Janus Particles at Fluid Interfaces: Stability and Interfacial Rheology
Abstract
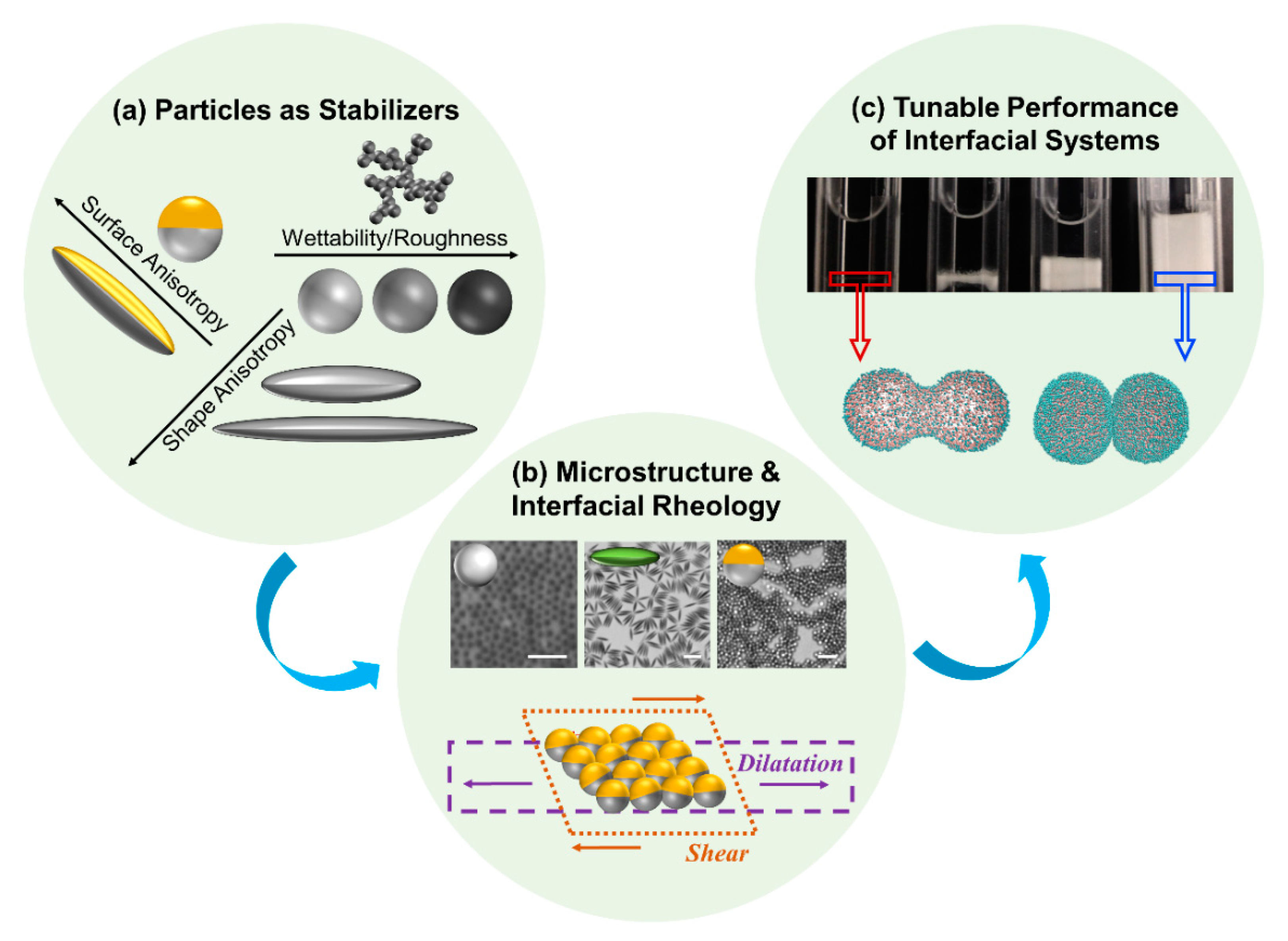
1. Introduction
2. Stability of Particles at Fluid Interfaces
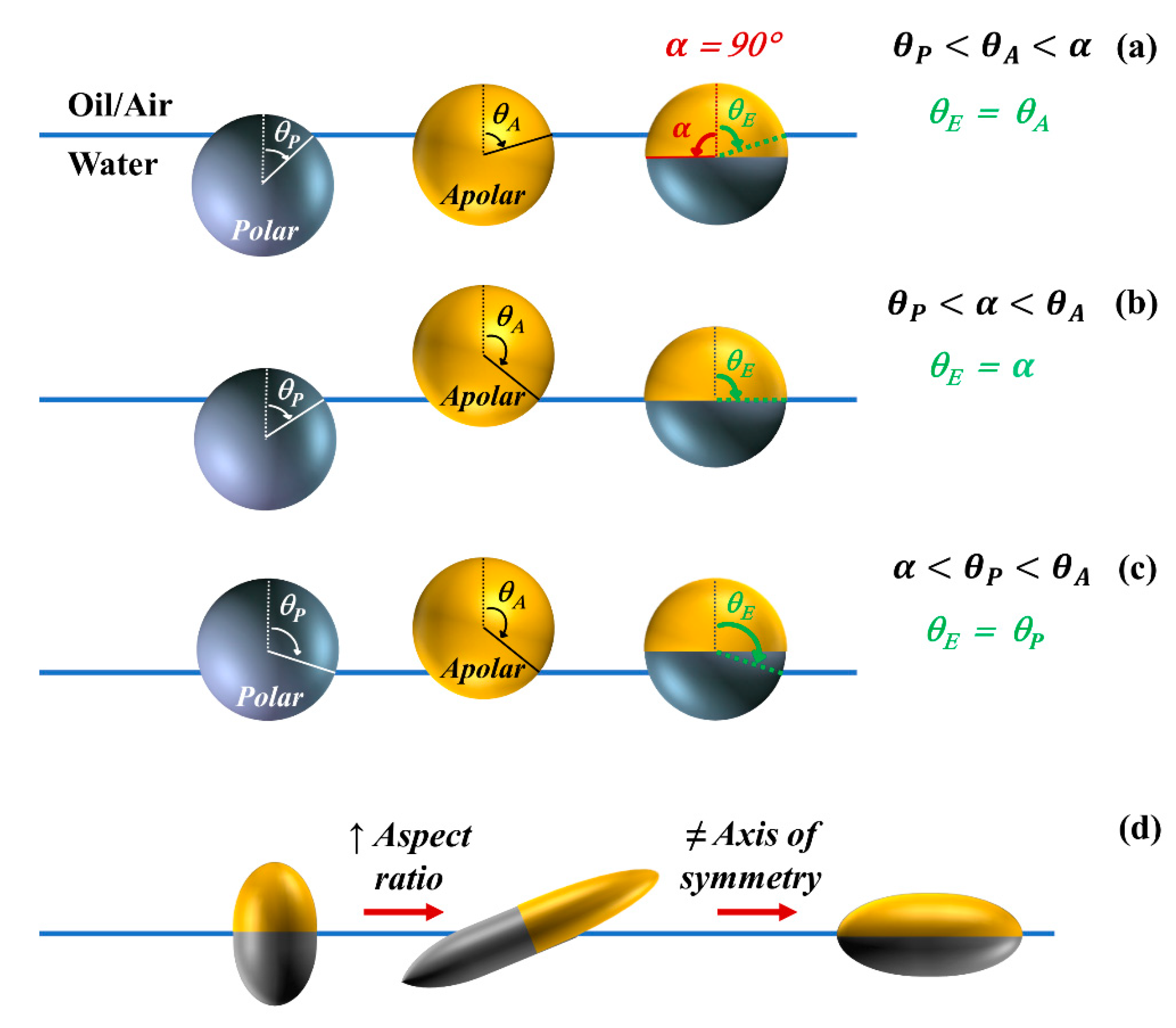
2.1. Equilibrium Position of Particles at Fluid Interfaces
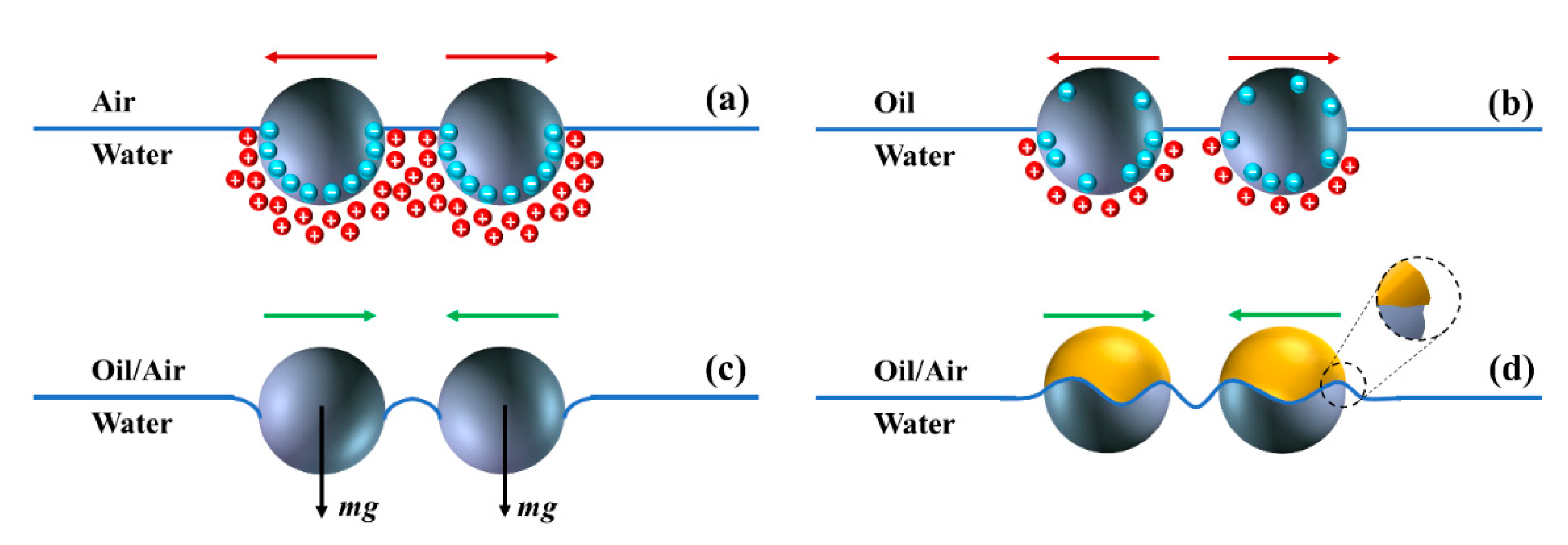
2.2. Interparticle Interactions at Fluid Interfaces
3. Interfacial Rheology
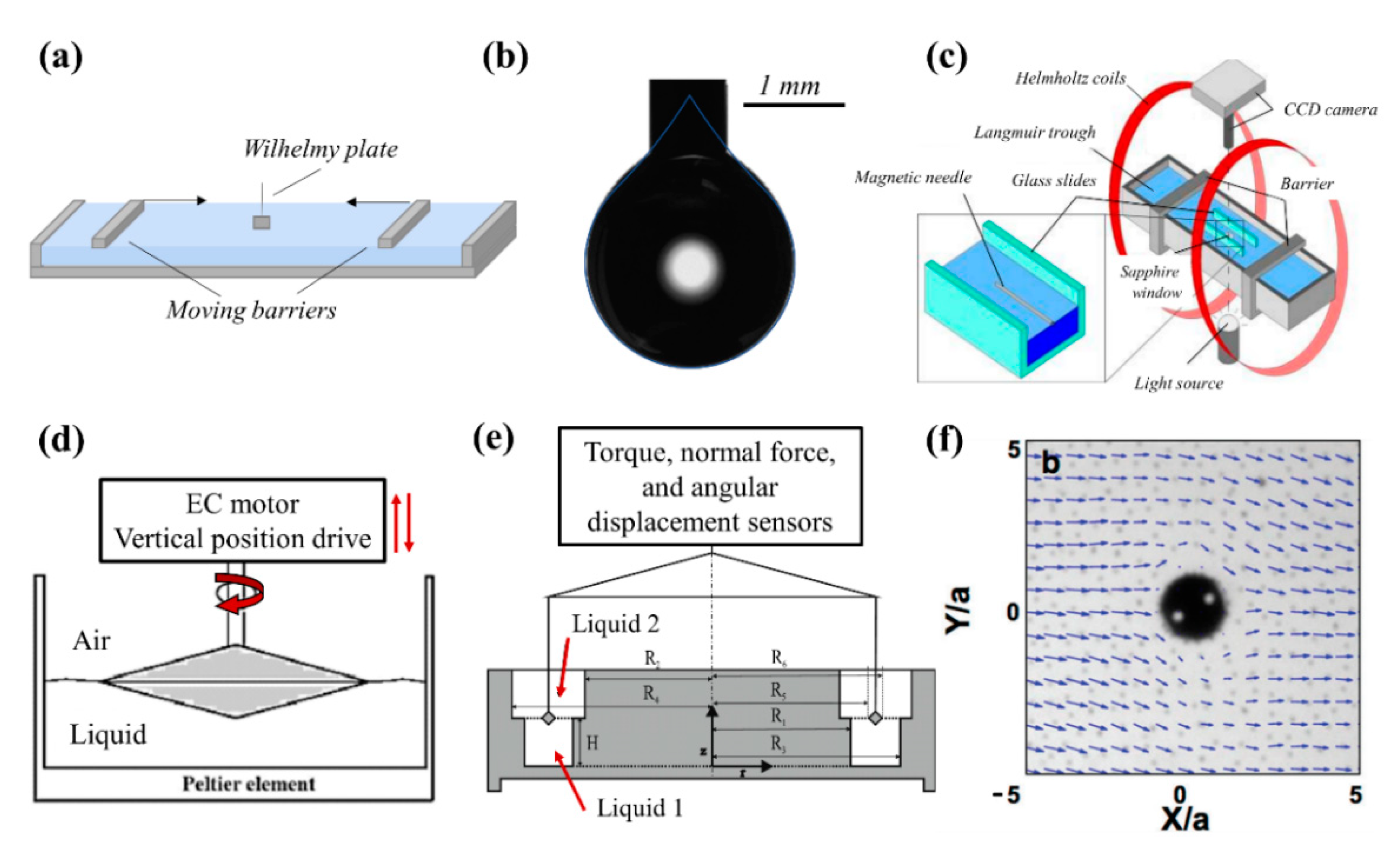
3.1. Tools and Techniques
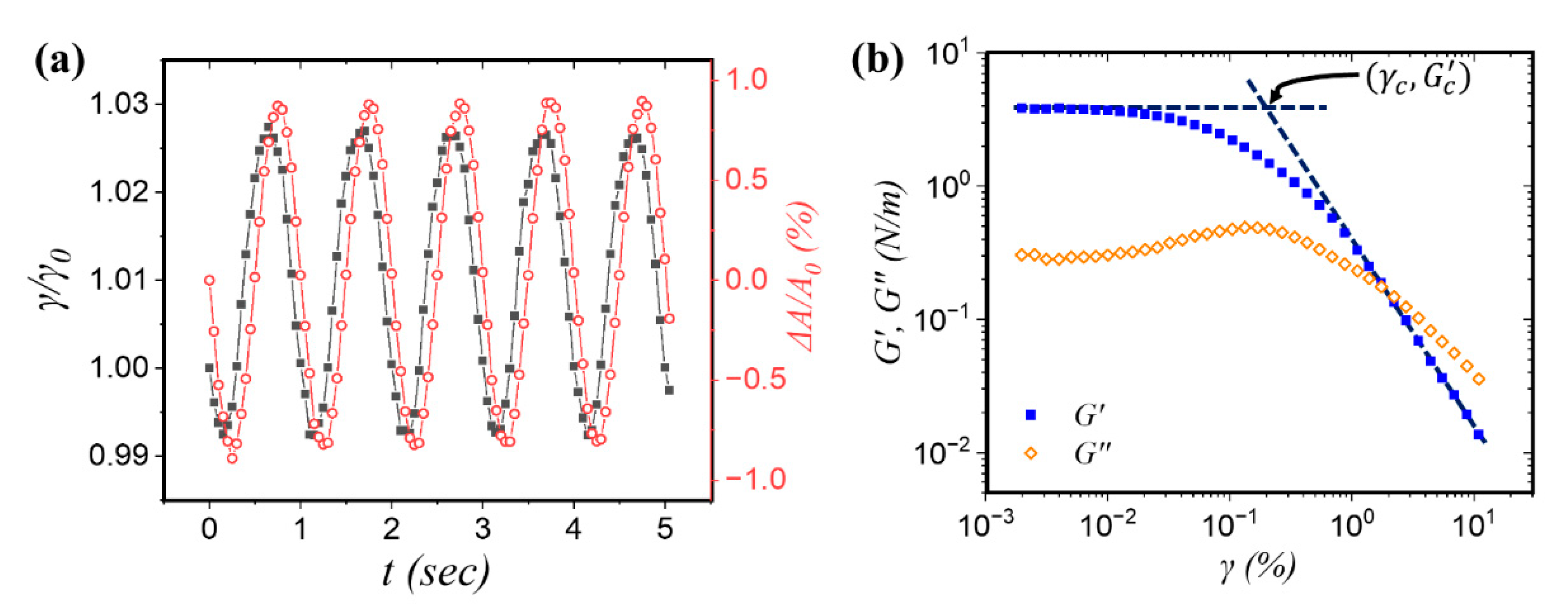
3.2. Interfacial Dilational Rheology
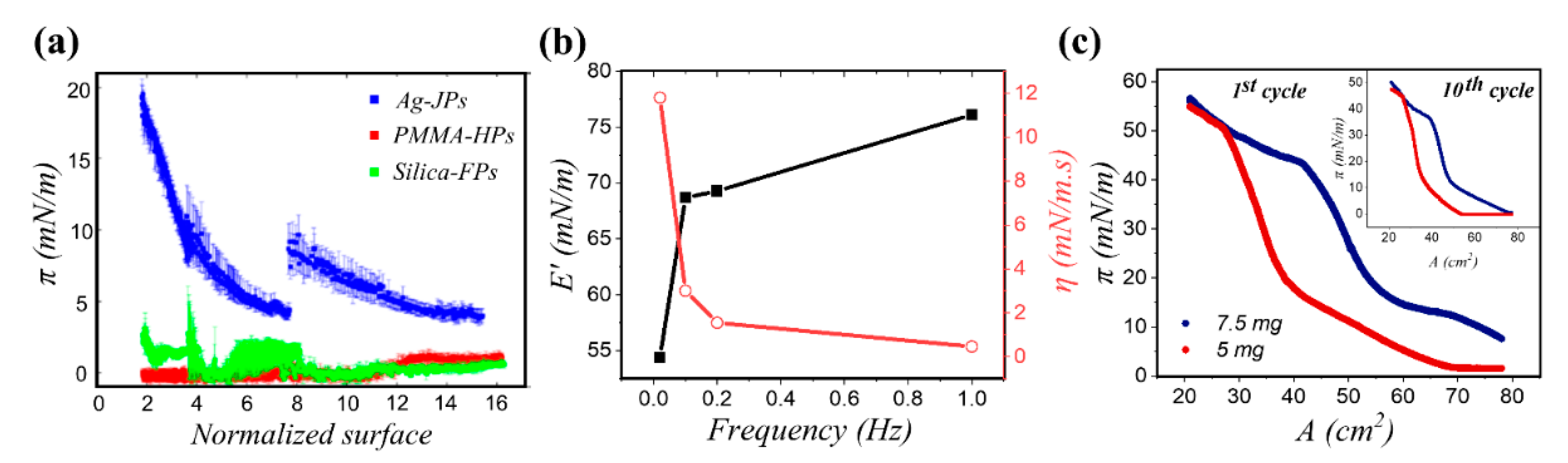
3.2.1. Homogeneous Particles
3.2.2. Janus Particles
3.3. Interfacial Shear Rheology
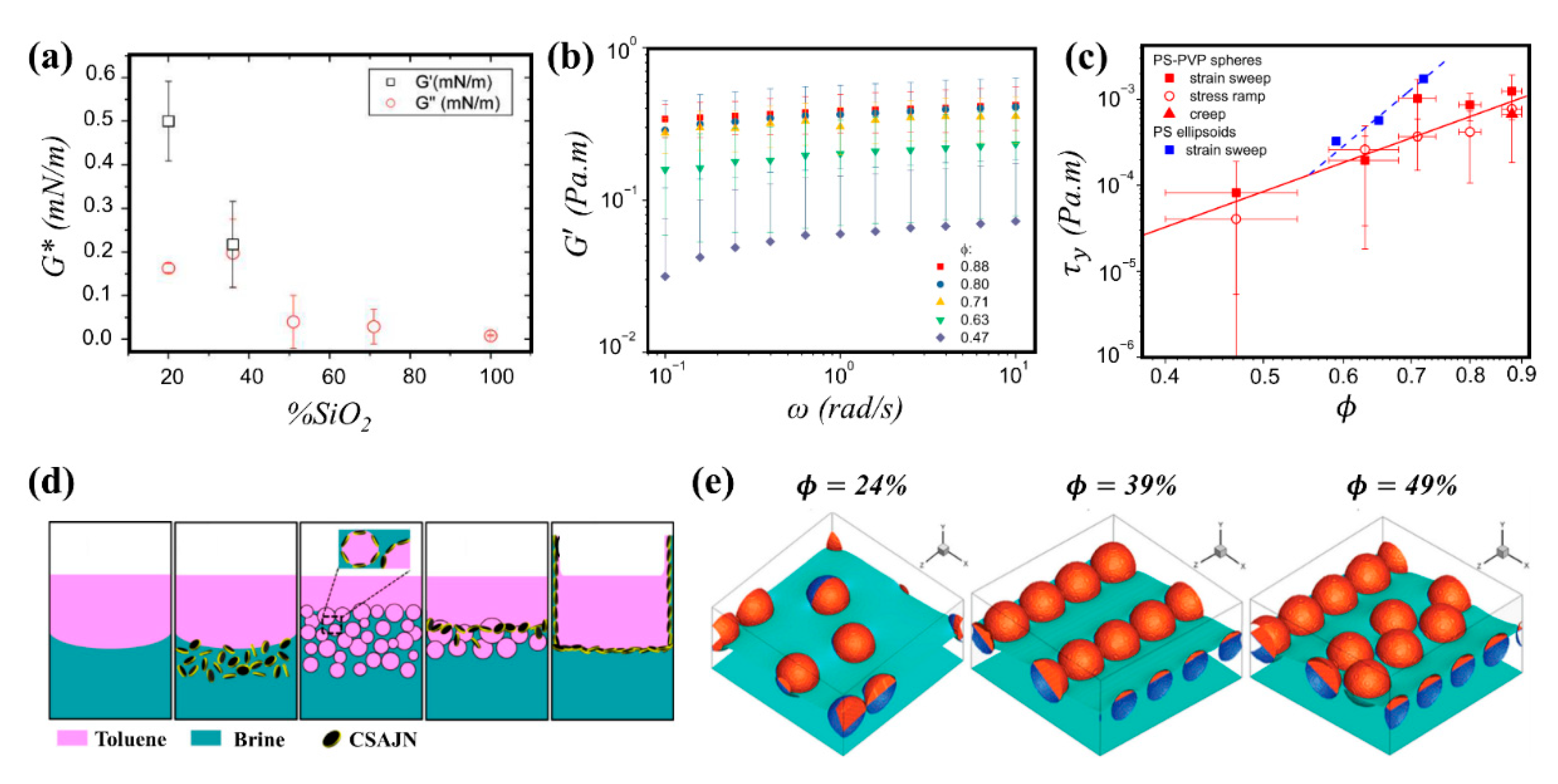
3.3.1. Homogeneous Particles
3.3.2. Janus Particles
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Ramsden, W. Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation)—Preliminary account. Proc. R. Soc. Lond. 1904, 72, 156–164. [Google Scholar]
- Pickering, S.U. CXCVI—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Mendoza, A.J.; Guzmán, E.; Martínez-Pedrero, F.; Ritacco, H.; Rubio, R.G.; Ortega, F.; Starov, V.M.; Miller, R. Particle laden fluid interfaces: Dynamics and interfacial rheology. Adv. Colloid Interface Sci. 2014, 206, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Horozov, T.S. Colloidal Particles at Liquid Interfaces; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Crossley, S.; Faria, J.; Shen, M.; Resasco, D.E. Solid Nanoparticles that Catalyze Biofuel Upgrade Reactions at the Water/Oil Interface. Science 2010, 327, 68–72. [Google Scholar] [CrossRef]
- Chen, C.L.; Wang, S.S.; Kadhum, M.J.; Harwell, J.H.; Shiau, B.J. Using carbonaceous nanoparticles as surfactant carrier in enhanced oil recovery: A laboratory study. Fuel 2018, 222, 561–568. [Google Scholar] [CrossRef]
- Qian, C.; Telmadarreie, A.; Dong, M.; Bryant, S. Synergistic Effect between Surfactant and Nanoparticles on the Stability of Methane Foam in EOR Processes. In SPE Western Regional Meeting; Society of Petroleum Engineers: San Jose, CA, USA, 2019; p. 16. [Google Scholar]
- Binks, B.P. Colloidal Particles at a Range of Fluid–Fluid Interfaces. Langmuir 2017, 33, 6947–6963. [Google Scholar] [CrossRef]
- Liu, R.C.; Raman, A.K.Y.; Shaik, I.; Aichele, C.; Kim, S.J. Inorganic microfiltration membranes incorporated with hydrophilic silica nanoparticles for oil-in-water emulsion separation. J. Water Process. Eng. 2018, 26, 124–130. [Google Scholar] [CrossRef]
- Park, B.J.; Lee, D. Particles at fluid–fluid interfaces: From single-particle behavior to hierarchical assembly of materials. MRS Bull. 2014, 39, 1089–1098. [Google Scholar] [CrossRef]
- Kaz, D.M. Colloidal Particles and Liquid Interfaces: A Spectrum of Interactions. Ph.D. Thesis, Harvard University, Manoharan Lab Publications, Cambridge, MA, USA, 2011. [Google Scholar]
- Zang, D.Y.; Rio, E.; Langevin, D.; Wei, B.; Binks, B.P. Viscoelastic properties of silica nanoparticle monolayers at the air-water interface. Eur. Phys. J. E 2010, 31, 125–134. [Google Scholar] [CrossRef]
- Böker, A.; He, J.; Emrick, T.; Russell, T.P. Self-assembly of nanoparticles at interfaces. Soft Matter 2007, 3, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Furst, E.M. Directing colloidal assembly at fluid interfaces. Proc. Natl. Acad. Sci. 2011, 108, 20853. [Google Scholar] [CrossRef] [PubMed]
- Komura, S.; Hirose, Y.; Nonomura, Y. Adsorption of colloidal particles to curved interfaces. J. Chem. Phys. 2006, 124, 241104. [Google Scholar] [CrossRef] [PubMed]
- Bresme, F.; Oettel, M. Nanoparticles at fluid interfaces. J. Phys. Condens. Matter 2007, 19, 413101. [Google Scholar] [CrossRef]
- Vishal, B.; Ghosh, P. The effect of silica nanoparticles on the stability of aqueous foams. J. Dispers. Sci. Technol. 2019, 40, 206–218. [Google Scholar] [CrossRef]
- Hu, N.; Li, Y.; Wu, Z.; Lu, K.; Huang, D.; Liu, W. Foams stabilization by silica nanoparticle with cationic and anionic surfactants in column flotation: Effects of particle size. J. Taiwan Inst. Chem. Eng. 2018, 88, 62–69. [Google Scholar] [CrossRef]
- Weston, J.S.; Jentoft, R.E.; Grady, B.P.; Resasco, D.E.; Harwell, J.H. Silica Nanoparticle Wettability: Characterization and Effects on the Emulsion Properties. Ind. Eng. Chem. Res. 2015, 54, 4274–4284. [Google Scholar] [CrossRef]
- McGorty, R.; Fung, J.; Kaz, D.; Manoharan, V.N. Colloidal self-assembly at an interface. Mater. Today 2010, 13, 34–42. [Google Scholar] [CrossRef]
- Zhou, J.; Qiao, X.; Binks, B.P.; Sun, K.; Bai, M.; Li, Y.; Liu, Y. Magnetic Pickering emulsions stabilized by Fe3O4 nanoparticles. Langmuir 2011, 27, 3308–3316. [Google Scholar] [CrossRef]
- Owoseni, O.; Nyankson, E.; Zhang, Y.; Adams, D.J.; He, J.; Spinu, L.; McPherson, G.L.; Bose, A.; Gupta, R.B.; John, V.T. Interfacial adsorption and surfactant release characteristics of magnetically functionalized halloysite nanotubes for responsive emulsions. J Colloid Interface Sci 2016, 463, 288–298. [Google Scholar] [CrossRef]
- Abkarian, M.; Subramaniam, A.B.; Kim, S.-H.; Larsen, R.J.; Yang, S.-M.; Stone, H.A. Dissolution Arrest and Stability of Particle-Covered Bubbles. Phys. Rev. Lett. 2007, 99, 188301. [Google Scholar] [CrossRef] [PubMed]
- Doroudian Rad, M.; Telmadarreie, A.; Xu, L.; Dong, M.; Bryant, S.L. Insight on Methane Foam Stability and Texture via Adsorption of Surfactants on Oppositely Charged Nanoparticles. Langmuir: ACS J. Surf. Colloids 2018, 34, 14274–14285. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Worthen, A.; Qajar, A.; Robert, I.; Bryant, S.L.; Huh, C.; Prodanović, M.; Johnston, K.P. Viscosity and stability of ultra-high internal phase CO2-in-water foams stabilized with surfactants and nanoparticles with or without polyelectrolytes. J. Colloid Interface Sci. 2016, 461, 383–395. [Google Scholar] [CrossRef]
- Arab, D.; Kantzas, A.; Bryant, S.L. Nanoparticle stabilized oil in water emulsions: A critical review. J. Pet. Sci. Eng. 2018, 163, 217–242. [Google Scholar] [CrossRef]
- Katepalli, H.; Bose, A. Response of Surfactant Stabilized Oil-in-Water Emulsions to the Addition of Particles in an Aqueous Suspension. Langmuir 2014, 30, 12736–12742. [Google Scholar] [CrossRef]
- Katepalli, H.; Bose, A.; Hatton, T.A.; Blankschtein, D. Destabilization of Oil-in-Water Emulsions Stabilized by Non-ionic Surfactants: Effect of Particle Hydrophilicity. Langmuir 2016, 32, 10694–10698. [Google Scholar] [CrossRef]
- Fan, H.; Striolo, A. Mechanistic study of droplets coalescence in Pickering emulsions. Soft Matter 2012, 8, 9533–9538. [Google Scholar] [CrossRef]
- Hunter, T.; Pugh, R.; Franks, G.; Jameson, G. The role of particles in stabilising foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef]
- Lin, Y.; Skaff, H.; Emrick, T.; Dinsmore, A.; Russell, T.P. Nanoparticle assembly and transport at liquid-liquid interfaces. Science 2003, 299, 226–229. [Google Scholar] [CrossRef]
- Raman, A.K.Y.; Aichele, C.P. Influence of non-ionic surfactant addition on the stability and rheology of particle-stabilized emulsions. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 585, 124084. [Google Scholar] [CrossRef]
- Van Hooghten, R.; Imperiali, L.; Boeckx, V.; Sharma, R.; Vermant, J. Rough nanoparticles at the oil–water interfaces: Their structure, rheology and applications. Soft Matter 2013, 9, 10791–10798. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Jia, W.; Li, Z.; Zhao, Y.; Ren, S. Demulsification of Crude Oil-in-Water Emulsions Driven by Graphene Oxide Nanosheets. Energy Fuels 2015, 29, 4644–4653. [Google Scholar] [CrossRef]
- Pawar, A.B.; Caggioni, M.; Hartel, R.W.; Spicer, P.T. Arrested coalescence of viscoelastic droplets with internal microstructure. Faraday Discuss. 2012, 158, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Madivala, B.; Vandebril, S.; Fransaer, J.; Vermant, J. Exploiting particle shape in solid stabilized emulsions. Soft Matter 2009, 5, 1717–1727. [Google Scholar] [CrossRef]
- Sacanna, S.; Korpics, M.; Rodriguez, K.; Colón-Meléndez, L.; Kim, S.-H.; Pine, D.J.; Yi, G.-R. Shaping colloids for self-assembly. Nat. Commun. 2013, 4, 1688. [Google Scholar] [CrossRef]
- Vu, T.V.; Papavassiliou, D.V. Modification of Oil–Water Interfaces by Surfactant-Stabilized Carbon Nanotubes. J. Phys. Chem. C 2018, 122, 27734–27744. [Google Scholar] [CrossRef]
- Hou, H.B.; Yu, D.M.; Tian, Q.; Hu, G.H. Preparation, Characterization, and Properties of Hollow Janus Particles with Tailored Shapes. Langmuir 2014, 30, 1741–1747. [Google Scholar] [CrossRef]
- Pawar, A.B.; Kretzschmar, I. Fabrication, Assembly, and Application of Patchy Particles. Macromol. Rapid Commun. 2010, 31, 150–168. [Google Scholar] [CrossRef]
- Zhang, J.; Grzybowski, B.A.; Granick, S. Janus Particle Synthesis, Assembly, and Application. Langmuir 2017, 33, 6964–6977. [Google Scholar] [CrossRef]
- Duguet, É.; Hubert, C.; Chomette, C.; Perro, A.; Ravaine, S. Patchy colloidal particles for programmed self-assembly. Comptes Rendus Chim. 2016, 19, 173–182. [Google Scholar] [CrossRef]
- Walther, A.; Müller, A.H.E. Janus Particles: Synthesis, Self-Assembly, Physical Properties, and Applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef] [PubMed]
- Lattuada, M.; Hatton, T.A. Synthesis, properties and applications of Janus nanoparticles. Nano Today 2011, 6, 286–308. [Google Scholar] [CrossRef]
- Glotzer, S.C.; Solomon, M.J. Anisotropy of building blocks and their assembly into complex structures. Nat Mater 2007, 6, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Ruhland, T.M.; Gröschel, A.H.; Ballard, N.; Skelhon, T.S.; Walther, A.; Müller, A.H.E.; Bon, S.A.F. Influence of Janus Particle Shape on Their Interfacial Behavior at Liquid–Liquid Interfaces. Langmuir 2013, 29, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, C.; Fabre, P.; Raphaël, E.; Veyssié, M. “Janus Beads”: Realization and Behaviour at Water/Oil Interfaces. Europhys. Lett. (EPL) 1989, 9, 251–255. [Google Scholar] [CrossRef]
- Chen, Q.; Whitmer, J.K.; Jiang, S.; Bae, S.C.; Luijten, E.; Granick, S. Supracolloidal reaction kinetics of Janus spheres. Science 2011, 331, 199–202. [Google Scholar] [CrossRef]
- Ren, B.; Ruditskiy, A.; Song, J.H.; Kretzschmar, I. Assembly Behavior of Iron Oxide-Capped Janus Particles in a Magnetic Field. Langmuir 2012, 28, 1149–1156. [Google Scholar] [CrossRef]
- Shah, A.A.; Schultz, B.; Zhang, W.; Glotzer, S.C.; Solomon, M.J. Actuation of shape-memory colloidal fibres of Janus ellipsoids. Nat. Mater. 2015, 14, 117–124. [Google Scholar] [CrossRef]
- Shemi, O.; Solomon, M.J. Effect of Surface Chemistry and Metallic Layer Thickness on the Clustering of Metallodielectric Janus Spheres. Langmuir 2014, 30, 15408–15415. [Google Scholar] [CrossRef]
- Yuet, K.P.; Hwang, D.K.; Haghgooie, R.; Doyle, P.S. Multifunctional Superparamagnetic Janus Particles. Langmuir 2010, 26, 4281–4287. [Google Scholar] [CrossRef]
- Smoukov, S.K.; Gangwal, S.; Marquez, M.; Velev, O.D. Reconfigurable responsive structures assembled from magnetic Janus particles. Soft Matter 2009, 5, 1285–1292. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Q.; Tripathy, M.; Luijten, E.; Schweizer, K.S.; Granick, S. Janus Particle Synthesis and Assembly. Adv. Mater. 2010, 22, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Nedev, S.; Carretero-Palacios, S.; Kühler, P.; Lohmüller, T.; Urban, A.S.; Anderson, L.J.E.; Feldmann, J. An Optically Controlled Microscale Elevator Using Plasmonic Janus Particles. ACS Photonics 2015, 2, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Pourrahimi, A.M.; Pumera, M. Multifunctional and self-propelled spherical Janus nano/micromotors: Recent advances. Nanoscale 2018, 10, 16398–16415. [Google Scholar] [CrossRef] [PubMed]
- Jalilvand, Z.; Haider, H.; Cui, J.; Kretzschmar, A.I. Pt-SiO2 Janus Particles and the Water/Oil Interface: A Competition between Motility and Thermodynamics. Langmuir 2020, 36, 6880–6887. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Garg, A.; Campbell, A.I.; Howse, J.; Sen, A.; Velegol, D.; Golestanian, R.; Ebbens, S.J. Boundaries can steer active Janus spheres. Nat. Commun. 2015, 6, 8999. [Google Scholar] [CrossRef] [PubMed]
- Paxton, W.F.; Kistler, K.C.; Olmeda, C.C.; Sen, A.; St. Angelo, S.K.; Cao, Y.; Mallouk, T.E.; Lammert, P.E.; Crespi, V.H. Catalytic Nanomotors: Autonomous Movement of Striped Nanorods. J. Am. Chem. Soc. 2004, 126, 13424–13431. [Google Scholar]
- Lee, J.G.; Brooks, A.M.; Shelton, W.A.; Bishop, K.J.M.; Bharti, B. Directed propulsion of spherical particles along three dimensional helical trajectories. Nat. Commun. 2019, 10, 2575. [Google Scholar] [CrossRef]
- Ye, Y.; Luan, J.; Wang, M.; Chen, Y.; Wilson, D.A.; Peng, F.; Tu, Y. Fabrication of Self-Propelled Micro- and Nanomotors Based on Janus Structures. Chem. A Eur. J. 2019, 25, 8663–8680. [Google Scholar] [CrossRef]
- Fei, W.; Gu, Y.; Bishop, K.J.M. Active colloidal particles at fluid-fluid interfaces. Curr. Opin. Colloid Interface Sci. 2017, 32, 57–68. [Google Scholar] [CrossRef]
- Yi, Y.; Sanchez, L.; Gao, Y.; Yu, Y. Janus particles for biological imaging and sensing. Analyst 2016, 141, 3526–3539. [Google Scholar] [CrossRef]
- Xing, Y.; Zhou, Y.; Fan, L.; Yang, Y.; Zhang, Y.; Deng, X.; Dong, C.; Shuang, S. Construction strategy for ratiometric fluorescent probe based on Janus silica nanoparticles as a platform toward intracellular pH detection. Talanta 2019, 205, 120021. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Villa, C.H.; Dishman, A.F.; Grabowski, M.E.; Pan, D.C.; Durmaz, H.; Misra, A.C.; Colón-Meléndez, L.; Solomon, M.J.; Muzykantov, V.R.; et al. Long-circulating Janus nanoparticles made by electrohydrodynamic co-jetting for systemic drug delivery applications. J Drug Target 2015, 23, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; She, Z.G.; Wang, S.; Sharma, G.; Smith, J.W. One-step fabrication of polymeric Janus nanoparticles for drug delivery. Langmuir 2012, 28, 4459–4463. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Lin, J.; Li, Y.; Li, Y.; Wu, H.; Yu, F.; Jia, M.; Yang, X.; Wu, S.; Xie, L.; et al. Bacillus-Shape Design of Polymer Based Drug Delivery Systems with Janus-Faced Function for Synergistic Targeted Drug Delivery and More Effective Cancer Therapy. Mol. Pharm. 2015, 12, 1318–1327. [Google Scholar] [CrossRef]
- Garbuzenko, O.B.; Winkler, J.; Tomassone, M.S.; Minko, T. Biodegradable Janus Nanoparticles for Local Pulmonary Delivery of Hydrophilic and Hydrophobic Molecules to the Lungs. Langmuir 2014, 30, 12941–12949. [Google Scholar] [CrossRef]
- Chen, B.; Jia, Y.; Gao, Y.; Sanchez, L.; Anthony, S.M.; Yu, Y. Janus Particles as Artificial Antigen-Presenting Cells for T Cell Activation. ACS Appl. Mater. Interfaces 2014, 6, 18435–18439. [Google Scholar] [CrossRef]
- Bradley, L.C.; Stebe, K.J.; Lee, D. Clickable Janus Particles. J. Am. Chem. Soc. 2016, 138, 11437–11440. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Y.; Wang, L.; Chen, D.; Bao, M.; Wang, Z. Amphiphilic Janus particles for efficient dispersion of oil contaminants in seawater. J. Colloid Interface Sci. 2019, 556, 54–64. [Google Scholar] [CrossRef]
- Månsson, L.K.; Peng, F.; Crassous, J.J.; Schurtenberger, P. A microgel-Pickering emulsion route to colloidal molecules with temperature-tunable interaction sites. Soft Matter 2020, 16, 1908–1921. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Fu, Z.; Zhao, X.; Li, Y.; Li, J. Rheology of Nanosilica-Compatibilized Immiscible Polymer Blends: Formation of a “Heterogeneous Network” Facilitated by Interfacially Anchored Hybrid Nanosilica. Macromolecules 2017, 50, 9494–9506. [Google Scholar] [CrossRef]
- Yang, L.L.; Wang, T.D.; Yang, X.; Jiang, G.C.; Luckham, P.F.; Xu, J.P.; Li, X.L.; Ni, X.X. Highly Stabilized Foam by Adding Amphiphilic Janus Particles for Drilling a High-Temperature and High-Calcium Geothermal Well. Ind. Eng. Chem. Res. 2019, 58, 9795–9805. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, G.; Dai, C.; Wu, Y.; Lyu, Y. Interfacial characteristics and the stability mechanism of a dispersed particle gel (DPG) three-phase foam. J. Mol. Liq. 2020, 301, 112425. [Google Scholar] [CrossRef]
- Tu, F.; Park, B.J.; Lee, D. Thermodynamically Stable Emulsions Using Janus Dumbbells as Colloid Surfactants. Langmuir 2013, 29, 12679–12687. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Park, B.J.; Tu, F.; Lee, D. Amphiphilic Janus particles at fluid interfaces. Soft Matter 2013, 9, 6604–6617. [Google Scholar] [CrossRef]
- Bradley, L.C.; Chen, W.-H.; Stebe, K.J.; Lee, D. Janus and patchy colloids at fluid interfaces. Curr. Opin. Colloid Interface Sci. 2017, 30, 25–33. [Google Scholar] [CrossRef]
- Razavi, S. Janus Colloidal Particles and Fluid Interfaces: Approach, Breach, and Flow Behavior. Ph.D. Thesis, The City College of New York, New York, NY, USA, 2015. [Google Scholar]
- Honciuc, A. Amphiphilic Janus Particles at Interfaces. In Flowing Matter; Federico, T., Sega, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 95–136. [Google Scholar]
- Binks, B.P.; Fletcher, P.D.I. Particles Adsorbed at the Oil−Water Interface: A Theoretical Comparison between Spheres of Uniform Wettability and “Janus” Particles. Langmuir 2001, 17, 4708–4710. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Tu, F.Q.; Lee, D. Shape-Changing and Amphiphilicity-Reversing Janus Particles with pH-Responsive Surfactant Properties. J. Am. Chem. Soc. 2014, 136, 9999–10006. [Google Scholar] [CrossRef]
- Glaser, N.; Adams, D.J.; Böker, A.; Krausch, G. Janus Particles at Liquid−Liquid Interfaces. Langmuir 2006, 22, 5227–5229. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.A.; Percebom, A.M.; Giner-Casares, J.J.; Rodríguez-Valverde, M.A.; Cabrerizo-Vílchez, M.A.; Liz-Marzán, L.M.; Hidalgo-Álvarez, R. Interfacial Activity of Gold Nanoparticles Coated with a Polymeric Patchy Shell and the Role of Spreading Agents. ACS Omega 2016, 1, 311–317. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, M.A.; Ramos, J.; Isa, L.; Rodriguez-Valverde, M.A.; Cabrerizo-Vilchez, M.A.; Hidalgo-Alvarez, R. Interfacial Activity and Contact Angle of Homogeneous, Functionalized, and Janus Nanoparticles at the Water/Decane Interface. Langmuir 2015, 31, 8818–8823. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Hernandez, L.M.; Read, A.; Vargas, W.L.; Kretzschmar, I. Surface tension anomaly observed for chemically-modified Janus particles at the air/water interface. J. Colloid Interface Sci. 2020, 558, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Choi, J.; Li, H.Y.; Jia, Y.K.; Huang, R.J.; Stebe, K.J.; Lee, D. Janus Particles with Varying Configurations for Emulsion Stabilization. Ind. Eng. Chem. Res. 2019, 58, 20961–20968. [Google Scholar] [CrossRef]
- Yin, T.; Yang, Z.; Dong, Z.; Lin, M.; Zhang, J. Physicochemical properties and potential applications of silica-based amphiphilic Janus nanosheets for enhanced oil recovery. Fuel 2019, 237, 344–351. [Google Scholar] [CrossRef]
- He, X.; Liang, C.; Liu, Q.; Xu, Z. Magnetically responsive Janus nanoparticles synthesized using cellulosic materials for enhanced phase separation in oily wastewaters and water-in-crude oil emulsions. Chem. Eng. J. 2019, 378, 122045. [Google Scholar] [CrossRef]
- Yu, X.; Huang, S.; Chen, K.; Zhou, Z.; Guo, X.; Li, L. Preparation of Functional Janus Particles with Response to Magnetic Force. Ind. Eng. Chem. Res. 2015, 54, 2690–2696. [Google Scholar] [CrossRef]
- Xue, W.; Yang, H.; Du, Z. Synthesis of pH-Responsive Inorganic Janus Nanoparticles and Experimental Investigation of the Stability of Their Pickering Emulsions. Langmuir 2017, 33, 10283–10290. [Google Scholar] [CrossRef]
- Haney, B.; Werner, J.G.; Weitz, D.A.; Ramakrishnan, S. Absorbent–Adsorbates: Large Amphiphilic Janus Microgels as Droplet Stabilizers. ACS Appl. Mater. Interfaces 2020, 12, 33439–33446. [Google Scholar] [CrossRef]
- Ku, K.H.; Lee, Y.J.; Yi, G.-R.; Jang, S.G.; Schmidt, B.V.K.J.; Liao, K.; Klinger, D.; Hawker, C.J.; Kim, B.J. Shape-Tunable Biphasic Janus Particles as pH-Responsive Switchable Surfactants. Macromolecules 2017, 50, 9276–9285. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, M.A.; Rodriguez-Valverde, M.A.; Cabrerizo-Vilchez, M.A.; Hidalgo-Alvarez, R. Surface activity of Janus particles adsorbed at fluid–fluid interfaces: Theoretical and experimental aspects. Adv. Colloid Interface Sci. 2016, 233, 240–254. [Google Scholar] [CrossRef]
- Wu, H.; Gao, K.; Lu, Y.; Meng, Z.; Gou, C.; Li, Z.; Yang, M.; Qu, M.; Liu, T.; Hou, J.; et al. Silica-based amphiphilic Janus nanofluid with improved interfacial properties for enhanced oil recovery. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 586, 124162. [Google Scholar] [CrossRef]
- Beltramo, P.J.; Gupta, M.; Alicke, A.; Liascukiene, I.; Gunes, D.Z.; Baroud, C.N.; Vermant, J. Arresting dissolution by interfacial rheology design. Proc. Natl. Acad. Sci. 2017, 114, 10373–10378. [Google Scholar] [CrossRef] [PubMed]
- Franck, A.; Hodder, P. Measuring the Rheological Properties of Ultrathin Films at the Water-Air and Water-Oil Interface by Using a Novel Double Wall Ring (DWR) Geometry. Annu. Trans. Nordic Rheol. Soc. 2009, 17, 1–4. [Google Scholar]
- Yin, T.; Shin, D.; Frechette, J.; Colosqui, C.; Drazer, G. Dynamic Effects on the Mobilization of a Deposited Nanoparticle by a Moving Liquid-Liquid Interface. Phys. Rev. Lett. 2018, 121, 1–6. [Google Scholar] [CrossRef]
- Rahman, S.E.; Laal-Dehghani, N.; Christopher, G.F. Interfacial Viscoelasticity of Self-Assembled Hydrophobic/Hydrophilic Particles at an Air/Water Interface. Langmuir 2019, 35, 13116–13125. [Google Scholar] [CrossRef]
- Safouane, M.; Langevin, D.; Binks, B.P. Effect of Particle Hydrophobicity on the Properties of Silica Particle Layers at the Air−Water Interface. Langmuir 2007, 23, 11546–11553. [Google Scholar] [CrossRef]
- Razavi, S.; Cao, K.D.; Lin, B.; Lee, K.Y.C.; Tu, R.S.; Kretzschmar, I. Collapse of Particle-Laden Interfaces under Compression: Buckling vs Particle Expulsion. Langmuir 2015, 31, 7764–7775. [Google Scholar] [CrossRef]
- Rezvantalab, H.; Shojaei-Zadeh, S. Capillary interactions between spherical Janus particles at liquid–fluid interfaces. Soft Matter 2013, 9, 3640–3650. [Google Scholar] [CrossRef]
- Correia, E.L.; Nguyen, T.D.; Vu, T.V.; Papavassiliou, D.V.; Razavi, S. Unpublished data.
- Ballard, N.; Law, A.D.; Bon, S.A.F. Colloidal particles at fluid interfaces: Behaviour of isolated particles. Soft Matter 2019, 15, 1186–1199. [Google Scholar] [CrossRef]
- Razavi, S.; Lin, B.; Lee, K.Y.C.; Tu, R.S.; Kretzschmar, I. Impact of surface amphiphilicity on the interfacial behavior of Janus particle layers under compression. Langmuir: ACS J. Surf. Colloids 2019, 35, 15813–15824. [Google Scholar] [CrossRef]
- Pieranski, P. Two-Dimensional Interfacial Colloidal Crystals. Phys. Rev. Lett. 1980, 45, 569–572. [Google Scholar] [CrossRef]
- Dinsmore, A.D.; Hsu, M.F.; Nikolaides, M.G.; Marquez, M.; Bausch, A.R.; Weitz, D.A. Colloidosomes: Selectively Permeable Capsules Composed of Colloidal Particles. Science 2002, 298, 1006. [Google Scholar] [CrossRef] [PubMed]
- Colosqui, C.E.; Morris, J.F.; Koplik, J. Colloidal Adsorption at Fluid Interfaces: Regime Crossover from Fast Relaxation to Physical Aging. Phys. Rev. Lett. 2013, 111, 028302. [Google Scholar] [CrossRef] [PubMed]
- Kaz, D.M.; McGorty, R.; Mani, M.; Brenner, M.P.; Manoharan, V.N. Physical ageing of the contact line on colloidal particles at liquid interfaces. Nat. Mater. 2012, 11, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.M.; Wang, A.; Manoharan, V.N.; Colosqui, C.E. Colloidal particle adsorption at liquid interfaces: Capillary driven dynamics and thermally activated kinetics. Soft Matter 2016, 12, 6365–6372. [Google Scholar] [CrossRef]
- Razavi, S.; Kretzschmar, I.; Koplik, J.; Colosqui, C.E. Nanoparticles at liquid interfaces: Rotational dynamics and angular locking. J. Chem. Phys. 2014, 140, 014904. [Google Scholar] [CrossRef] [PubMed]
- Anjali, T.G.; Basavaraj, M.G. Contact angle and detachment energy of shape anisotropic particles at fluid-fluid interfaces. J. Colloid Interface Sci. 2016, 478, 63–71. [Google Scholar] [CrossRef]
- Ondarçuhu, T.; Fabre, P.; Raphaël, E.; Veyssié, M. Specific properties of amphiphilic particles at fluid interfaces. J. Phys. Fr. 1990, 51, 1527–1536. [Google Scholar] [CrossRef]
- Jiang, S.; Granick, S. Janus balance of amphiphilic colloidal particles. J. Chem. Phys. 2007, 127, 161102. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, Y. Macrophage Uptake of Janus Particles Depends upon Janus Balance. Langmuir 2015, 31, 2833–2838. [Google Scholar] [CrossRef]
- Khanum, R. Effect of Janus particle concentration on water-repellent finish of textile fabric. J. Text. Inst. 2017, 108, 543–546. [Google Scholar] [CrossRef]
- Fujii, S.; Yokoyama, Y.; Miyanari, Y.; Shiono, T.; Ito, M.; Yusa, S.-i.; Nakamura, Y. Micrometer-Sized Gold–Silica Janus Particles as Particulate Emulsifiers. Langmuir 2013, 29, 5457–5465. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Brugarolas, T.; Lee, D. Janus particles at an oil–water interface. Soft Matter 2011, 7, 6413–6417. [Google Scholar] [CrossRef]
- Knapp, E.M.; Dagastine, R.R.; Tu, R.S.; Kretzschmar, I. Effect of Orientation and Wetting Properties on the Behavior of Janus Particles at the Air-Water Interface. ACS Appl. Mater. Interfaces 2020, 12, 5128–5135. [Google Scholar] [CrossRef]
- Wu, D.; Chew, J.W.; Honciuc, A. Polarity Reversal in Homologous Series of Surfactant-Free Janus Nanoparticles: Toward the Next Generation of Amphiphiles. Langmuir 2016, 32, 6376–6386. [Google Scholar] [CrossRef]
- Gao, H.-M.; Lu, Z.-Y.; Liu, H.; Sun, Z.-Y.; An, L.-J. Orientation and surface activity of Janus particles at fluid-fluid interfaces. J. Chem. Phys. 2014, 141, 134907. [Google Scholar] [CrossRef]
- Koplik, J.; Maldarelli, C. Molecular dynamics study of the translation and rotation of amphiphilic Janus nanoparticles at a vapor-liquid surface. Phys. Rev. Fluids 2019, 4, 23. [Google Scholar] [CrossRef]
- Cheung, D.L.; Bon, S.A.F. Stability of Janus nanoparticles at fluid interfaces. Soft Matter 2009, 5, 3969–3976. [Google Scholar] [CrossRef]
- Ruhland, T.M.; Gröschel, A.H.; Walther, A.; Müller, A.H.E. Janus Cylinders at Liquid–Liquid Interfaces. Langmuir 2011, 27, 9807–9814. [Google Scholar] [CrossRef]
- Ballard, N.; Bon, S.A.F. Equilibrium orientations of non-spherical and chemically anisotropic particles at liquid–liquid interfaces and the effect on emulsion stability. J. Colloid Interface Sci. 2015, 448, 533–544. [Google Scholar] [CrossRef]
- Borówko, M.; Słyk, E.; Sokołowski, S.; Staszewski, T. Janus Dimers at Liquid–Liquid Interfaces. J. Phys. Chem. B 2019, 123, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; In, M.; Blanc, C.; Malgaretti, P.; Nobili, M.; Stocco, A. Wetting and orientation of catalytic Janus colloids at the surface of water. Faraday Discuss. 2016, 191, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Günther, F.; Xie, Q.; Harting, J. Equilibrium Orientation and Adsorption of an Ellipsoidal Janus Particle at a Fluid–Fluid Interface. Colloids Interfaces 2020, 4, 55. [Google Scholar] [CrossRef]
- Bleibel, J.; Domínguez, A.; Oettel, M. Colloidal particles at fluid interfaces: Effective interactions, dynamics and a gravitation–like instability. Eur. Phys. J. Spec. Top. 2013, 222, 3071–3087. [Google Scholar] [CrossRef]
- Hurd, A.J. The electrostatic interaction between interfacial colloidal particles. J. Phys. A: Math. Gen. 1985, 18, L1055–L1060. [Google Scholar] [CrossRef]
- Hurd, A.J.; Schaefer, D.W. Diffusion-Limited Aggregation in Two Dimensions. Phys. Rev. Lett. 1985, 54, 1043–1046. [Google Scholar] [CrossRef]
- Girotto, M.; dos Santos, A.P.; Levin, Y. Interaction of Charged Colloidal Particles at the Air–Water Interface. J. Phys. Chem. B 2016, 120, 5817–5822. [Google Scholar] [CrossRef]
- Park, B.J.; Vermant, J.; Furst, E.M. Heterogeneity of the electrostatic repulsion between colloids at the oil–water interface. Soft Matter 2010, 6, 5327–5333. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H.; Fletcher, P.D.I.; Horozov, T.S.; Neumann, B.; Paunov, V.N.; Annesley, J.; Botchway, S.W.; Nees, D.; et al. Measurement of Long-Range Repulsive Forces between Charged Particles at an Oil-Water Interface. Phys. Rev. Lett. 2002, 88, 246102. [Google Scholar] [CrossRef]
- Park, B.J.; Pantina, J.P.; Furst, E.M.; Oettel, M.; Reynaert, S.; Vermant, J. Direct Measurements of the Effects of Salt and Surfactant on Interaction Forces between Colloidal Particles at Water−Oil Interfaces. Langmuir 2008, 24, 1686–1694. [Google Scholar] [CrossRef]
- Oettel, M.; Dietrich, S. Colloidal Interactions at Fluid Interfaces. Langmuir 2008, 24, 1425–1441. [Google Scholar] [CrossRef]
- Park, B.J.; Furst, E.M. Attractive interactions between colloids at the oil–water interface. Soft Matter 2011, 7, 7676–7682. [Google Scholar] [CrossRef]
- Quesada-Pérez, M.; Moncho-Jordá, A.; Martínez-López, F.; Hidalgo-Álvarez, R. Probing interaction forces in colloidal monolayers: Inversion of structural data. J. Chem. Phys. 2001, 115, 10897–10902. [Google Scholar] [CrossRef]
- Horozov, T.S.; Aveyard, R.; Binks, B.P.; Clint, J.H. Structure and Stability of Silica Particle Monolayers at Horizontal and Vertical Octane−Water Interfaces. Langmuir 2005, 21, 7405–7412. [Google Scholar] [CrossRef]
- Xu, H.; Liu, X.; Su, G.; Zhang, B.; Wang, D. Electrostatic Repulsion-Controlled Formation of Polydopamine–Gold Janus Particles. Langmuir 2012, 28, 13060–13065. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. 6—Van der Waals Forces. In Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J.N., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 107–132. [Google Scholar]
- Fernández-Toledano, J.C.; Moncho-Jordá, A.; Martínez-López, F.; Hidalgo-Álvarez, R. Theory for Interactions between Particles in Monolayers. In Colloidal Particles at Liquid Interfaces; Binks, B.P., Horozov, T.S., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 108–151. [Google Scholar]
- Ghezzi, F.; Earnshaw, J.C. Formation of meso-structures in colloidal monolayers. J. Phys. Condens. Matter 1997, 9, L517–L523. [Google Scholar] [CrossRef]
- Ghezzi, F.; Earnshaw, J.C.; Finnis, M.; McCluney, M. Pattern Formation in Colloidal Monolayers at the Air–Water Interface. J. Colloid Interface Sci. 2001, 238, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garcia, J.; Gámez-Corrales, R.; Ivlev, B.I. Foam and cluster structure formation by latex particles at the air/water interface. Phys. A: Stat. Mech. Its Appl. 1997, 236, 97–104. [Google Scholar] [CrossRef]
- Ruiz-García, J.; Gámez-Corrales, R.; Ivlev, B.I. Formation of two-dimensional colloidal voids, soap froths, and clusters. Phys. Rev. E 1998, 58, 660–663. [Google Scholar] [CrossRef]
- Onoda, G.Y. Direct observation of two-dimensional, dynamic clustering and ordering with colloids. Phys. Rev. Lett. 1985, 55, 226–229. [Google Scholar] [CrossRef]
- Kralchevsky, P.A.; Paunov, V.N.; Ivanov, I.B.; Nagayama, K. Capillary meniscus interaction between colloidal particles attached to a liquid—fluid interface. J. Colloid Interface Sci. 1992, 151, 79–94. [Google Scholar] [CrossRef]
- Paunov, V.N.; Kralchevsky, P.A.; Denkov, N.D.; Nagayama, K. Lateral Capillary Forces between Floating Submillimeter Particles. J. Colloid Interface Sci. 1993, 157, 100–112. [Google Scholar] [CrossRef]
- Danov, K.D.; Kralchevsky, P.A.; Naydenov, B.N.; Brenn, G. Interactions between particles with an undulated contact line at a fluid interface: Capillary multipoles of arbitrary order. J. Colloid Interface Sci. 2005, 287, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Kralchevsky, P.A.; Nagayama, K. Capillary interactions between particles bound to interfaces, liquid films and biomembranes. Adv. Colloid Interface Sci. 2000, 85, 145–192. [Google Scholar] [CrossRef]
- Vassileva, N.D.; van den Ende, D.; Mugele, F.; Mellema, J. Capillary Forces between Spherical Particles Floating at a Liquid−Liquid Interface. Langmuir 2005, 21, 11190–11200. [Google Scholar] [CrossRef]
- Oettel, M.; Domínguez, A.; Dietrich, S. Effective capillary interaction of spherical particles at fluid interfaces. Phys. Rev. E 2005, 71, 051401. [Google Scholar] [CrossRef]
- Stamou, D.; Duschl, C.; Johannsmann, D. Long-range attraction between colloidal spheres at the air-water interface: The consequence of an irregular meniscus. Phys. Rev. E 2000, 62, 5263–5272. [Google Scholar] [CrossRef]
- Danov, K.D.; Pouligny, B.; Kralchevsky, P.A. Capillary Forces between Colloidal Particles Confined in a Liquid Film: The Finite-Meniscus Problem. Langmuir 2001, 17, 6599–6609. [Google Scholar] [CrossRef]
- Nikolaides, M.G.; Bausch, A.R.; Hsu, M.F.; Dinsmore, A.D.; Brenner, M.P.; Gay, C.; Weitz, D.A. Electric-field-induced capillary attraction between like-charged particles at liquid interfaces. Nature 2002, 420, 299–301. [Google Scholar] [CrossRef]
- Megens, M.; Aizenberg, J. Like-charged particles at liquid interfaces. Nature 2003, 424, 1014-1014. [Google Scholar] [CrossRef]
- Goggin, D.M.; Samaniuk, J.R. Dynamics of Pristine Graphite and Graphene at an Air-Water Interface. AICHE J. 2018, 64, 3177–3187. [Google Scholar] [CrossRef]
- Vella, D.; Mahadevan, L. The “Cheerios effect”. Am. J. Phys. 2005, 73, 817–825. [Google Scholar] [CrossRef]
- Kralchevsky, P.A.; Nagayama, K. Capillary forces between colloidal particles. Langmuir 1994, 10, 23–36. [Google Scholar] [CrossRef]
- Danov, K.D.; Kralchevsky, P.A.; Boneva, M.P. Electrodipping Force Acting on Solid Particles at a Fluid Interface. Langmuir 2004, 20, 6139–6151. [Google Scholar] [CrossRef]
- Lewandowski, E.P.; Cavallaro, M.; Botto, L.; Bernate, J.C.; Garbin, V.; Stebe, K.J. Orientation and Self-Assembly of Cylindrical Particles by Anisotropic Capillary Interactions. Langmuir 2010, 26, 15142–15154. [Google Scholar] [CrossRef]
- Kang, D.W.; Choi, K.H.; Lee, S.J.; Park, B.J. Mapping Anisotropic and Heterogeneous Colloidal Interactions via Optical Laser Tweezers. J. Phys. Chem. Lett. 2019, 10, 1691–1697. [Google Scholar] [CrossRef]
- Lee, M.; Xia, M.; Park, B.J. Transition Behaviors of Configurations of Colloidal Particles at a Curved Oil-Water Interface. Materials 2016, 9, 138. [Google Scholar] [CrossRef]
- Ferrar, J.A.; Bedi, D.S.; Zhou, S.; Zhu, P.; Mao, X.; Solomon, M.J. Capillary-driven binding of thin triangular prisms at fluid interfaces. Soft Matter 2018, 14, 3902–3918. [Google Scholar] [CrossRef]
- Liu, I.B.; Sharifi-Mood, N.; Stebe, K.J. Capillary Assembly of Colloids: Interactions on Planar and Curved Interfaces. Annu. Rev. Condens. Matter Phys. 2018, 9, 283–305. [Google Scholar] [CrossRef]
- Botto, L.; Lewandowski, E.P.; Cavallaro, M.; Stebe, K.J. Capillary interactions between anisotropic particles. Soft Matter 2012, 8, 9957–9971. [Google Scholar] [CrossRef]
- Brugarolas, T.; Park, B.; Lee, M.; Lee, D. Generation of Amphiphilic Janus Bubbles and Their Behavior at an Air–Water Interface. Adv. Funct. Mater. 2011, 21, 3924–3931. [Google Scholar] [CrossRef]
- Katepalli, H.; John, V.T.; Tripathi, A.; Bose, A. Microstructure and rheology of particle stabilized emulsions: Effects of particle shape and inter-particle interactions. J. Colloid Interface Sci. 2017, 485, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, H.; Shojaei-Zadeh, S. Role of Geometry and Amphiphilicity on Capillary-Induced Interactions between Anisotropic Janus Particles. Langmuir 2013, 29, 14962–14970. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Cacciuto, A.; Luijten, E.; Granick, S. Clusters of Charged Janus Spheres. Nano Lett. 2006, 6, 2510–2514. [Google Scholar] [CrossRef] [PubMed]
- Aveyard, R.; Clint, J.H.; Nees, D.; Quirke, N. Structure and Collapse of Particle Monolayers under Lateral Pressure at the Octane/Aqueous Surfactant Solution Interface. Langmuir 2000, 16, 8820–8828. [Google Scholar] [CrossRef]
- Luo, M.; Olivier, G.K.; Frechette, J. Electrostatic interactions to modulate the reflective assembly of nanoparticles at the oil–water interface. Soft Matter 2012, 8, 11923–11932. [Google Scholar] [CrossRef]
- Deshmukh, O.S.; van den Ende, D.; Stuart, M.C.; Mugele, F.; Duits, M.H.G. Hard and soft colloids at fluid interfaces: Adsorption, interactions, assembly & rheology. Adv. Colloid Interface Sci. 2015, 222, 215–227. [Google Scholar]
- Pawar, A.B.; Caggioni, M.; Ergun, R.; Hartel, R.W.; Spicer, P.T. Arrested coalescence in Pickering emulsions. Soft Matter 2011, 7, 7710–7716. [Google Scholar] [CrossRef]
- Fuller, G.G.; Vermant, J. Complex Fluid-Fluid Interfaces: Rheology and Structure. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 519–543. [Google Scholar] [CrossRef]
- Miller, R.; Wüstneck, R.; Krägel, J.; Kretzschmar, G. Dilational and shear rheology of adsorption layers at liquid interfaces. Colloids Surf. A: Physicochem. Eng. Asp. 1996, 111, 75–118. [Google Scholar] [CrossRef]
- Krägel, J.; Derkatch, S.R. Interfacial shear rheology. Curr. Opin. Colloid Interface Sci. 2010, 15, 246–255. [Google Scholar] [CrossRef]
- Ravera, F.; Loglio, G.; Kovalchuk, V.I. Interfacial dilational rheology by oscillating bubble/drop methods. Curr. Opin. Colloid Interface Sci. 2010, 15, 217–228. [Google Scholar] [CrossRef]
- Derkach, S.R.; Krägel, J.; Miller, R. Methods of measuring rheological properties of interfacial layers (Experimental methods of 2D rheology). Colloid J. 2009, 71, 1–17. [Google Scholar] [CrossRef]
- Maestro, A.; Santini, E.; Zabiegaj, D.; Llamas, S.; Ravera, F.; Liggieri, L.; Ortega, F.; Rubio, R.G.; Guzman, E. Particle and Particle-Surfactant Mixtures at Fluid Interfaces: Assembly, Morphology, and Rheological Description. Adv. Condens. Matter Phys. 2015, 2015, 917516. [Google Scholar] [CrossRef]
- Miller, R.; Ferri, J.K.; Javadi, A.; Krägel, J.; Mucic, N.; Wüstneck, R. Rheology of interfacial layers. Colloid Polym. Sci. 2010, 288, 937–950. [Google Scholar] [CrossRef]
- Miller, R.; Liggieri, L. Interfacial Rheology; CRC Press: Boca Raton, FL, USA, 2009; Volume 1. [Google Scholar]
- Correia, E.L.; Razavi, S. Unpublished data.
- Banks, H.T.; Hu, S.; Kenz, Z.R. A Brief Review of Elasticity and Viscoelasticity for Solids. Adv. Appl. Math. Mech. 2011, 3, 1–51. [Google Scholar] [CrossRef]
- Zasadzinski, J.A.; Ding, J.; Warriner, H.E.; Bringezu, F.; Waring, A.J. The physics and physiology of lung surfactants. Curr. Opin. Colloid Interface Sci. 2001, 6, 506–513. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kristl, J.; Rošic, R.; Baumgartner, S.; Kocbek, P. Interfacial rheology: An overview of measuring techniques and its role in dispersions and electrospinning. Acta Pharm. (Zagrebcroatia) 2012, 62, 123–140. [Google Scholar] [CrossRef]
- Erk, K.A.; Martin, J.D.; Schwalbe, J.T.; Phelan, F.R.; Hudson, S.D. Shear and dilational interfacial rheology of surfactant-stabilized droplets. J. Colloid Interface Sci. 2012, 377, 442–449. [Google Scholar] [CrossRef]
- Jaensson, N.; Vermant, J. Tensiometry and rheology of complex interfaces. Curr. Opin. Colloid Interface Sci. 2018, 37, 136–150. [Google Scholar] [CrossRef]
- Sashuk, V.; Hołyst, R.; Wojciechowski, T.; Fiałkowski, M. Close-packed monolayers of charged Janus-type nanoparticles at the air–water interface. J. Colloid Interface Sci. 2012, 375, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.R.; Bognet, B.; Patanwala, H.S.; Chinesta, F.; Ma, A.W.K. Surface Pressure and Microstructure of Carbon Nanotubes at an Air–Water Interface. Langmuir 2015, 31, 4663–4672. [Google Scholar] [CrossRef] [PubMed]
- Gopal, A.; Lee, K.Y.C. Headgroup Percolation and Collapse of Condensed Langmuir Monolayers. J. Phys. Chem. B 2006, 110, 22079–22087. [Google Scholar] [CrossRef] [PubMed]
- Kwok, D.Y.; Vollhardt, D.; Miller, R.; Li, D.; Neumann, A.W. Axisymmetric drop shape analysis as a film balance. Colloids Surf. A: Physicochem. Eng. Asp. 1994, 88, 51–58. [Google Scholar] [CrossRef]
- Ferdous, S.; Ioannidis, M.A.; Henneke, D. Adsorption kinetics of alkanethiol-capped gold nanoparticles at the hexane–water interface. J. Nanoparticle Res. 2011, 13, 6579–6589. [Google Scholar] [CrossRef]
- Garbin, V.; Crocker, J.C.; Stebe, K.J. Forced Desorption of Nanoparticles from an Oil–Water Interface. Langmuir 2012, 28, 1663–1667. [Google Scholar] [CrossRef]
- Alvarez, N.J.; Anna, S.L.; Saigal, T.; Tilton, R.D.; Walker, L.M. Interfacial Dynamics and Rheology of Polymer-Grafted Nanoparticles at Air–Water and Xylene–Water Interfaces. Langmuir 2012, 28, 8052–8063. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, M.A.; Song, Y.; Rodríguez-Valverde, M.Á.; Chen, S.; Cabrerizo-Vilchez, M.A.; Hidalgo-Alvarez, R. Comparison of the Interfacial Activity between Homogeneous and Janus Gold Nanoparticles by Pendant Drop Tensiometry. Langmuir 2014, 30, 1799–1804. [Google Scholar] [CrossRef]
- Reynaert, S.; Brooks, C.F.; Moldenaers, P.; Vermant, J.; Fuller, G.G. Analysis of the magnetic rod interfacial stress rheometer. J. Rheol. 2008, 52, 261–285. [Google Scholar] [CrossRef]
- Fitzgibbon, S.; Shaqfeh, E.S.G.; Fuller, G.G.; Walker, T.W. Scaling analysis and mathematical theory of the interfacial stress rheometer. J. Rheol. 2014, 58, 999–1038. [Google Scholar] [CrossRef]
- Tajuelo, J.; Pastor, J.; Martínez-Pedrero, F.; Vázquez, M.; Ortega, F.; Rubio, R.; Rubio, M. Magnetic microwire probes for the magnetic rod interfacial stress rheometer. Langmuir 2015, 31, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Erni, P.; Fischer, P.; Windhab, E.J.; Kusnezov, V.; Stettin, H.; Läuger, J. Stress- and strain-controlled measurements of interfacial shear viscosity and viscoelasticity at liquid/liquid and gas/liquid interfaces. Rev. Sci. Instrum. 2003, 74, 4916–4924. [Google Scholar] [CrossRef]
- Vandebril, S.; Franck, A.; Fuller, G.G.; Moldenaers, P.; Vermant, J. A double wall-ring geometry for interfacial shear rheometry. Rheol. Acta 2010, 49, 131–144. [Google Scholar] [CrossRef]
- Karbaschi, M.; Lotfi, M.; Krägel, J.; Javadi, A.; Bastani, D.; Miller, R. Rheology of interfacial layers. Curr. Opin. Colloid Interface Sci. 2014, 19, 514–519. [Google Scholar] [CrossRef]
- Zell, Z.A.; Mansard, V.; Wright, J.; Kim, K.; Choi, S.Q.; Squires, T.M. Linear and nonlinear microrheometry of small samples and interfaces using microfabricated probes. J. Rheol. 2016, 60, 141–159. [Google Scholar] [CrossRef]
- Chang, C.-C.; Nowbahar, A.; Mansard, V.; Williams, I.; Mecca, J.; Schmitt, A.K.; Kalantar, T.H.; Kuo, T.-C.; Squires, T.M. Interfacial Rheology and Heterogeneity of Aging Asphaltene Layers at the Water–Oil Interface. Langmuir 2018, 34, 5409–5415. [Google Scholar] [CrossRef]
- Renggli, D.; Alicke, A.; Ewoldt, R.H.; Vermant, J. Operating windows for oscillatory interfacial shear rheology. J. Rheol. 2020, 64, 141–160. [Google Scholar] [CrossRef]
- Garbin, V.; Crocker, J.C.; Stebe, K.J. Nanoparticles at fluid interfaces: Exploiting capping ligands to control adsorption, stability and dynamics. J. Colloid Interface Sci. 2012, 387, 1–11. [Google Scholar] [CrossRef]
- Wang, H.; Singh, V.; Behrens, S.H. Image Charge Effects on the Formation of Pickering Emulsions. J. Phys. Chem Lett 2012, 3, 2986–2990. [Google Scholar] [CrossRef]
- Leunissen, M.E.; van Blaaderen, A.; Hollingsworth, A.D.; Sullivan, M.T.; Chaikin, P.M. Electrostatics at the oil–water interface, stability, and order in emulsions and colloids. Proc. Natl. Acad. Sci. 2007, 104, 2585–2590. [Google Scholar] [CrossRef]
- Maestro, A.; Bonales, L.J.; Ritacco, H.; Rubio, R.G.; Ortega, F. Effect of the spreading solvent on the three-phase contact angle of microparticles attached at fluid interfaces. Phys. Chem. Chem. Phys. 2010, 12, 14115–14120. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, M.A.; Rodríguez-Valverde, M.A.; Cabrerizo-Vílchez, M.; Hidalgo-Alvarez, R. Surface activity and collective behaviour of colloidally stable Janus-like particles at the air–water interface. Soft Matter 2014, 10, 3471–3476. [Google Scholar] [CrossRef] [PubMed]
- Beloqui Redondo, A.; Jordan, I.; Ziazadeh, I.; Kleibert, A.; Giorgi, J.B.; Wörner, H.J.; May, S.; Abbas, Z.; Brown, M.A. Nanoparticle-Induced Charge Redistribution of the Air–Water Interface. J. Phys. Chem. C 2015, 119, 2661–2668. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, H.G.; Biggs, S.; Xu, Z.H.; Cayre, O.J.; Harbottle, D. The rheology of polyvinylpyrrolidone-coated silica nanoparticles positioned at an air-aqueous interface. J. Colloid Interface Sci. 2018, 527, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Zang, D.Y.; Rio, E.; Delon, G.; Langevin, D.; Wei, B.; Binks, B.P. Influence of the contact angle of silica nanoparticles at the air–water interface on the mechanical properties of the layers composed of these particles. Mol. Phys. 2011, 109, 1057–1066. [Google Scholar] [CrossRef]
- Schultz, D.G.; Lin, X.-M.; Li, D.; Gebhardt, J.; Meron, M.; Viccaro, J.; Lin, B. Structure, Wrinkling, and Reversibility of Langmuir Monolayers of Gold Nanoparticles. J. Phys. Chem. B 2006, 110, 24522–24529. [Google Scholar] [CrossRef] [PubMed]
- Kostakis, T.; Ettelaie, R.; Murray, B.S. Effect of High Salt Concentrations on the Stabilization of Bubbles by Silica Particles. Langmuir 2006, 22, 1273–1280. [Google Scholar] [CrossRef]
- Kim, K.; Kim, B.Q.; Kim, J.Q.; Choi, S.Q. New Collapse Mechanism of Colloidal Particle Monolayers via Depletion Pressure: Formation of Large-Area Particle Multilayers at the Air–Water Interface. J. Phys. Chem. C 2019, 123, 27862–27867. [Google Scholar] [CrossRef]
- Garbin, V. Collapse mechanisms and extreme deformation of particle-laden interfaces. Curr. Opin. Colloid Interface Sci. 2019, 39, 202–211. [Google Scholar] [CrossRef]
- Yazhgur, P.A.; Noskov, B.A.; Liggieri, L.; Lin, S.Y.; Loglio, G.; Miller, R.; Ravera, F. Dynamic properties of mixed nanoparticle/surfactant adsorption layers. Soft Matter 2013, 9, 3305–3314. [Google Scholar] [CrossRef]
- Ravera, F.; Ferrari, M.; Liggieri, L.; Loglio, G.; Santini, E.; Zanobini, A. Liquid–liquid interfacial properties of mixed nanoparticle–surfactant systems. Colloids Surf. A: Physicochem. Eng. Asp. 2008, 323, 99–108. [Google Scholar] [CrossRef]
- Lu, T.; Li, Z.; Zhou, Y. Flow Behavior and Displacement Mechanisms of Nanoparticle Stabilized Foam Flooding for Enhanced Heavy Oil Recovery. Energies 2017, 10, 560. [Google Scholar]
- Vella, D.; Aussillous, P.; Mahadevan, L. Elasticity of an interfacial particle raft. EPL (Europhys. Lett.) 2004, 68, 212. [Google Scholar] [CrossRef]
- Lefebure, S.; Ménager, C.; Cabuil, V.; Assenheimer, M.; Gallet, F.; Flament, C. Langmuir Monolayers of Monodispersed Magnetic Nanoparticles Coated with a Surfactant. J. Phys. Chem. B 1998, 102, 2733–2738. [Google Scholar] [CrossRef]
- Bykov, A.G.; Loglio, G.; Miller, R.; Noskov, B.A. Dilational surface elasticity of monolayers of charged polystyrene nano- and microparticles at liquid/fluid interfaces. Colloids Surf. A: Physicochem. Eng. Asp. 2015, 485, 42–48. [Google Scholar] [CrossRef]
- Yang, X.; Bournival, G.; Ata, S. Effect of polydispersity on the behaviour of the particle-laden interface. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 607, 125494. [Google Scholar] [CrossRef]
- Miller, R.; Fainerman, V.B.; Kovalchuk, V.I.; Grigoriev, D.O.; Leser, M.E.; Michel, M. Composite interfacial layers containing micro-size and nano-size particles. Adv. Colloid Interface Sci. 2006, 128–130, 17–26. [Google Scholar] [CrossRef]
- Lam, S.; Velikov, K.P.; Velev, O.D. Pickering stabilization of foams and emulsions with particles of biological origin. Curr. Opin. Colloid Interface Sci. 2014, 19, 490–500. [Google Scholar] [CrossRef]
- Dickinson, E. Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocoll. 2017, 68, 219–231. [Google Scholar] [CrossRef]
- Bertsch, P.; Fischer, P. Interfacial Rheology of Charged Anisotropic Cellulose Nanocrystals at the Air–Water Interface. Langmuir 2019, 35, 7937–7943. [Google Scholar] [CrossRef]
- van den Berg, M.E.H.; Kuster, S.; Windhab, E.J.; Sagis, L.M.C.; Fischer, P. Nonlinear shear and dilatational rheology of viscoelastic interfacial layers of cellulose nanocrystals. Phys. Fluids 2018, 30, 072103. [Google Scholar] [CrossRef]
- Maestro, A.; Santini, E.; Guzmán, E. Physico-chemical foundations of particle-laden fluid interfaces. Eur. Phys. J. E 2018, 41, 97. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A. Tailoring the interfacial assembly of colloidal particles by engineering the mechanical properties of the interface. Curr. Opin. Colloid Interface Sci. 2019, 39, 232–250. [Google Scholar] [CrossRef]
- Wang, A.; Dimiduk, T.G.; Fung, J.; Razavi, S.; Kretzschmar, I.; Chaudhary, K.; Manoharan, V.N. Using the discrete dipole approximation and holographic microscopy to measure rotational dynamics of non-spherical colloidal particles. J. Quant. Spectrosc. Radiat. Transf. 2014, 146, 499–509. [Google Scholar] [CrossRef]
- Imperiali, L.; Liao, K.-H.; Clasen, C.; Fransaer, J.; Macosko, C.W.; Vermant, J. Interfacial Rheology and Structure of Tiled Graphene Oxide Sheets. Langmuir 2012, 28, 7990–8000. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.Á.; Rahmani, S.; Yu, C.K.J.; Rodríguez-Valverde, M.Á.; Cabrerizo-Vílchez, M.Á.; Michel, C.A.; Lahann, J.; Hidalgo-Álvarez, R. Synthesis and interfacial activity of PMMA/PtBMA Janus and homogeneous nanoparticles at water/oil interfaces. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 536, 259–265. [Google Scholar]
- Kadam, R.; Zilli, M.; Maas, M.; Rezwan, K. Nanoscale Janus Particles with Dual Protein Functionalization. Part. Part. Syst. Charact. 2018, 35, 1700332. [Google Scholar] [CrossRef]
- Lenis, J.; Razavi, S.; Cao, K.D.; Lin, B.; Lee, K.Y.C.; Tu, R.S.; Kretzschmar, I. Mechanical Stability of Polystyrene and Janus Particle Monolayers at the Air/Water Interface. J. Am. Chem. Soc. 2015, 137, 15370–15373. [Google Scholar] [CrossRef]
- Van Hooghten, R.; Blair, V.E.; Vananroye, A.; Schofield, A.B.; Vermant, J.; Thijssen, J.H.J. Interfacial Rheology of Sterically Stabilized Colloids at Liquid Interfaces and Its Effect on the Stability of Pickering Emulsions. Langmuir 2017, 33, 4107–4118. [Google Scholar] [CrossRef]
- Vishal, B.; Ghosh, P. Nonlinear Viscoelastic Behavior of Air-Water Interface Containing Surfactant-Laden Nanoparticles. Nihon Reoroji Gakkaishi 2020, 48, 15–25. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, K.; Cayre, O.J.; Harbottle, D. Interfacial Particle Dynamics: One and Two Step Yielding in Colloidal Glass. Langmuir 2016, 32, 13472–13481. [Google Scholar] [CrossRef] [PubMed]
- Cicuta, P.; Stancik, E.J.; Fuller, G.G. Shearing or Compressing a Soft Glass in 2D: Time-Concentration Superposition. Phys. Rev. Lett. 2003, 90, 236101. [Google Scholar] [CrossRef] [PubMed]
- de Schepper, I.M.; Smorenburg, H.E.; Cohen, E.G.D. Viscoelasticity in dense hard sphere colloids. Phys. Rev. Lett. 1993, 70, 2178–2181. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Christopher, G.F. Simultaneous Interfacial Rheology and Microstructure Measurement of Densely Aggregated Particle Laden Interfaces Using a Modified Double Wall Ring Interfacial Rheometer. Langmuir 2014, 30, 9752–9760. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Christopher, G.F. Role of capillarity and microstructure on interfacial viscoelasticity of particle laden interfaces. J. Rheol. 2016, 60, 35–45. [Google Scholar] [CrossRef]
- Reynaert, S.; Moldenaers, P.; Vermant, J. Interfacial rheology of stable and weakly aggregated two-dimensional suspensions. Phys. Chem. Chem. Phys. 2007, 9, 6463–6475. [Google Scholar] [CrossRef] [PubMed]
- Buttinoni, I.; Steinacher, M.; Spanke, H.T.; Pokki, J.; Bahmann, S.; Nelson, B.; Foffi, G.; Isa, L. Colloidal polycrystalline monolayers under oscillatory shear. Phys. Rev. E 2017, 95, 012610. [Google Scholar] [CrossRef] [PubMed]
- Keim, N.C.; Arratia, P.E. Yielding and microstructure in a 2D jammed material under shear deformation. Soft Matter 2013, 9, 6222–6225. [Google Scholar] [CrossRef]
- Madivala, B.; Fransaer, J.; Vermant, J. Self-Assembly and Rheology of Ellipsoidal Particles at Interfaces. Langmuir 2009, 25, 2718–2728. [Google Scholar] [CrossRef]
- Basavaraj, M.G.; Fuller, G.G.; Fransaer, J.; Vermant, J. Packing, Flipping, and Buckling Transitions in Compressed Monolayers of Ellipsoidal Latex Particles. Langmuir 2006, 22, 6605–6612. [Google Scholar] [CrossRef]
- Stancik, E.J.; Widenbrant, M.J.O.; Laschitsch, A.T.; Vermant, J.; Fuller, G.G. Structure and dynamics of particle monolayers at a liquid-liquid interface subjected to extensional flow. Langmuir 2002, 18, 4372–4375. [Google Scholar] [CrossRef]
- Kaganyuk, M.; Mohraz, A. Shear-induced deformation and interfacial jamming of solid-stabilized droplets. Soft Matter 2020, 16, 4431–4443. [Google Scholar] [CrossRef] [PubMed]
- Zang, D.; Langevin, D.; Binks, B.P.; Wei, B. Shearing particle monolayers: Strain-rate frequency superposition. Phys. Rev. E 2010, 81, 011604. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Zhang, H.; Forman, N.A.; Maynor, B.W.; Betts, D.E.; DeSimone, J.M.; Jaeger, H.M. Shear thickening and jamming in densely packed suspensions of different particle shapes. Phys. Rev. E 2011, 84, 031408. [Google Scholar] [CrossRef] [PubMed]
- Stancik, E.J.; Gavranovic, G.T.; Widenbrant, M.J.O.; Laschitsch, A.T.; Vermant, J.; Fuller, G.G. Structure and dynamics of particle monolayers at a liquid–liquid interface subjected to shear flow. Faraday Discuss. 2003, 123, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A.; Guzmán, E. Colloids at Fluid Interfaces. Processes 2019, 7, 942. [Google Scholar] [CrossRef]
- Rezvantalab, H.; Connington, K.W.; Shojaei-Zadeh, S. Shear-induced interfacial assembly of Janus particles. Phys. Rev. Fluids 2016, 1, 074205. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Arai, N.; Nikoubashman, A. Structure and dynamics of amphiphilic Janus spheres and spherocylinders under shear. Soft Matter 2020, 16, 476–486. [Google Scholar] [CrossRef]
- Bianchi, E.; Panagiotopoulos, A.Z.; Nikoubashman, A. Self-assembly of Janus particles under shear. Soft Matter 2015, 11, 3767–3771. [Google Scholar] [CrossRef]
- DeLaCruz-Araujo, R.A.; Beltran-Villegas, D.J.; Larson, R.G.; Córdova-Figueroa, U.M. Rich Janus colloid phase behavior under steady shear. Soft Matter 2016, 12, 4071–4081. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, G.; Chen, P.; Hou, C.; Yan, L.-T. Plastic Crystal-to-Crystal Transition of Janus Particles under Shear. Phys. Rev. Lett. 2019, 122, 198002. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Arai, N. Self-Assembly and Viscosity Behavior of Janus Nanoparticles in Nanotube Flow. Langmuir 2017, 33, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, H.; Shojaei-Zadeh, S. Tilting and Tumbling of Janus Nanoparticles at Sheared Interfaces. ACS Nano 2016, 10, 5354–5361. [Google Scholar] [CrossRef] [PubMed]
- Paiva, F.L.; Hore, M.J.A.; Secchi, A.; Calado, V.; Maia, J.; Khani, S. Dynamic Interfacial Trapping of Janus Nanorod Aggregates. Langmuir 2020, 36, 4184–4193. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, E.L.; Brown, N.; Razavi, S. Janus Particles at Fluid Interfaces: Stability and Interfacial Rheology. Nanomaterials 2021, 11, 374. https://doi.org/10.3390/nano11020374
Correia EL, Brown N, Razavi S. Janus Particles at Fluid Interfaces: Stability and Interfacial Rheology. Nanomaterials. 2021; 11(2):374. https://doi.org/10.3390/nano11020374
Chicago/Turabian StyleCorreia, Elton L., Nick Brown, and Sepideh Razavi. 2021. "Janus Particles at Fluid Interfaces: Stability and Interfacial Rheology" Nanomaterials 11, no. 2: 374. https://doi.org/10.3390/nano11020374
APA StyleCorreia, E. L., Brown, N., & Razavi, S. (2021). Janus Particles at Fluid Interfaces: Stability and Interfacial Rheology. Nanomaterials, 11(2), 374. https://doi.org/10.3390/nano11020374







