Biomaterials for Three-Dimensional Cell Culture: From Applications in Oncology to Nanotechnology
Abstract
1. Introduction
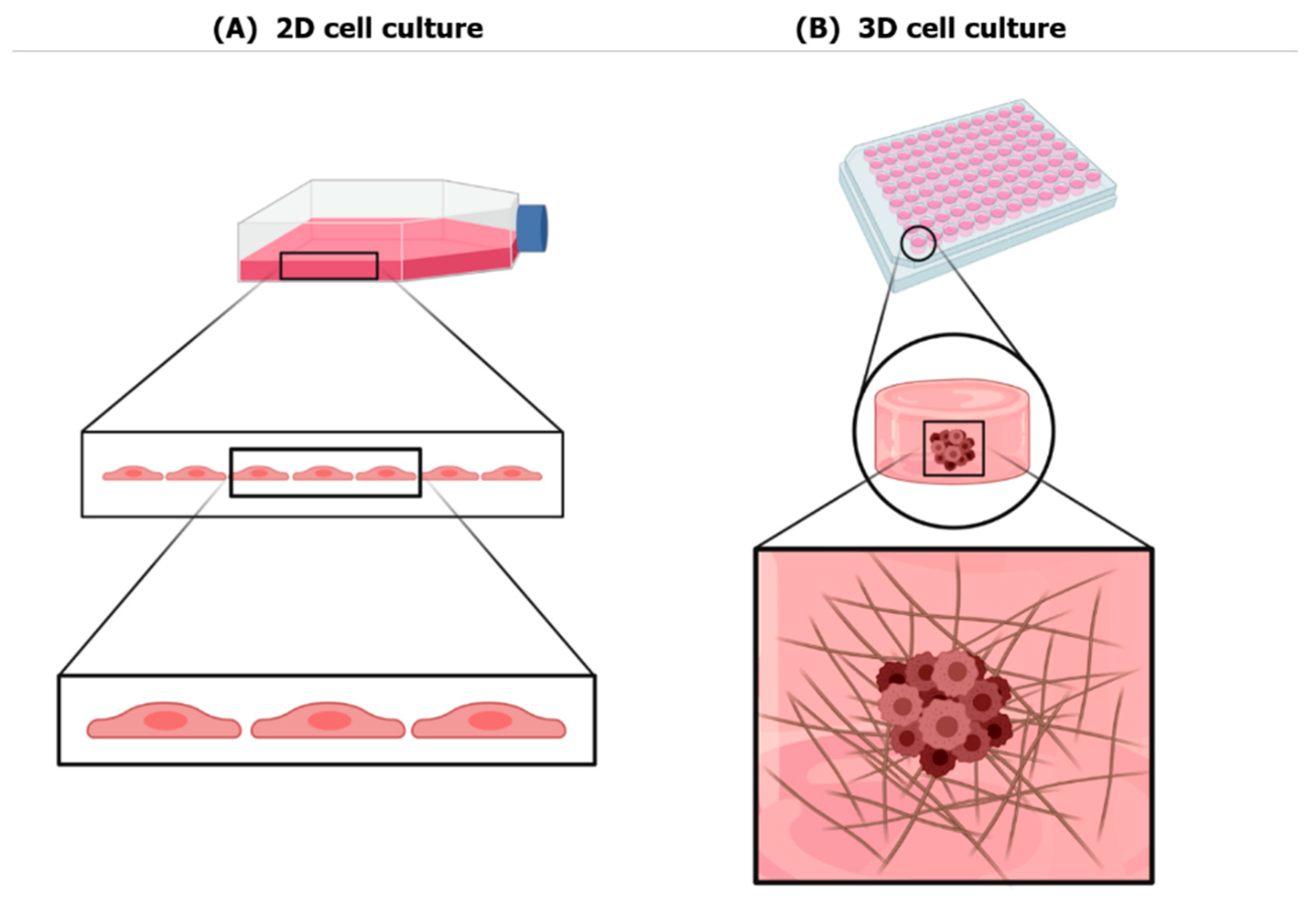
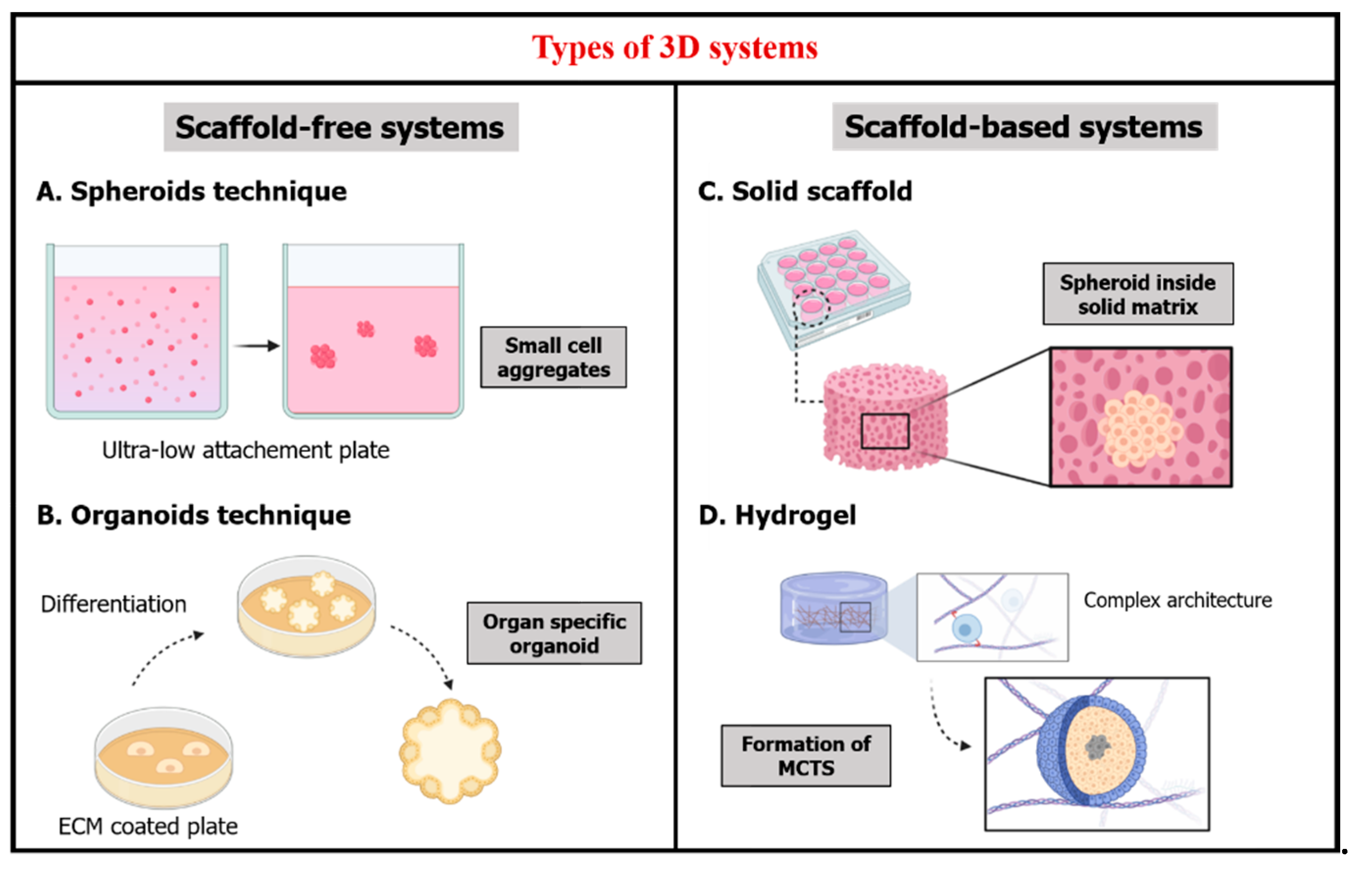
2. Three-Dimensional Culture Systems
2.1. Scaffold-Free Systems
2.2. Scaffold-Based Systems
- -
- Solid scaffold-based technology that provides a 3D space hosting cells and allowing them to create 3D tissue-like structures. Natural or synthetic, they consist of porous membranes or fibrous scaffolds that have been widely studied in the field of stem cells and regenerative medicine, for example, porous membranes produced by thermally induced phase separation [28,29]. Owing to their porous structure, these 3D matrixes facilitate tissue regeneration (e.g., cornea, skin and bone) [30,31,32]. Other types of solid scaffold-based technologies are 3D tissue models made of paper-based microfluidics. The latter are materials retrieved from plant tissue in the perspectives of developing human tissue structures compatible for 3D culture of mammalian cells [33]. Nanocellulose-based scaffolds, in particular nanocrystalline cellulose and nanofibrillated cellulose [34], and silk-based composite scaffolds [35] have proven their potential in regenerative medicine such as wound healing and organ reparation, due to their special permeability and hemocompatibility.
- -
- Hydrogels that can be designed as a soft scaffold for hosting cells.
- Proteins used historically in stem cell-based tissue engineering [2,9,22,45] such as collagen used for example in differentiation of human embryonic stem cells into hepatocytes [48,49], gelatin proved efficient in chondrogenic differentiation of adipose-derived adult stem cells [50,51], fibrin for differentiation of murine embryonic stem cells into neural lineage cells [52,53], and silk [54,55].
- Polysaccharides that are naturally found in the ECM [9,45] such as hyaluronic acid that is either used as an integral scaffold for tissue engineering [56,57] or used as a functionalization tool of synthetic biomaterials to create a hybrid more biomimetic matrix [58] and chitosan for tissue engineering applications [59].
- Matrigels, that originated from murine sarcomas ECM, are basement membrane matrixes composed mainly of four ECM proteins: laminin (60%), collagen IV (30%), entactin (8%) and heparin sulfate proteoglycan perlecan (2%) [60]. By virtue of their built-in bioactivity, they exhibited diverse applications in cellular biology [61]. They have been used in cancer invasion studies to assess the metastasis capabilities of cancer cells [62]. Matrigels were used in cell culture of human pluripotent stem cells (hPSCs) to understand the impact of the micro-environmental factors on cell differentiation [63], for cardiomyocytes as in vitro models of heart activity [64] and organoid production [65]. However, matrigels construction requires a large number of structural proteins but also involves other factors such as growth factors [66] and transcription factors [67]. Previous studies displayed major inconvenience that can limit cellular behavior studies linked to the matrigel’s complex, unclear and highly fluctuating composition [66,67,68].
- Non-biodegradable biomaterials such as poly(ethylene glycol) (PEG) [75], 2-hydroxyethyl methacrylate (HEMA) and acrylamide (AAm) [76]. They maintain physical and mechanical integrity for non-biodegradable applications in tissue engineering [45]. Today non-biodegradable biomaterials are more used combined with biodegradable materials to help tune and control some of their properties such as porosity and permeability.
3. Cancer Applications of Hydrogels
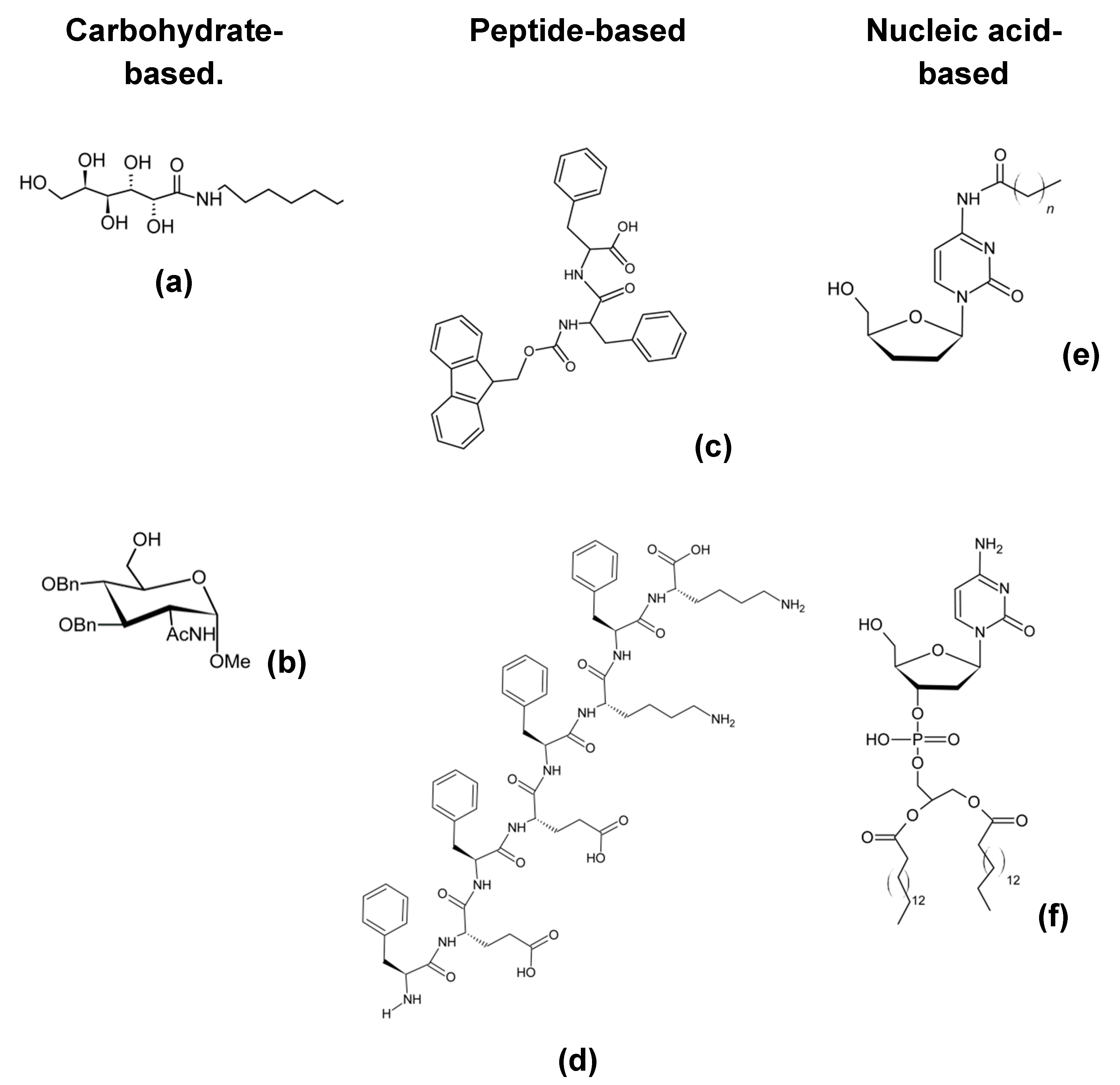
4. Nanoparticles Emergence
4.1. Definition of Nanoparticles
4.2. Nanoparticles Origin and Environmental Dispersion
4.3. Why Are NPs a Center of Attention Today?
4.4. In Vitro Cellular Models for Nanoparticles Toxicity Assays
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.; Anuradha, E.; Solomon, F.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Muthuswamy, S.K. 3D culture reveals a signaling network. Breast Cancer Res. 2011, 13, 103. [Google Scholar] [CrossRef]
- Sittampalam, S.; Eglen, R.; Ferguson, S.; Maynes, J.T.; Olden, K.; Schrader, L.; Shelper, T.; Ferrer, M. Three-Dimensional Cell Culture Assays: Are They More Predictive of In Vivo Efficacy than 2D Monolayer Cell-Based Assays? Assay Drug Dev. Technol. 2015, 13, 254–261. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Cekanova, M.; Rathore, K. Animal models and therapeutic molecular targets of cancer: Utility and limitations. Drug Des. Dev. Ther. 2014, 8, 1911–1921. [Google Scholar] [CrossRef]
- Voskoglou-Nomikos, T.; Pater, J.L.; Seymour, L. Clinical Predictive Value of the in Vitro Cell Line, Human Xenograft, and Mouse Allograft Preclinical Cancer Models. Clin. Cancer Res. 2003, 9, 4227. [Google Scholar]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Bielecka, Z.F.; Maliszewska-Olejniczak, K.; Safir, I.J.; Szczylik, C.; Czarnecka, A.M. Three-dimensional cell culture model utilization in cancer stem cell research. Biol. Rev. 2017, 92, 1505–1520. [Google Scholar] [CrossRef]
- Nguyen, H.T.-L.; Nguyen, S.T.; Van Pham, P. Concise Review: 3D cell culture systems for anticancer drug screening. Biomed. Res. Ther. 2016, 3, 1–8. [Google Scholar] [CrossRef]
- Till, U.; Gibot, L.; Vicendo, P.; Rols, M.-P.; Gaucher, M.; Violleau, F.; Mingotaud, A.-F. Crosslinked polymeric self-assemblies as an efficient strategy for photodynamic therapy on a 3D cell culture. RSC Adv. 2016, 6, 69984–69998. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar] [PubMed]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Borm, P.J.; Castranova, V.; Gulumian, M. The limits of testing particle-mediated oxidative stress in vitro in predicting diverse pathologies; relevance for testing of nanoparticles. Part. Fibre Toxicol. 2009, 6, 13. [Google Scholar] [CrossRef]
- Mapanao, A.K.; Voliani, V. Three-dimensional tumor models: Promoting breakthroughs in nanotheranostics translational research. Appl. Mater. Today 2020, 19, 100552. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- A Multicellular 3D Heterospheroid Model of Liver Tumor and Stromal Cells in Collagen Gel for Anti-Cancer Drug Testing—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0006291x13004026?via%3Dihub (accessed on 1 July 2020).
- Soares, C.P.; Midlej, V.; De Oliveira, M.E.W.; Benchimol, M.; Costa, M.L.; Mermelstein, C. 2D and 3D-Organized Cardiac Cells Shows Differences in Cellular Morphology, Adhesion Junctions, Presence of Myofibrils and Protein Expression. PLoS ONE 2012, 7, e38147. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, M.; Carnachan, R.J.; Cameron, N.R.; Przyborski, S.A. Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J. Anat. 2007, 211, 567–576. [Google Scholar] [CrossRef]
- Multi-Channel 3-D Cell Culture Device Integrated on a Silicon Chip for Anticancer Drug Sensitivity Test—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0142961204005046?via%3Dihub (accessed on 1 July 2020).
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. Slas Discov. Adv. Life Sci. R. D 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012, 10, 29. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Osteochondral Tissue Engineering. SpringerLink. Available online: https://link.springer.com/book/10.1007%2F978-3-319-76711-6 (accessed on 1 July 2020).
- Matrice Poreuse et Culture de Cellules Primaires: Un Même Concept Pour La Reconstruction Cutanée et Cornéenne—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0369811408001272?via%3Dihub (accessed on 1 July 2020).
- Jiang, S.; Lyu, C.; Zhao, P.; Li, W.; Kong, W.; Huang, C.; Genin, G.M.; Du, Y. Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Alghuwainem, A.; Alshareeda, A.T.; Alsowayan, B. Scaffold-Free 3-D Cell Sheet Technique Bridges the Gap between 2-D Cell Culture and Animal Models. Int. J. Mol. Sci. 2019, 20, 4926. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 226–236. [Google Scholar] [CrossRef]
- The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies: Trends in Biotechnology. Available online: https://www.cell.com/trends/biotechnology/fulltext/S0167-7799(18)30026-X?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS016777991830026X%3Fshowall%3Dtrue (accessed on 1 July 2020).
- Youn, B.; Sen, A.; Behie, L.; Girgis-Gabardo, A.; Hassell, J. Scale-Up of Breast Cancer Stem Cell Aggregate Cultures to Suspension Bioreactors. Biotechnol. Prog. 2006, 22, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.M.; Larson, T.S.; Lockett, M.R. Tissue Papers: Leveraging Paper-Based Microfluidics for the Next Generation of 3D Tissue Models. Anal. Chem. 2019, 91, 10916–10926. [Google Scholar] [CrossRef]
- Luo, H.; Cha, R.; Li, J.; Hao, W.; Zhang, Y.; Zhou, F. Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydr. Polym. 2019, 224, 115144. [Google Scholar] [CrossRef]
- Tandon, S.; Kandasubramanian, B.; Yakout, S.M. Silk-based Composite Scaffolds for Tissue Engineering Applications. Ind. Eng. Chem. Res. 2020, 59, 17593–17611. [Google Scholar] [CrossRef]
- Chirani, N.; Gritsch, L.; Motta, F.L.; Fare, S. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4. [Google Scholar] [CrossRef]
- Cell Encapsulation Using Biopolymer Gels for Regenerative Medicine. SpringerLink. Available online: https://link.springer.com/article/10.1007%2Fs10529-010-0221-0 (accessed on 1 July 2020).
- Hydrogels for Biomedical Applications—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0169409X01002393?via%3Dihub (accessed on 1 July 2020).
- Nelson, S.R.; Zhang, C.; Roche, S.; O’Neill, F.; Swan, N.; Luo, Y.; Larkin, A.; Crown, J.; Walsh, N. Modelling of pancreatic cancer biology: Transcriptomic signature for 3D PDX-derived organoids and primary cell line organoid development. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Kalabis, J.; Wong, G.S.; Vega, M.E.; Natsuizaka, M.; Robertson, E.S.; Herlyn, M.; Nakagawa, H.; Rustgi, A.K. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat. Protoc. 2012, 7, 235–246. [Google Scholar] [CrossRef]
- Nisbet, D.R.; Forsythe, J.S.; Shen, W.; Finkelstein, D.I.; Horne, M. Review Paper: A Review of the Cellular Response on Electrospun Nanofibers for Tissue Engineering. J. Biomater. Appl. 2008, 24, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Kopeček, J. Swell gels. Nat. Cell Biol. 2002, 417, 389–391. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Buxboim, A.; Ivanovska, I.L.; Discher, D.E. Matrix elasticity, cytoskeletal forces and physics of the nucleus: How deeply do cells ‘feel’ outside and in? J. Cell Sci. 2010, 123, 297–308. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Osswald, A.; Hedrich, V.; Sommergruber, W. 3D-3 Tumor Models in Drug Discovery for Analysis of Immune Cell Infiltration. Methods Mol. Biol. 2019, 1953, 151–162. [Google Scholar] [PubMed]
- Zou, L.; Luo, Y.; Chen, M.; Wang, G.; Ding, M.; Petersen, C.C.; Kang, R.; Dagnaes-Hansen, F.; Zeng, Y.; Lv, N.; et al. A simple method for deriving functional MSCs and applied for osteogenesis in 3D scaffolds. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Baharvand, H.; Hashemi, S.M.; Ashtiani, S.K.; Farrokhi, A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int. J. Dev. Biol. 2006, 50, 645–652. [Google Scholar] [CrossRef]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef]
- Sakai, S.; Hirose, K.; Taguchi, K.; Ogushi, Y.; Kawakami, K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials 2009, 30, 3371–3377. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.A.; Wickham, M.Q.; Leddy, H.A.; Gimble, J.M.; Guilak, F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 2004, 25, 3211–3222. [Google Scholar] [CrossRef]
- Willerth, S.M.; Arendas, K.J.; Gottlieb, D.I.; Sakiyama-Elbert, S.E. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials 2006, 27, 5990–6003. [Google Scholar] [CrossRef] [PubMed]
- Osathanon, T.; Linnes, M.L.; Rajachar, R.M.; Ratner, B.D.; Somerman, M.J.; Giachelli, C.M. Microporous nanofibrous fibrin-based scaffolds for bone tissue engineering. Biomaterials 2008, 29, 4091–4099. [Google Scholar] [CrossRef]
- Mauney, J.R.; Nguyen, T.; Gillen, K.; Kirker-Head, C.; Gimble, J.M.; Kaplan, D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 2007, 28, 5280–5290. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.-J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef]
- Gerecht, S.; Burdick, J.A.; Ferreira, L.S.; Townsend, S.A.; Langer, R.; Vunjak-Novakovic, G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11298–11303. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Qu, Y.; Hua, X.; Zhang, L.; Liu, Z.; Pflugfelder, S.C.; Li, D.-Q. A hyaluronan hydrogel scaffold-based xeno-free culture system for ex vivo expansion of human corneal epithelial stem cells. Eye 2017, 31, 962–971. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.R.; Tsai, M.-C.; Frampton, J.P. Fabrication of thin-layer matrigel-based constructs for three-dimensional cell culture. Biotechnol. Prog. 2019, 35, e2733. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Siemann, D. C-Src is required for hypoxia-induced metastasis-associated functions in prostate cancer cells. OncoTargets Ther. 2019, 12, 3519–3529. [Google Scholar] [CrossRef]
- Bogacheva, M.S.; Khan, S.; Kanninen, L.K.; Yliperttula, M.; Leung, A.W.; Lou, Y.-R. Differences in definitive endoderm induction approaches using growth factors and small molecules. J. Cell. Physiol. 2018, 233, 3578–3589. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.J.S.; Ang, Y.-S.; Fu, J.-D.; Rivas, R.N.; Mohamed, T.M.A.; Higgs, G.C.; Srivastava, D.; Pruitt, B.L. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 12705–12710. [Google Scholar] [CrossRef]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Kerjaschki, D.; Penninger, J.M. Generation of blood vessel organoids from human pluripotent stem cells. Nat. Protoc. 2019, 14, 3082–3100. [Google Scholar] [CrossRef]
- Talbot, N.C.; Caperna, T.J. Proteome array identification of bioactive soluble proteins/peptides in Matrigel: Relevance to stem cell responses. Cytotechnology 2014, 67, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.C.; Kiemele, L.; Maller, O.; O’Brien, J.; Shankar, A.; Fornetti, J.; Schedin, P. An In-solution Ultrasonication-assisted Digestion Method for Improved Extracellular Matrix Proteome Coverage *. Mol. Cell. Proteom. 2009, 8, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Soofi, S.S.; Last, J.A.; Liliensiek, S.J.; Nealey, P.F.; Murphy, C.J. The elastic modulus of Matrigel™ as determined by atomic force microscopy. J. Struct. Biol. 2009, 167, 216–219. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 1–10. [Google Scholar] [CrossRef]
- Ruedinger, F.; Lavrentieva, A.; Blume, C.; Pepelanova, I.; Scheper, T. Hydrogels for 3D mammalian cell culture: A starting guide for laboratory practice. Appl. Microbiol. Biotechnol. 2015, 99, 623–636. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, W.; Wang, Y.; Wang, C.; Zhang, X.; Li, Q.; Han, D. Three-Dimensional-Printed Poly-L-Lactic Acid Scaffolds with Different Pore Sizes Influence Periosteal Distraction Osteogenesis of a Rabbit Skull. BioMed Res. Int. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Ossipov, D.A.; Brännvall, K.; Forsberg-Nilsson, K.; Hilborn, J. Formation of the first injectable poly(vinyl alcohol) hydrogel by mixing of functional PVA precursors. J. Appl. Polym. Sci. 2007, 106, 60–70. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Hayward, A.S.; Sano, N.; Przyborski, S.A.; Cameron, N.R. Acrylic-Acid-Functionalized PolyHIPE Scaffolds for Use in 3D Cell Culture. Macromol. Rapid Commun. 2013, 34, 1844–1849. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Latxague, L.; Ramin, M.A.; Appavoo, A.; Berto, P.; Maisani, M.; Ehret, C.; Chassande, O.; Barthélémy, P. Control of Stem-Cell Behavior by Fine Tuning the Supramolecular Assemblies of Low-Molecular-Weight Gelators. Angew. Chem. Int. Ed. 2015, 54, 4517–4521. [Google Scholar] [CrossRef] [PubMed]
- A Versatile Carbohydrate Based Gelator for Oil Water Separation, Nanoparticle Synthesis and Dye Removal—New Journal of Chemistry (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2017/NJ/C6NJ03520E (accessed on 1 July 2020).
- Rajkamal, R.; Pathak, N.P.; Chatterjee, D.; Paul, A.; Yadav, S. Arabinose based gelators: Rheological characterization of the gels and phase selective organogelation of crude-oil. RSC Adv. 2016, 6, 92225–92234. [Google Scholar] [CrossRef]
- Chalard, A.; Vaysse, L.; Joseph, P.; Malaquin, L.; Souleille, S.; Lonetti, B.; Sol, J.-C.; Loubinoux, I.; Fitremann, J. Simple Synthetic Molecular Hydrogels from Self-Assembling Alkylgalactonamides as Scaffold for 3D Neuronal Cell Growth. ACS Appl. Mater. Interfaces 2018, 10, 17004–17017. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, H.; Ren, C.; Wang, J.; Tan, M.; Shen, J.; Yang, Z.; Wang, P.G.; Wang, L. A saccharide-based supramolecular hydrogel for cell culture. Carbohydr. Res. 2011, 346, 1013–1017. [Google Scholar] [CrossRef]
- Ryadnov, M. Peptide α-helices for synthetic nanostructures. Biochem. Soc. Trans. 2007, 35, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Soto, P.; Gazit, E. Peptide self-assembly at the nanoscale: A challenging target for computational and experimental biotechnology. Trends Biotechnol. 2007, 25, 211–218. [Google Scholar] [CrossRef]
- Manandhar, A.; Kang, M.; Chakraborty, K.; Tang, P.K.; LoVerde, S.M. Molecular simulations of peptide amphiphiles. Org. Biomol. Chem. 2017, 15, 7993–8005. [Google Scholar] [CrossRef]
- Jayawarna, V.; Richardson, S.M.; Hirst, A.R.; Hodson, N.W.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Introducing chemical functionality in Fmoc-peptide gels for cell culture. Acta Biomater. 2009, 5, 934–943. [Google Scholar] [CrossRef]
- Gao, J.; Tang, C.; ElSawy, M.A.; Smith, A.M.; Miller, A.F.; Saiani, A. Controlling Self-Assembling Peptide Hydrogel Properties through Network Topology. Biomacromolecules 2017, 18, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Yoshikawa, I. Nucleobase-Containing Gelators. Top. Curr. Chem. 2005, 256, 133–165. [Google Scholar] [CrossRef] [PubMed]
- Skilling, K.J.; Ndungu, A.; Kellam, B.; Ashford, M.; Bradshaw, T.D.; Marlow, M. Gelation properties of self-assembling N-acyl modified cytidine derivatives. J. Mater. Chem. B 2014, 2, 8412–8417. [Google Scholar] [CrossRef]
- Alies, B.; Ouelhazi, M.A.; Patwa, A.N.; Verget, J.; Navailles, L.; Desvergnes, V.; Barthelemy, P. Cytidine- and guanosine-based nucleotide–lipids. Org. Biomol. Chem. 2018, 16, 4888–4894. [Google Scholar] [CrossRef]
- Campins, N.; Dieudonné, P.; Grinstaff, M.W.; Barthélémy, P. Nanostructured assemblies from nucleotide-based amphiphiles. New J. Chem. 2007, 31, 1928–1934. [Google Scholar] [CrossRef]
- Patel, S.; Volpe, A.B.; Awwad, S.; Schätzlein, A.G.; Haider, S.; Liu, B.; Uchegbu, I.F. A Self-Assembling Lipidic Peptide and Selective Partial V2 Receptor Agonist Inhibits Urine Production. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Zhang, S. Discovery and design of self-assembling peptides. Interface Focus 2017, 7, 20170028. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, H.; Zhao, X. Designer Self-Assembling Peptide Hydrogels to Engineer 3D Cell Microenvironments for Cell Constructs Formation and Precise Oncology Remodeling in Ovarian Cancer. Adv. Sci. 2020, 7, 1903718. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, Y.; Tang, C.; Zhao, X. Amphiphilic peptides as novel nanomaterials: Design, self-assembly and application. Int. J. Nanomed. 2018, 13, 5003–5022. [Google Scholar] [CrossRef]
- Latxague, L.; Gaubert, A.; Maleville, D.; Baillet, J.; Ramin, M.A.; Barthélémy, P. Carbamate-Based Bolaamphiphile as Low-Molecular-Weight Hydrogelators. Gels 2016, 2, 25. [Google Scholar] [CrossRef]
- Latxague, L.; Dalila, M.-J.; Patwa, A.; Ziane, S.; Chassande, O.; Godeau, G.; Barthélémy, P. Glycoside nucleoside lipids (GNLs): An intrusion into the glycolipids’ world? Comptes Rendus Chim. 2012, 15, 29–36. [Google Scholar] [CrossRef]
- Nagarajan, R. Self-Assembly of Bola Amphiphiles. Chem. Eng. Commun. 1987, 55, 251–273. [Google Scholar] [CrossRef]
- Ochi, R.; Kurotani, K.; Ikeda, M.; Kiyonaka, S.; Hamachi, I. Supramolecular hydrogels based on bola-amphiphilic glycolipids showing color change in response to glycosidases. Chem. Commun. 2013, 49, 2115–2117. [Google Scholar] [CrossRef]
- Ramakanth, I.; Patnaik, A. Novel Two-Component Gels of Cetylpyridinium Chloride and the Bola-amphiphile 6-Amino Caproic Acid: Phase Evolution and Mechanism of Gel Formation. J. Phys. Chem. B 2012, 116, 2722–2729. [Google Scholar] [CrossRef] [PubMed]
- Nebot, V.J.; Armengol, J.; Smets, J.; Prieto, S.F.; Escuder, B.; Miravet, J.F. Molecular Hydrogels from Bolaform Amino Acid Derivatives: A Structure-Properties Study Based on the Thermodynamics of Gel Solubilization. Chem. Eur. J. 2012, 18, 4063–4072. [Google Scholar] [CrossRef]
- Ramin, M.A.; Latxague, L.; Sindhu, K.R.; Chassande, O.; Barthélémy, P. Low molecular weight hydrogels derived from urea based-bolaamphiphiles as new injectable biomaterials. Biomaterials 2017, 145, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fuchs, H. Soft Matter Nanotechnology: From Structure to Function; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 9783527682140. [Google Scholar]
- Jain, N.; Goldschmidt, V.; Oncul, S.; Arntz, Y.; Duportail, G.; Mély, Y.; Klymchenko, A.S. Lactose-ornithine bolaamphiphiles for efficient gene delivery in vitro. Int. J. Pharm. 2012, 423, 392–400. [Google Scholar] [CrossRef]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; Di Carlo, D.; Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015, 14, 737–744. [Google Scholar] [CrossRef]
- Ziane, S.; Schlaubitz, S.; Miraux, S.; Patwa, A.; Lalande, C.; Bilem, I.; Lepreux, S.; Rousseau, B.; Le Meins, J.-F.; Latxague, L.; et al. A thermosensitive low molecular weight hydrogel as scaffold for tissue engineering. Eur. Cells Mater. 2012, 23, 147–160. [Google Scholar] [CrossRef]
- Mirabelli, P.; Coppola, L.; Salvatore, M. Cancer Cell Lines Are Useful Model Systems for Medical Research. Cancers 2019, 11, 1098. [Google Scholar] [CrossRef]
- Shetab-Bou, S.V.; Abdollahi, M. Current Concerns on the Validity of in vitro Models that use Transformed Neoplastic Cells in Pharmacology and Toxicology. Int. J. Pharmacol. 2012, 8, 594–595. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, L.; Thakkar, S.; Roberts, R.; Tong, W. Can Transcriptomic Profiles from Cancer Cell Lines Be Used for Toxicity Assessment? Chem. Res. Toxicol. 2019, 33, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Hamilton, G.; Rath, B. Applicability of tumor spheroids for in vitro chemosensitivity assays. Expert Opin. Drug Metab. Toxicol. 2018, 15, 15–23. [Google Scholar] [CrossRef]
- Ediriwickrema, A.; Saltzman, W.M. Nanotherapy for Cancer: Targeting and Multifunctionality in the Future of Cancer Therapies. ACS Biomater. Sci. Eng. 2015, 1, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.S.; Garlin, M.A.; Weissleder, R.; Miller, M.A. Improving nanotherapy delivery and action through image-guided systems pharmacology. Theranostics 2020, 10, 968–997. [Google Scholar] [CrossRef]
- Schwachöfer, J.H. Multicellular tumor spheroids in radiotherapy research (review). Anticancer Res. 1990, 10, 963–969. [Google Scholar] [PubMed]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Fröhlich, E. Comparison of conventional and advanced in vitro models in the toxicity testing of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1091–1107. [Google Scholar] [CrossRef]
- Peng, F.; Setyawati, M.I.; Tee, J.K.; Ding, X.; Wang, J.; Nga, M.E.; Ho, H.K.; Leong, D.T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019, 14, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Von Maltzahn, G.; Park, J.-H.; Lin, K.Y.-M.; Singh, N.; Schwöppe, C.; Mesters, R.M.; Berdel, W.E.; Ruoslahti, E.; Sailor, M.J.; Bhatia, S.N. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat. Mater. 2011, 10, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Prince, E.; Kumacheva, E. Design and applications of man-made biomimetic fibrillar hydrogels. Nat. Rev. Mater. 2019, 4, 99–115. [Google Scholar] [CrossRef]
- Saleh, A.; Marhuenda, E.; Fabre, C.; Hassani, Z.; De Weille, J.; Boukhaddaoui, H.; Guelfi, S.; Maldonado, I.L.; Hugnot, J.-P.; Duffau, H.; et al. A novel 3D nanofibre scaffold conserves the plasticity of glioblastoma stem cell invasion by regulating galectin-3 and integrin-β1 expression. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kumacheva, E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018, 4, eaas8998. [Google Scholar] [CrossRef]
- Huang, B.-W.; Gao, J.-Q. Application of 3D cultured multicellular spheroid tumor models in tumor-targeted drug delivery system research. J. Control. Release 2018, 270, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef]
- Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D.J. Engineering tumors with 3D scaffolds. Nat. Methods 2007, 4, 855–860. [Google Scholar] [CrossRef]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef]
- Balcão, V.M.; Barreira, S.V.P.; Nunes, T.M.; Chaud, M.V.; Tubino, M.; Vila, M.M.D.C. Carbohydrate Hydrogels with Stabilized Phage Particles for Bacterial Biosensing: Bacterium Diffusion Studies. Appl. Biochem. Biotechnol. 2013, 172, 1194–1214. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, D.; Wagner, D.; Sitta, C.E.; Thoma, R.; Felekyan, S.; Hermes, H.E.; Janiak, C.; Amadeu, N.D.S.; Kühnemuth, R.; Löwen, H.; et al. Diffusion of macromolecules in a polymer hydrogel: From microscopic to macroscopic scales. Phys. Chem. Chem. Phys. 2016, 18, 12860–12876. [Google Scholar] [CrossRef] [PubMed]
- Engberg, K.; Frank, C.W. Protein diffusion in photopolymerized poly(ethylene glycol) hydrogel networks. Biomed. Mater. 2011, 6, 055006. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, J.; Zhang, Y.; Dong, D.; Zhang, E.; Ji, F.; Qin, Z.; Yang, J.; Yao, F. Establishment of a Physical Model for Solute Diffusion in Hydrogel: Understanding the Diffusion of Proteins in Poly(sulfobetaine methacrylate) Hydrogel. J. Phys. Chem. B 2017, 121, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Golmohamadi, M.; Wilkinson, K.J. Diffusion of ions in a calcium alginate hydrogel-structure is the primary factor controlling diffusion. Carbohydr. Polym. 2013, 94, 82–87. [Google Scholar] [CrossRef]
- Faucher, S.; Le Coustumer, P.; Lespes, G. Nanoanalytics: History, concepts, and specificities. Environ. Sci. Pollut. Res. 2018, 26, 5267–5281. [Google Scholar] [CrossRef]
- 14:00–17:00 ISO/TS 80004-4. 2011. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/05/21/52195.html (accessed on 2 July 2020).
- Bruinink, A.; Wang, J.; Wick, P. Effect of particle agglomeration in nanotoxicology. Arch. Toxicol. 2015, 89, 659–675. [Google Scholar] [CrossRef]
- Sharma, V.K. Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment—A Review. J. Environ. Sci. Health Part A 2009, 44, 1485–1495. [Google Scholar] [CrossRef]
- Baalousha, M.; Yang, Y.; Vance, M.E.; Colman, B.P.; McNeal, S.; Xu, J.; Blaszczak, J.; Steele, M.; Bernhardt, E.; Hochella, M.F. Outdoor urban nanomaterials: The emergence of a new, integrated, and critical field of study. Sci. Total Environ. 2016, 557–558, 740–753. [Google Scholar] [CrossRef]
- Shandilya, N.; Le Bihan, O.; Bressot, C.; Morgeneyer, M. Emission of Titanium Dioxide Nanoparticles from Building Materials to the Environment by Wear and Weather. Environ. Sci. Technol. 2015, 49, 2163–2170. [Google Scholar] [CrossRef]
- Babaizadeh, H.; Hassan, M. Life cycle assessment of nano-sized titanium dioxide coating on residential windows. Constr. Build. Mater. 2013, 40, 314–321. [Google Scholar] [CrossRef]
- Windler, L.; Lorenz, C.; Von Goetz, N.; Hungerbühler, K.; Amberg, M.; Heuberger, M.; Nowack, B. Release of Titanium Dioxide from Textiles during Washing. Environ. Sci. Technol. 2012, 46, 8181–8188. [Google Scholar] [CrossRef]
- Osmond, M.J.; McCall, M.J. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2009, 4, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Marcellini, F.; Nepote, E.; Damiani, E.; Danovaro, R. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Sci. Total Environ. 2018, 637–638, 1279–1285. [Google Scholar] [CrossRef]
- Sadik, O.A. Anthropogenic nanoparticles in the environment. Environ. Sci. Process. Impacts 2012, 15, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.M.; MacKenzie, A.R.; Xu, H.; Alam, M.S.; Nikolova, I.; Zhong, J.; Singh, A.; Zeraati-Rezaei, S.; Stark, C.; Beddows, D.C.S.; et al. Diesel exhaust nanoparticles and their behaviour in the atmosphere. Proc. R. Soc. A Math. Phys. Eng. Sci. 2018, 474, 20180492. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Becheri, A.; Dürr, M.; Nostro, P.L.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanoparticle Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef]
- Egerton, T.; Christensen, P.; Kosa, S.; Onoka, B.; Harper, J.; Tinlin, J. Photoelectrocatalysis by titanium dioxide for water treatment. Int. J. Environ. Pollut. 2006, 27, 2–19. [Google Scholar] [CrossRef]
- Chertok, B.; Moffat, B.A.; David, A.E.; Yu, F.; Bergemann, C.; Ross, B.D.; Yang, V.C. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 2008, 29, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Poon, C.-S. Photocatalytic activity of titanium dioxide modified concrete materials—Influence of utilizing recycled glass cullets as aggregates. J. Environ. Manag. 2009, 90, 3436–3442. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Burton, M.; Jobson, B.; Haselbach, L. Pervious concrete with titanium dioxide as a photocatalyst compound for a greener urban road environment. Constr. Build. Mater. 2012, 35, 874–883. [Google Scholar] [CrossRef]
- You, H.; Yang, S.; Ding, B.; Yang, H. Synthesis of colloidal metal and metal alloy nanoparticles for electrochemical energy applications. Chem. Soc. Rev. 2013, 42, 2880–2904. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef]
- Webb, J.A.; Bardhan, R. Emerging advances in nanomedicine with engineered gold nanostructures. Nanoscale 2014, 6, 2502–2530. [Google Scholar] [CrossRef]
- Das, S.; Dowding, J.M.; Klump, K.E.; McGinnis, J.F.; Self, W.; Seal, S. Cerium oxide nanoparticles: Applications and prospects in nanomedicine. Nanomedicine 2013, 8, 1483–1508. [Google Scholar] [CrossRef]
- Guarino-Hotz, M.; Zhang, J.Z. Structural control and biomedical applications of plasmonic hollow gold nanospheres: A mini review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, e1694. [Google Scholar] [CrossRef]
- Ramazanov, M.; Karimova, A.; Shirinova, H. Magnetism for Drug Delivery, MRI and Hyperthermia Applications: A Review. Biointerface Res. Appl. Chem. 2020, 11, 8654–8668. [Google Scholar] [CrossRef]
- Cormode, D.P.; Naha, P.C.; Fayad, Z.A. Nanoparticle contrast agents for computed tomography: A focus on micelles. Contrast Media Mol. Imaging 2014, 9, 37–52. [Google Scholar] [CrossRef]
- Hochella, M.F., Jr.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Q.; Lohani, M.; Dopp, E.; Pemsel, H.; Jonas, L.; Weiss, D.G.; Schiffmann, D. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ. Health Perspect. 2002, 110, 797–800. [Google Scholar] [CrossRef]
- Yamamoto, A.; Honma, R.; Sumita, M.; Hanawa, T. Cytotoxicity evaluation of ceramic particles of different sizes and shapes. J. Biomed. Mater. Res. 2003, 68, 244–256. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Vevers, W.F.; Jha, A.N. Genotoxic and cytotoxic potential of titanium dioxide (TiO2) nanoparticles on fish cells in vitro. Ecotoxicology 2008, 17, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2008, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.B.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2010, 85, 743–750. [Google Scholar] [CrossRef]
- Park, M.V.; Neigh, A.M.; Vermeulen, J.P.; De La Fonteyne, L.J.; Verharen, H.W.; Briedé, J.J.; Van Loveren, H.; De Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef] [PubMed]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.A.; Köller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alrokayan, S.A. MgO nanoparticles cytotoxicity caused primarily by GSH depletion in human lung epithelial cells. J. Trace Elem. Med. Biol. 2018, 50, 283–290. [Google Scholar] [CrossRef]
- Lovern, S.B.; Klaper, R. Daphnia Magna Mortality When Exposed to Titanium Dioxide and Fullerene (C60) Nanoparticles. Environ. Toxicol. Chem. 2006, 25, 1132–1137. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, G.; Tiancheng, W.; Yu, H.; Wang, T.; Ma, Y.; Jiangxue, W.; Gao, Y.; Li, Y.; Sun, J. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef]
- Chen, T.-H.; Lin, C.-Y.; Tseng, M.-C. Behavioral effects of titanium dioxide nanoparticles on larval zebrafish (Danio rerio). Mar. Pollut. Bull. 2011, 63, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.K.; Jin, T.; Behari, J. Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol. Mech. Methods 2010, 21, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the In Vitro and In Vivo Toxicity of Superparamagnetic Iron Oxide Nanoparticles. Chem. Rev. 2011, 112, 2323–2338. [Google Scholar] [CrossRef]
- Simpson, C.A.; Salleng, K.J.; Cliffel, D.E.; Feldheim, D.L. In vivo toxicity, biodistribution, and clearance of glutathione-coated gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 257–263. [Google Scholar] [CrossRef]
- Askri, D.; Ouni, S.; Galai, S.; Chovelon, B.; Arnaud, J.; Lehmann, S.G.; Sakly, M.; Sève, M.; Amara, S. Sub-acute intravenous exposure to Fe2O3 nanoparticles does not alter cognitive performances and catecholamine levels, but slightly disrupts plasma iron level and brain iron content in rats. J. Trace Elem. Med. Biol. 2018, 50, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Hess, K.; Gearhart, J.; Geiss, K.; Schlager, J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro 2005, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.S.; Simões, A.M.; Duarte, F.; Rolo, A.P.; Murdoch, R.C.; Hussain, S.M.; Palmeira, C.M. Assessment of the toxicity of silver nanoparticles in vitro: A mitochondrial perspective. Toxicol. In Vitro 2011, 25, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Avalos, A.; Haza, A.I.; Mateo, D.; Morales, P. Cytotoxicity and ROS production of manufactured silver nanoparticles of different sizes in hepatoma and leukemia cells. J. Appl. Toxicol. 2013, 34, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Kaba, S.I.; Egorova, E.M. In vitro studies of the toxic effects of silver nanoparticles on HeLa and U937 cells. Nanotechnol. Sci. Appl. 2015, 8, 19–29. [Google Scholar] [CrossRef]
- Reeves, J.F.; Davies, S.J.; Dodd, N.J.; Jha, A.N. Hydroxyl radicals (OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat. Res. Mol. Mech. Mutagen. 2008, 640, 113–122. [Google Scholar] [CrossRef]
- Osman, I.F.; Baumgartner, A.; Cemeli-Carratala, E.; Fletcher, J.N.; Anderson, D. Genotoxicity and cytotoxicity of zinc oxide and titanium dioxide in HEp-2 cells. Nanomedicine 2010, 5, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Thurn, K.T.; Arora, H.; Paunesku, T.; Wu, A.; Brown, E.M.; Doty, C.; Kremer, J.; Woloschak, G. Endocytosis of titanium dioxide nanoparticles in prostate cancer PC-3M cells. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 123–130. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, L.; Liu, Y.; Deng, S.; Wu, H.; Wang, G. Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii. Ecotoxicol. Environ. Saf. 2012, 84, 155–162. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Rahman, Q.; Kashyap, M.P.; Singh, A.K.; Jain, G.; Jahan, S.; Lohani, M.; Lantow, M.; Pant, A.B. Nano-titanium dioxide induces genotoxicity and apoptosis in human lung cancer cell line, A549. Hum. Exp. Toxicol. 2012, 32, 153–166. [Google Scholar] [CrossRef] [PubMed]
- El-Said, K.S.; Ali, E.M.; Kanehira, K.; Taniguchi, A. Molecular mechanism of DNA damage induced by titanium dioxide nanoparticles in toll-like receptor 3 or 4 expressing human hepatocarcinoma cell lines. J. Nanobiotechnol. 2014, 12, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Zhou, J.; Li, F.; Wang, J.; Chen, M.; Liu, Q. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ. Sci. Pollut. Res. 2014, 22, 5519–5530. [Google Scholar] [CrossRef]
- Biondi, M.; Guarnieri, D.; Yu, H.; Belli, V.; Netti, P.A. Sub-100 nm biodegradable nanoparticles: In vitro release features and toxicity testing in 2D and 3D cell cultures. Nanotechnology 2013, 24, 045101. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.; Loitto, V.; Audinot, J.-N.; Bayat, N.; Gutleb, A.C.; Cristobal, S. Dose-dependent autophagic effect of titanium dioxide nanoparticles in human HaCaT cells at non-cytotoxic levels. J. Nanobiotechnol. 2016, 14, 22. [Google Scholar] [CrossRef]
- Sun, Q.; Tan, D.; Ze, Y.; Sang, X.; Liu, X.; Gui, S.; Cheng, Z.; Cheng, J.; Hu, R.; Gao, G.; et al. Pulmotoxicological effects caused by long-term titanium dioxide nanoparticles exposure in mice. J. Hazard. Mater. 2012, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, Y.; Chen, Z.; Xu, H.; Zhou, J.; Tang, S.; Xu, Z.; Kong, F.; Li, X.; Zhang, Y.; et al. Cardiopulmonary effects induced by occupational exposure to titanium dioxide nanoparticles. Nanotoxicology 2018, 12, 169–184. [Google Scholar] [CrossRef]
- Qu, Y.; Lü, X. Aqueous synthesis of gold nanoparticles and their cytotoxicity in human dermal fibroblasts–fetal. Biomed. Mater. 2009, 4, 025007. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Escudero-Francos, M.A.; Cepas, V.; González-Menédez, P.; Badía-Laíño, R.; Díaz-García, M.E.; Sainz, R.M.; Mayo, J.C.; Hevia, D. Cellular Uptake and Tissue Biodistribution of Functionalized Gold Nanoparticles and Nanoclusters. J. Biomed. Nanotechnol. 2017, 13, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Susewind, J.; Carvalho-Wodarz, C.D.S.; Repnik, U.; Collnot, E.-M.; Schneider-Daum, N.; Griffiths, G.W.; Lehr, C.-M. A 3D co-culture of three human cell lines to model the inflamed intestinal mucosa for safety testing of nanomaterials. Nanotoxicology 2015, 10, 1–10. [Google Scholar] [CrossRef]
- Theumer, A.; Grafe, C.; Bähring, F.; Bergemann, C.; Hochhaus, A.; Clement, J.H. Superparamagnetic iron oxide nanoparticles exert different cytotoxic effects on cells grown in monolayer cell culture versus as multicellular spheroids. J. Magn. Magn. Mater. 2015, 380, 27–33. [Google Scholar] [CrossRef]
- Le, V.-M.; Lang, M.-D.; Shi, W.-B.; Liu, J.-W. A collagen-based multicellular tumor spheroid model for evaluation of the efficiency of nanoparticle drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guan, R.; Tao, M.; Lyu, F.; Cao, G.; Liu, M.; Gao, J. Assessment of the toxicity and inflammatory effects of different-sized zinc oxide nanoparticles in 2D and 3D cell cultures. RSC Adv. 2017, 7, 12437–12445. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Chen, Y.; Ding, J.; Xia, G.; Gao, C.; Cheng, J.; Jin, N.; Zhou, Y.; Li, X.; et al. Pharmacokinetic parameters and tissue distribution of magnetic Fe3O4 nanoparticles in mice. Int. J. Nanomed. 2010, 5, 861–866. [Google Scholar] [CrossRef]
- Coccini, T.; Caloni, F.; Cando, L.J.R.; De Simone, U. Cytotoxicity and proliferative capacity impairment induced on human brain cell cultures after short- and long-term exposure to magnetite nanoparticles. J. Appl. Toxicol. 2016, 37, 361–373. [Google Scholar] [CrossRef]
- Sambale, F.; Lavrentieva, A.; Stahl, F.; Blume, C.; Stiesch, M.; Kasper, C.; Bahnemann, D.; Scheper, T. Three dimensional spheroid cell culture for nanoparticle safety testing. J. Biotechnol. 2015, 205, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and Polymer Polyethylene Glycol (PEG) Coating Reduce Both 5 and 30 nm Iron Oxide Nanoparticle Cytotoxicity in 2D and 3D Cell Culture. Int. J. Mol. Sci. 2012, 13, 5554–5570. [Google Scholar] [CrossRef]
- Chia, S.L.; Tay, C.Y.; Setyawati, M.I.; Leong, D.T. Biomimicry 3D Gastrointestinal Spheroid Platform for the Assessment of Toxicity and Inflammatory Effects of Zinc Oxide Nanoparticles. Small 2014, 11, 702–712. [Google Scholar] [CrossRef]
- De Simone, U.; Roccio, M.; Gribaldo, L.; Spinillo, A.; Caloni, F.; Coccini, T. Human 3D Cultures as Models for Evaluating Magnetic Nanoparticle CNS Cytotoxicity after Short- and Repeated Long-Term Exposure. Int. J. Mol. Sci. 2018, 19, 1993. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lilly, G.D.; Doty, R.C.; Podsiadlo, P.; Kotov, N.A. In vitro Toxicity Testing of Nanoparticles in 3D Cell Culture. Small 2009, 5, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef]
- He, H.; Liu, C.; Liu, Y.; Liu, X.; Wu, Y.; Fan, J.; Zhao, L.; Cao, Y. Mathematical modeling of the heterogeneous distributions of nanomedicines in solid tumors. Eur. J. Pharm. Biopharm. 2019, 142, 153–164. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Lu, H.; Stenzel, M.H. Multicellular Tumor Spheroids (MCTS) as a 3D In Vitro Evaluation Tool of Nanoparticles. Small 2018, 14, e1702858. [Google Scholar] [CrossRef]
- Cao, Y.; Gong, Y.; Liu, L.; Zhou, Y.; Fang, X.; Zhang, C.; Li, Y.; Li, J. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: A review. J. Appl. Toxicol. 2017, 37, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; Macmillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Gollwitzer, C.; Bartczak, D.; Goenaga-Infante, H.; Kestens, V.; Krumrey, M.; Minelli, C.; Pálmai, M.; Ramaye, Y.; Roebben, G.; Sikora, A.; et al. A comparison of techniques for size measurement of nanoparticles in cell culture medium. Anal. Methods 2016, 8, 5272–5282. [Google Scholar] [CrossRef]
- Chen, Z.P.; Xu, R.Z.; Zhang, Y.; Gu, N. Effects of Proteins from Culture Medium on Surface Property of Silanes-Functionalized Magnetic Nanoparticles. Nanoscale Res. Lett. 2008, 4, 204–209. [Google Scholar] [CrossRef]
- Kato, H.; Fujita, K.; Horie, M.; Suzuki, M.; Nakamura, A.; Endoh, S.; Yoshida, Y.; Iwahashi, H.; Takahashi, K.; Kinugasa, S. Dispersion characteristics of various metal oxide secondary nanoparticles in culture medium for in vitro toxicology assessment. Toxicol. In Vitro 2010, 24, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Dubiak-Szepietowska, M.; Karczmarczyk, A.; Jönsson-Niedziółka, M.; Winckler, T.; Feller, K.-H. Development of complex-shaped liver multicellular spheroids as a human-based model for nanoparticle toxicity assessment in vitro. Toxicol. Appl. Pharmacol. 2016, 294, 78–85. [Google Scholar] [CrossRef]
- Chauhan, S.; Manivasagam, G.; Kumar, P.; Ambasta, R.K. Cellular Toxicity of Mesoporous Silica Nanoparticle in SHSY5Y and BMMNCs Cell. Pharm. Nanotechnol. 2019, 6, 245–252. [Google Scholar] [CrossRef]
- Braun, K.; Stürzel, C.M.; Biskupek, J.; Kaiser, U.; Kirchhoff, F.; Lindén, M. Comparison of different cytotoxicity assays for in vitro evaluation of mesoporous silica nanoparticles. Toxicol. In Vitro 2018, 52, 214–221. [Google Scholar] [CrossRef]
- Bonnier, F.; Keating, M.; Wróbel, T.; Majzner, K.; Baranska, M.; Garcia-Munoz, A.; Blanco, A.; Byrne, H.J. Cell viability assessment using the Alamar blue assay: A comparison of 2D and 3D cell culture models. Toxicol. In Vitro 2015, 29, 124–131. [Google Scholar] [CrossRef]
- Eustaquio, T.; Leary, J.F. Single-Cell Nanotoxicity Assays of Superparamagnetic Iron Oxide Nanoparticles. Adv. Struct. Saf. Stud. 2012, 926, 69–85. [Google Scholar] [CrossRef]
- Sonmez, E.; Cacciatore, I.; Bakan, F.; Turkez, H.; I Mohtar, Y.; Togar, B.; Stefano, A.D. Toxicity assessment of hydroxyapatite nanoparticles in rat liver cell model in vitro. Hum. Exp. Toxicol. 2016, 35, 1073–1083. [Google Scholar] [CrossRef]
- Mota, A.; Hemati-Dinarvand, M.; Taheraghdam, A.A.; Nejabati, H.R.; Ahmadi, R.; Ghasemnejad, T.; Hasanpour, M.; Valilo, M. Association of Paraoxonse1 (PON1) Genotypes with the Activity of PON1 in Patients with Parkinson’s Disease. Acta Neurol. Taiwanica 2019, 28, 66–74. [Google Scholar]
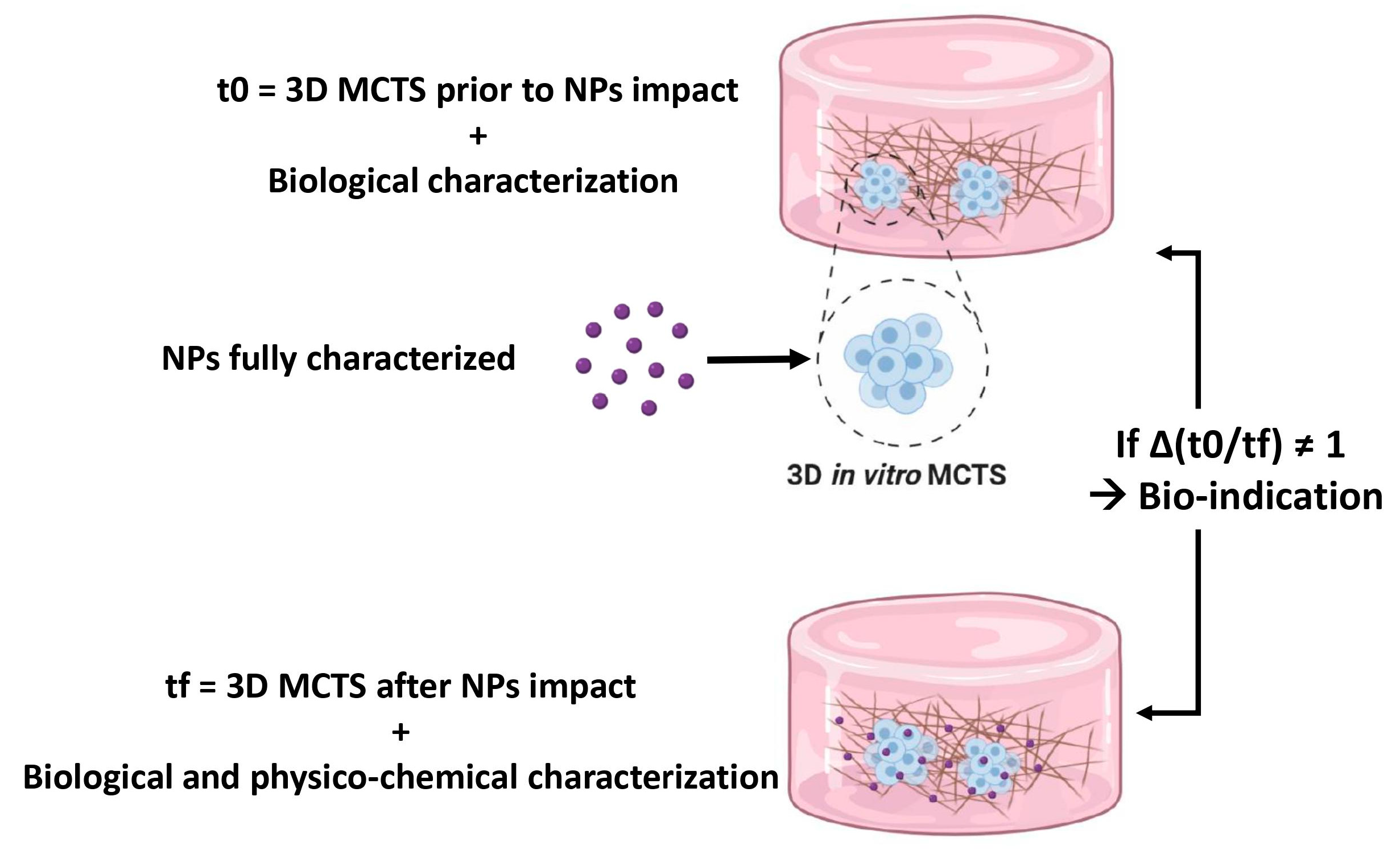
| NPs | NP Size (nm) | NP Coating | NP Concentration | Cell Line | 3D System | Cytotoxicity Assays | Cytotoxicity and Comparison to 2D Cell Culture | Ref. |
|---|---|---|---|---|---|---|---|---|
| ZnO | 24; 56; 87 | - | 10; 25; 50; 75; 100 µg mL−1 | Caco-2 (colorectal adenocarcinoma ) | Agarose gel | ROS expression (CellROX orange reagent); measurement of pro-inflammatory cytokines (IL-8 and IL-1β); cell proliferation (PicoGreen); modes of cell death (AnnexinV_FITC/PI) | Increased ROS expression; size-independent toxicity; decreased DNA amount at high concentrations; cell death: apoptosis in 3D vs. necrosis in 2D; less toxic in 3D than in 2D | [187] |
| 222.0 (in water); 142.2 (in FBS-DMEM) | - | 31.5; 125; 500; 1000 µM | SW480 and NCM460 (colorectal cancer) | Agarose gel | Mode of cell death (annexinV-PI); Immunofluorescence staining of NF-κB; metabolic activity (MTT method) | Increased ROS expression in NCM460 cells upon NPs uptake but no ROS increased in 3D SW480; higher basal ROS expression in 3D SW480 than in 2D; ROS-independent inflammatory response increased in both cell lines in 2D and 3D; 3D is more resistant to DNA damage than 2D | [192] | |
| ZnO TiO2 | ZnO: 41 ± 5 (in water); 190 ± 3 (in DMEM); 106 ± 11 (in standard culture medium); TiO2: 79 ± 25 (in water); 142 ± 29 (in DMEM); 118 ± 28 (in culture medium) | - | 0.5–2.5 µg mL−1 | A549 (human lung carcinoma); NIH-3T3 (mouse fibroblasts) | ULA well plates (spheroids) | Cell viability (CellTiter-Blue viability test; reduction of blue resazurin to purple resofurin monitored by fluorescence); (CellTiter-Glo luciferase reaction in presence of Mg/ATP/oxygen; monitored by luminescence); cell morphology (microscopy) | ZnO: similar toxicity in NIH-3T3 in 2D and 3D;A549 more sensitive in 3D than in 2D; both toxicity displayed in concentration-dependent manner; TiO2: small toxic effect in 3D; no toxicity in 2D | [190] |
| TiO2 Au Ag | Au “15 nm”: 51 ± 6; Au “80 nm”: 116 ± 5; TiO2: 896 ± 133; Ag: 120 ± 4 (in DMEM) | Au: phosphine TiO2: PVP | 1.25–625 µg cm−2 | Caco2 (colorectal adenocarcinoma) mono-cultured of co-cultured with THP-1 (human macrophages) and MUTZ-3 (human dendritic cells) | Collagen hydrogel | Inflammatory response (IL-8); cell viability (alamar blue assay; LDH assay) | No impact of Au on cell viability or inflammation in Caco2 mono-culture but slight increase of IL-8 in co-cultured cells; mono-cultured cells more sensitive to Ag; inflammatory response from Ag at any concentration in co-culture; no toxic or inflammatory effect for TiO2 in both mono- and co-cultured cells | [184] |
| Ag ZnO SiO2 | Ag: 6.873 ± 3.330 (in water); 6.887 ± 4.176 (in cell culture medium); ZnO: 43.58 ± 9.113 and 292 ± 42.50 (in water); 134.7 ± 108.2 and 3902.0 ± 1119.0 (in cell culture medium); SiO2: 30.99 ± 20.0 (in water); 41.65 ± 22.09 (in cell culture medium) | - | Ag: 5–50 µg mL−1; ZnO: 10–120 µg mL−1; SiO2: 0–2000 µg mL−1 | HepG2 (hepatocarcinoma) | Gelatin; collagen hydrogel; Matrigel | Metabolic activity (albumin and urea assays); cell viability (trypan blue and alamar blue); colorimetric cell proliferation assay (CellTiter96); morphology (microscopy) | 3D show less toxicity than 2D; FBS hinder toxic effect of NPs but no change cell survival; Increase spheroids disaggregation at higher frequency of low concentration of NPs uptake compared to lower frequency of high concentration | [204] |
| CdTe Au | CdTe: 2.4–6; Au: 3.5 ± 0.7 (in citrate); 5.5 ± 0.6 (in CTAB1) | CdTe-NPs: L-cysteine monolayer; Au-NPs: citrate/CTAB | CdTe: 10 µg mL−1; Au: 98.5 µg mL−1 | HepG2 (hepatocarcinoma) | Polyacrylamide hydrogel | Cell morphology (SEM; light microscopy); membrane integrity; metabolic activity (LDH and MTT methods); cell death mechanism (caspase assay for apoptotis; LDH for necrosis); live/dead assays (confocal microscopy) | Drop of cells metabolism and change in phenotype upon CdTe and CTAB-Au uptake; no toxicity reported for citrate-Au; less toxic in 3D than in 2D | [194] |
| IO | fluidMAG-D: 150; fluidMAG- PEI: 100; fluidMAG- CMX: 150 | D: neutral starch PEI: cationic polyethyleneimine: anionic carboxymethydextran | 25 µg cm−2 | HBMEC (Human brain microvascular endothelial cells) | Spheroids suspension in inverted plate | Real-time cell analysis based on electronic impedance measurement via electrode at the bottom of wells; immunoblotting (Akt signalling); stained cell observation (confocal microscope) | Increased Akt activation in 2D vs. 3D upon NPs uptake; NPs distribution is coating charge-dependent; cell death more pronounced in 2D vs. in 3D | [185] |
| Fe3O4: 48.7 (in sodium citrate); 10; 25; 70; 700 (in cell culture medium) | Polyvinylpyrrolidone (PVP) | 0.1–25 µg mL−1 (long term exposure); 1–100 µg mL−1 (short term exposure) | D384 (astrocyte); SH-SY5Y (neuroblastoma) | ULA well plates (spheroids) | Cell viability (Trypan blue); cell morphology (microscopy) | Concentration-dependent cell mortality and spheroid disaggregation; at highest concentration: 50% and 34% cell viability decrease of D384 and SH-SY5Y respectively in 3D vs 75% and 45% in 2D | [188,193] | |
| 5 and 30 | Dextran; PEG or no coating | 100; 250; 500 µg mL−1 | PAEC (Porcine aortic endothelial cells) | Alginate hydrogel | Cell viability (live/dead assay; alamar blue assay); ROS level; cell shape (actin cytoskeleton labeled and microscopy) | Bare NPs decrease cell viability and increase ROS expression in dose-dependent manner; any coating reduces cytotoxicity and ROS expression with no effect of the size in both 2D and 3D; bare NPs more toxic at low concentration in 3D vs. 2D | [184] | |
| PELGA 2 | PELGA10: 79.2 ± 5.7; PELGA20: 90.5 ± 5.0; PELGA40: 175.8 ± 4.3 | - | 2 µg mL−1 | HeLa (human epithelioid cervix carcinoma) | Collagen hydrogel | Cell viability (alamar blue assay); cell morphology (microscopy) | Sub-100 nm NPs more internalized and toxic in 3D than in 2D | [177] |
| Polymicells drug nanocarrier | 151.9 | - | 100 µg mL−1 | 95-D (lung cancer); U87 (glioblastoma); HCT 116 (colorectal cancer) | Collagen hydrogel | Metabolic activity (MTT method); cell morphology (microscopy) | Attenuation of antitumoral effect and drug sensitivity in 3D vs. in 2D. | [186] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saydé, T.; El Hamoui, O.; Alies, B.; Gaudin, K.; Lespes, G.; Battu, S. Biomaterials for Three-Dimensional Cell Culture: From Applications in Oncology to Nanotechnology. Nanomaterials 2021, 11, 481. https://doi.org/10.3390/nano11020481
Saydé T, El Hamoui O, Alies B, Gaudin K, Lespes G, Battu S. Biomaterials for Three-Dimensional Cell Culture: From Applications in Oncology to Nanotechnology. Nanomaterials. 2021; 11(2):481. https://doi.org/10.3390/nano11020481
Chicago/Turabian StyleSaydé, Tarek, Omar El Hamoui, Bruno Alies, Karen Gaudin, Gaëtane Lespes, and Serge Battu. 2021. "Biomaterials for Three-Dimensional Cell Culture: From Applications in Oncology to Nanotechnology" Nanomaterials 11, no. 2: 481. https://doi.org/10.3390/nano11020481
APA StyleSaydé, T., El Hamoui, O., Alies, B., Gaudin, K., Lespes, G., & Battu, S. (2021). Biomaterials for Three-Dimensional Cell Culture: From Applications in Oncology to Nanotechnology. Nanomaterials, 11(2), 481. https://doi.org/10.3390/nano11020481






