protein extract. Green Chem. 2012, 14, 1322–1334. [Google Scholar] [CrossRef]
- Goya, G.F.; Leite, E.R. Ferrimagnetism and spin canting of (ZnFe2O4)-Fe-57 nanoparticles embedded in ZnO matrix. J. Phys. Condens. Mat. 2003, 15, 641–651. [Google Scholar] [CrossRef]
- Makhlouf, S.A.; Parker, F.T.; Berkowitz, A.E. Magnetic hysteresis anomalies in ferritin. Phys. Rev. B 1997, 55, 14717–14720. [Google Scholar] [CrossRef]
- de Miguel, D.; Lemke, J.; Anel, A.; Walczak, H.; Martinez-Lostao, L. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016, 23, 733–747. [Google Scholar] [CrossRef]
- Barbault, F.; Maurel, F. Simulation with quantum mechanics/molecular mechanics for drug discovery. Expert Opin. Drug Discov. 2015, 10, 1047–1057. [Google Scholar] [CrossRef] [PubMed]

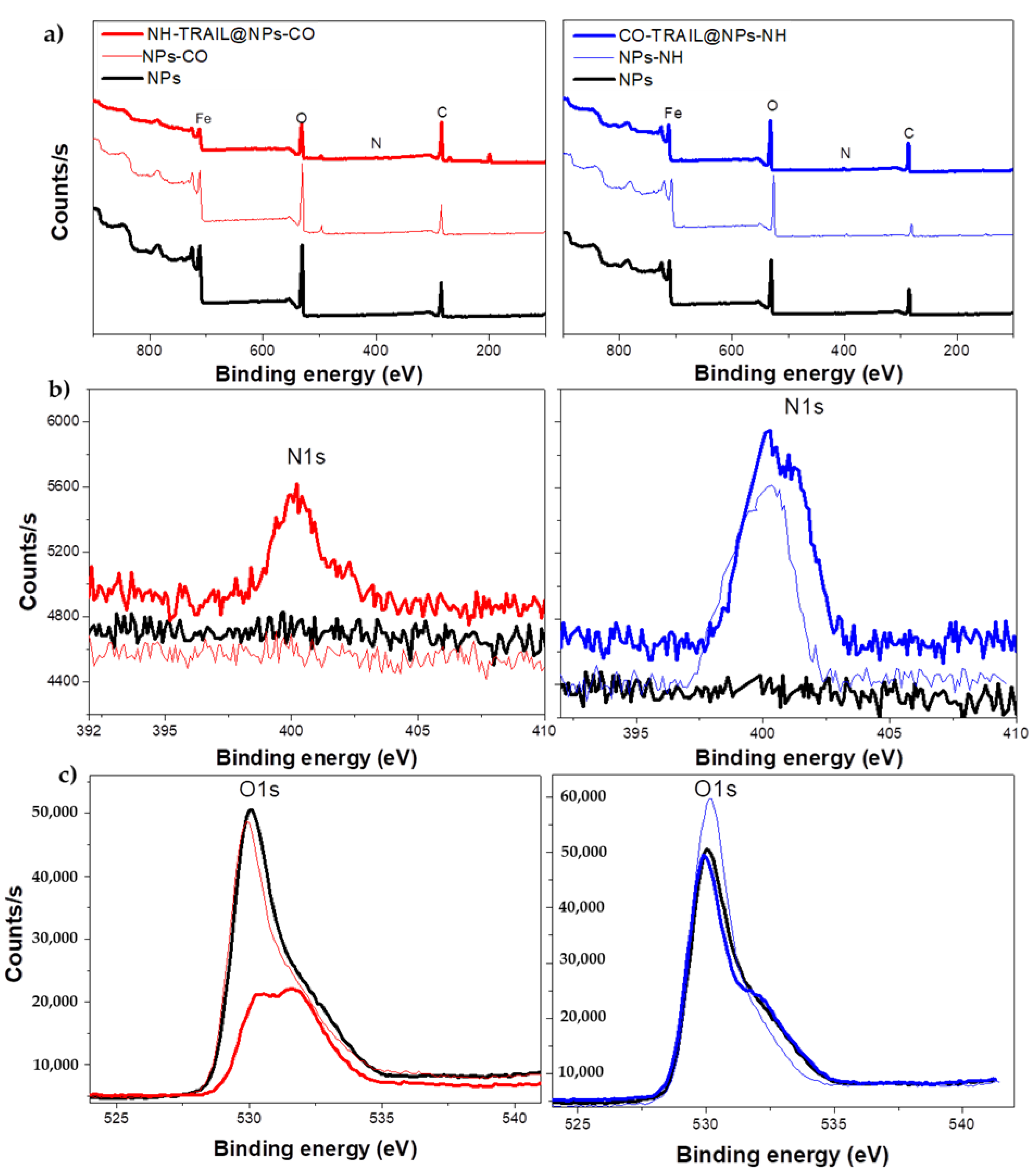

| Ratio | NP | NPs-CO | NH-TRAIL@NP-CO | NPs-NH | CO-TRAIL@NPs-NH |
|---|---|---|---|---|---|
| at.-N/Fe | 0 | 0 | 0.25 | 0.12 | 0.20 |
| at.-O/Fe | 2.88 | 2.96 | 5.59 | 2.17 | 3.69 |
| Samples | Magnetometry Analysis | TG | Bio-Rad Assay | |||
|---|---|---|---|---|---|---|
| Nanovectors | 5K-Msat (emu g−1) | Diamagnetic content (organic coating) (wt%) | TRAIL content (mol./part.) | Weight loss (wt%) | TRAIL content (mol./part.) | TRAIL content (mol./part.) |
| NH-TRAIL@NPs-CO | 65 ± 4 (*) | 22.8 | 15 ± 3 | 15.8 | 12 ± 4 | 13 ± 3 |
| CO-TRAIL@NPs-NH | 71 ± 5 (*) | 15.7 | 11 ± 2 | 13.0 | 10 ± 3 | 10 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkahla, H.; Constantinescu, A.A.; Gharbi, T.; Barbault, F.; Chevillot-Biraud, A.; Decorse, P.; Micheau, O.; Hémadi, M.; Ammar, S. Grafting TRAIL through Either Amino or Carboxylic Groups onto Maghemite Nanoparticles: Influence on Pro-Apoptotic Efficiency. Nanomaterials 2021, 11, 502. https://doi.org/10.3390/nano11020502
Belkahla H, Constantinescu AA, Gharbi T, Barbault F, Chevillot-Biraud A, Decorse P, Micheau O, Hémadi M, Ammar S. Grafting TRAIL through Either Amino or Carboxylic Groups onto Maghemite Nanoparticles: Influence on Pro-Apoptotic Efficiency. Nanomaterials. 2021; 11(2):502. https://doi.org/10.3390/nano11020502
Chicago/Turabian StyleBelkahla, Hanene, Andrei Alexandru Constantinescu, Tijani Gharbi, Florent Barbault, Alexandre Chevillot-Biraud, Philippe Decorse, Olivier Micheau, Miryana Hémadi, and Souad Ammar. 2021. "Grafting TRAIL through Either Amino or Carboxylic Groups onto Maghemite Nanoparticles: Influence on Pro-Apoptotic Efficiency" Nanomaterials 11, no. 2: 502. https://doi.org/10.3390/nano11020502
APA StyleBelkahla, H., Constantinescu, A. A., Gharbi, T., Barbault, F., Chevillot-Biraud, A., Decorse, P., Micheau, O., Hémadi, M., & Ammar, S. (2021). Grafting TRAIL through Either Amino or Carboxylic Groups onto Maghemite Nanoparticles: Influence on Pro-Apoptotic Efficiency. Nanomaterials, 11(2), 502. https://doi.org/10.3390/nano11020502









