Synthesis of MnO/C/NiO-Doped Porous Multiphasic Composites for Lithium-Ion Batteries by Biomineralized Mn Oxides from Engineered Pseudomonas putida Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Bacterial Strains, Culture Conditions, and Mn2+-Oxidizing Activity Assay
2.2. Preparation of MnO/C/NiOComposite
2.3. Material Characterization
2.4. Assays of Electrochemical Properties
3. Results and Discussion
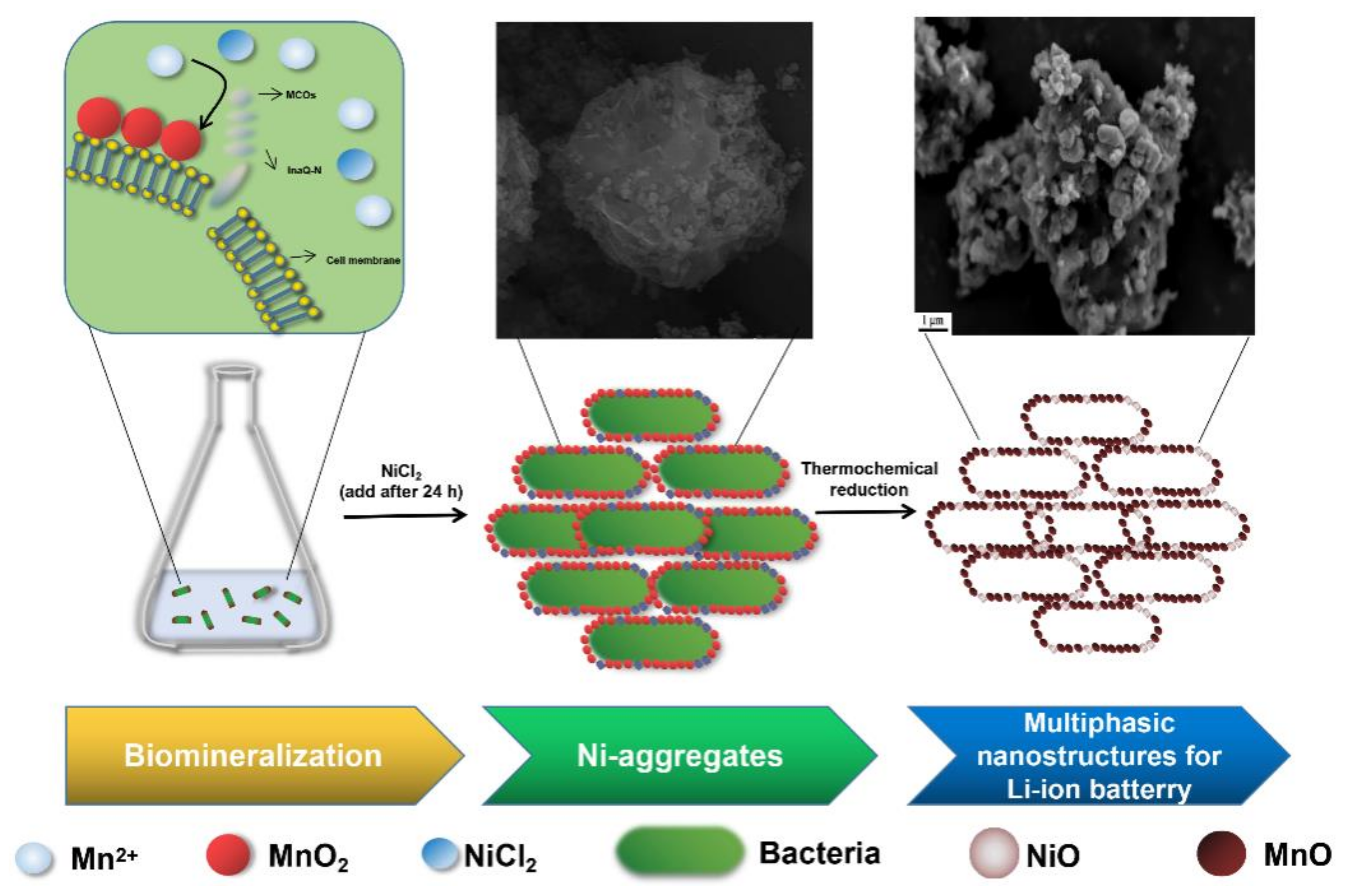
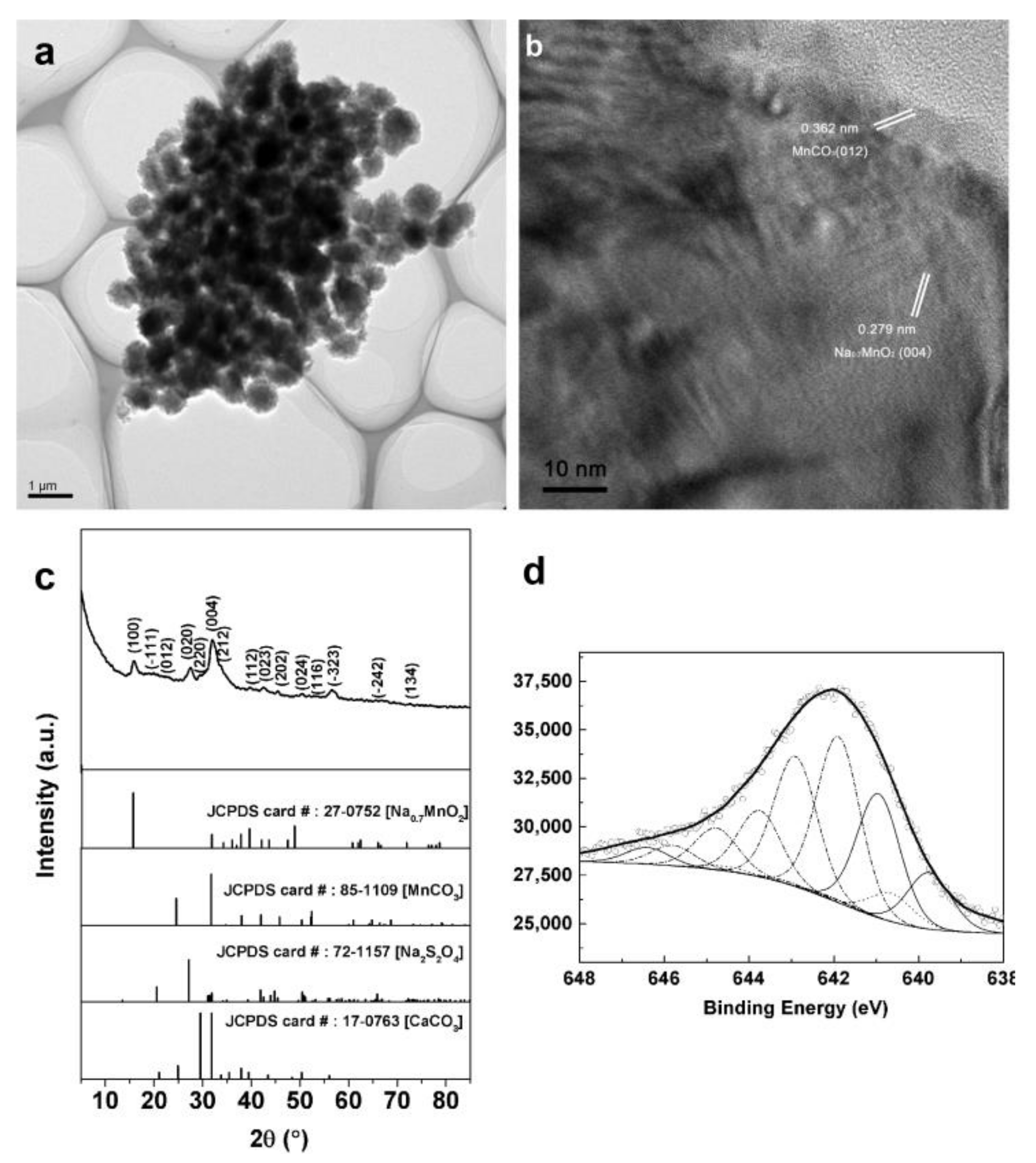
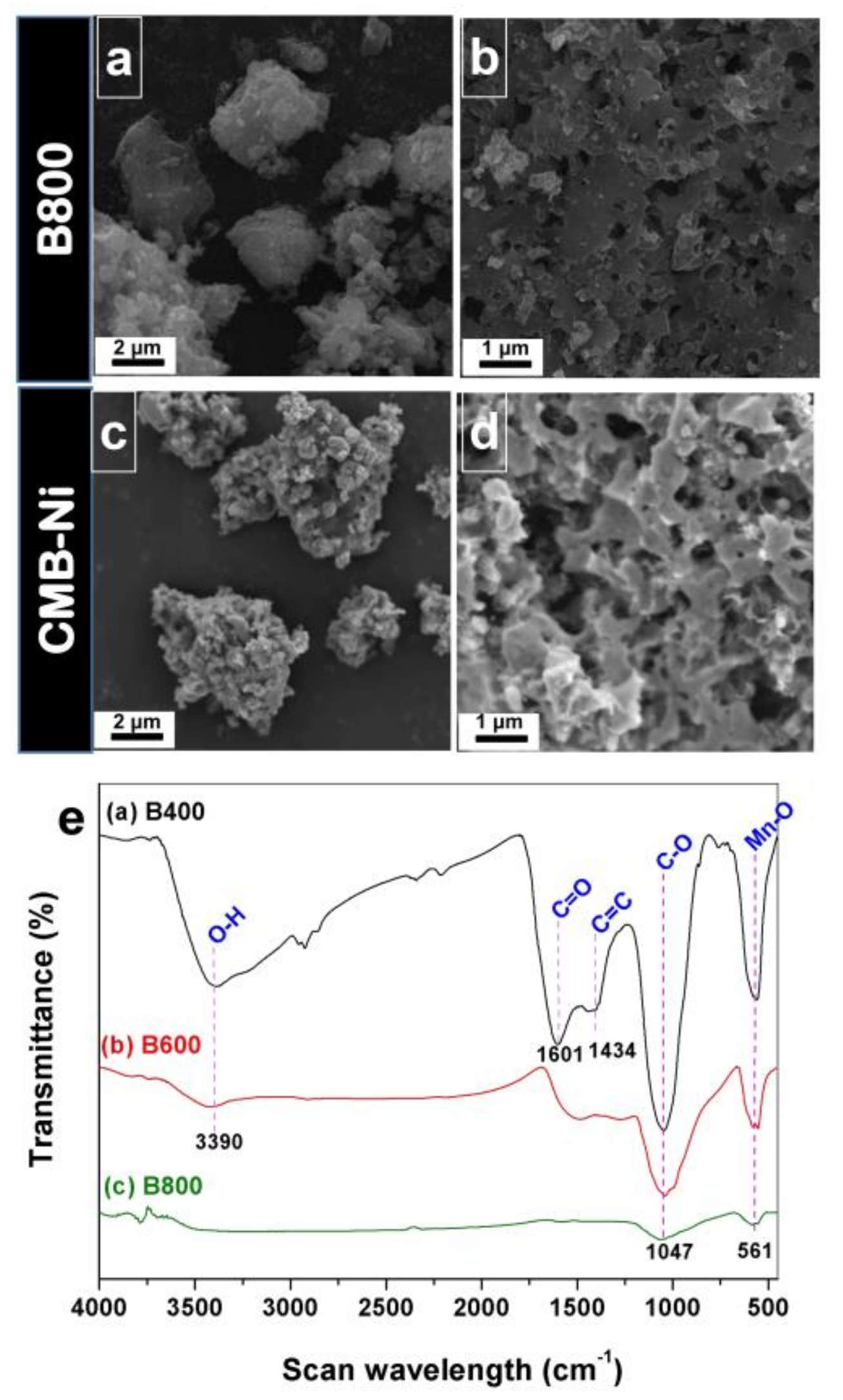
3.1. Formation and Characterization of BMB-Ni Aggregates
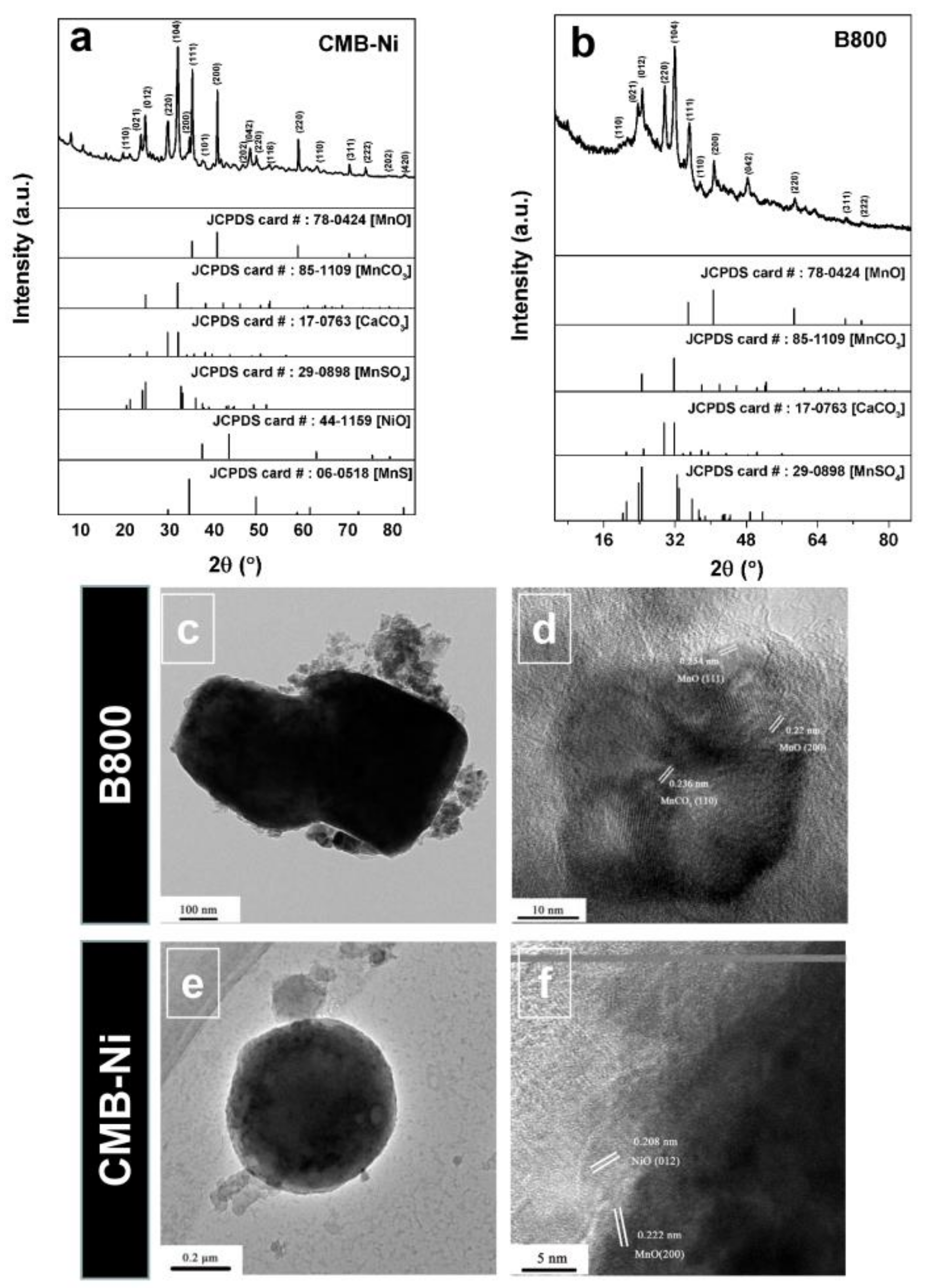
3.2. Preparation and Characterization of MnO/C/NiO Hollow Porous Composite
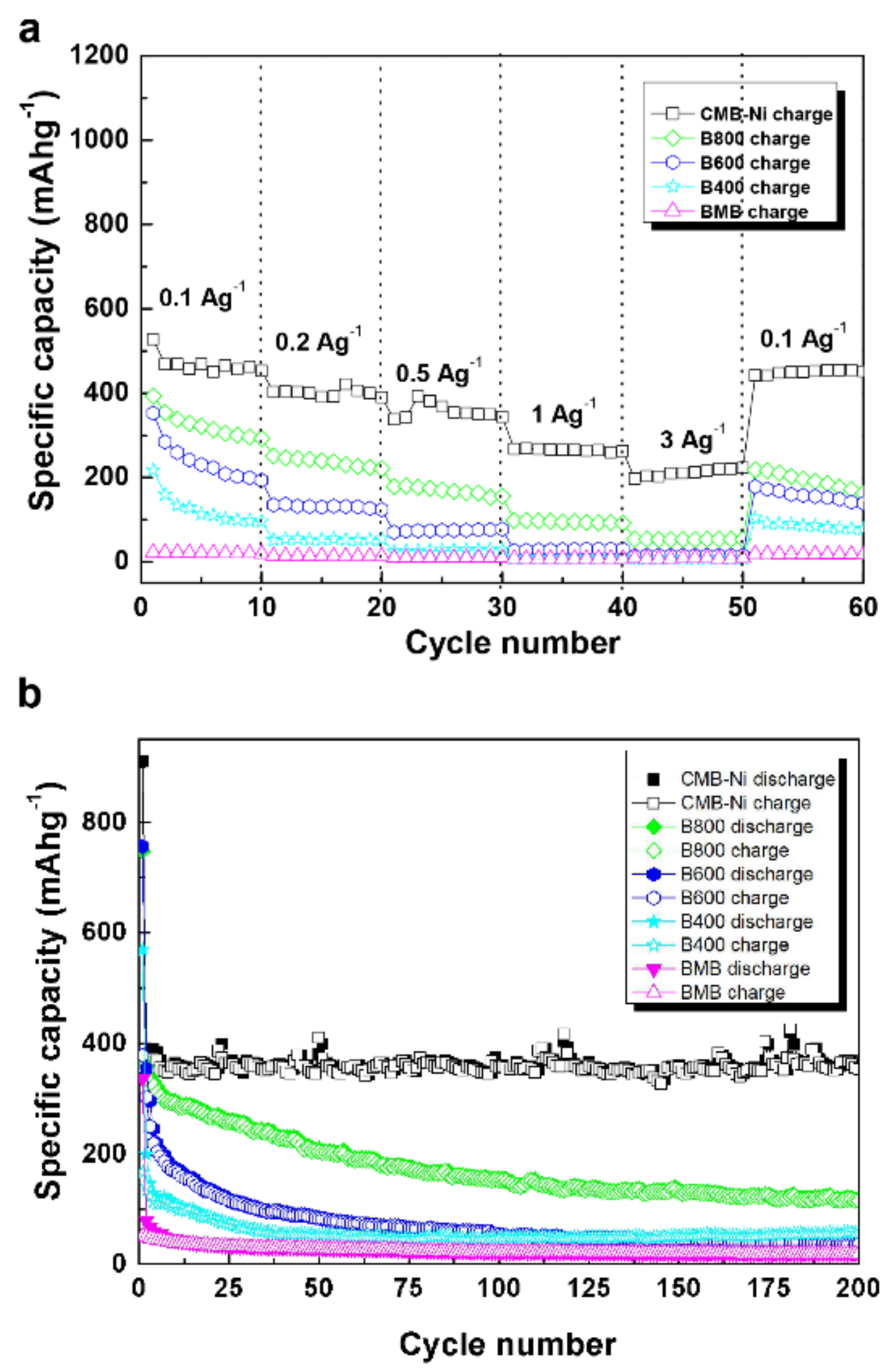
3.3. Electrochemical Determination of the Composites as Anodes for Lithium-Ion Batteries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Y.; Xiao, Z.; Dou, X.; Huang, H.; Lu, X.; Yan, R.; Gan, Y.; Zhu, W.; Gu, C.; Zhang, W.; et al. Green and Facile Fabrication of Hollow Porous MnO/C Microspheres from Microalgaes for Lithium-Ion Batteries. ACS Nano 2013, 7, 7083–7092. [Google Scholar] [CrossRef]
- Wu, M.S.; Chang, H.W. Self-Assembly of NiO-Coated ZnO Nanorod Electrodes with Core–Shell Nanostructures as Anode Materials for Rechargeable Lithium-Ion Batteries. J. Phys. Chem. 2013, 117, 2590–2599. [Google Scholar] [CrossRef]
- Tao, X.; Wu, R.; Xia, Y.; Huang, H.; Chai, W.; Feng, T.; Gan, Y.; Zhang, W. Biotemplated Fabrication of Sn@C Anode Materials Based on the Unique Metal Biosorption Behavior of Microalgae. ACS Appl. Mater. Interfaces 2014, 6, 3696–3702. [Google Scholar] [CrossRef]
- Zhang, K.; Han, X.; Hu, Z.; Zhang, X.; Tao, Z.; Chen, J. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 2015, 44, 699–728. [Google Scholar] [CrossRef]
- Zeng, J.; Wang, S.; Yu, J.; Cheng, H.; Tan, H.; Liu, Q.; Wu, J. Al and/or Ni-doped nanomanganese dioxide with anisotropic expansion and their electrochemical characterisation in primary Li–MnO2 batteries. J. Solid State Electrochem. 2014, 18, 1585–1591. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacin, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef]
- Opra, D.; Gnedenkov, S.; Sokolov, A.; Podgorbunsky, A.; Ustinov, A.; Mayorov, V.; Kuryavyi, V.; Sinebryukhov, S. Vanadium-doped TiO2-B/anatase mesoporous nanotubes with improved rate and cycle performance for rechargeable lithium and sodium batteries. J. Mater. Sci. Technol. 2020, 54, 181–189. [Google Scholar] [CrossRef]
- Opra, D.; Gnedenkov, S.; Sinebryukhov, S.; Podgorbunsky, A.; Sokolov, A.; Ustinov, A.; Kuryavyi, V.; Mayorov, V.; Zheleznov, V. Doping of titania with manganese for improving cycling and rate performances in lithium-ion batteries. Chem. Phys. 2020, 538, 110864. [Google Scholar] [CrossRef]
- Liu, W.; Yao, T.-H.; Xie, S.; She, Y.; Wang, H. Integrating TiO2/SiO2 into Electrospun Carbon Nanofibers towards Superior Lithium Storage Performance. Nanomaterials 2019, 9, 68. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.-Y.; Tang, R.; Qi, Y.-X.; Lun, N.; Bai, Y.-J.; Fan, R.-H. Carbon-Coated Fe–Mn–O Composites as Promising Anode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 9470–9477. [Google Scholar] [CrossRef]
- Han, L.; Shao, C.; Liang, B.; Liu, A. Genetically engineered phage-templated MnO2 nanowires: Synthesis and their application in electrochemical glucose biosensor operated atneutral pH condition. ACS Appl. Mater. Interfaces 2016, 8, 13768–13776. [Google Scholar] [CrossRef]
- Cao, B.; Yang, M.; Mao, C. Phage as a Genetically Modifiable Supramacromolecule in Chemistry, Materials and Medicine. ACC Chem. Res. 2016, 49, 1111–1120. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Chen, H.; Liu, J.; Liu, C.; Ni, H.; Zhao, C.; Ali, M.; Liu, F.; Li, L. Surface Mn(II) oxidation actuated by a multicopper oxidase in a soil bacterium leads to the formation of manganese oxide minerals. Sci. Rep. 2015, 5, 10895. [Google Scholar] [CrossRef]
- Takahashi, Y.; Manceau, A.; Geoffroy, N.; Marcus, M.A.; Usui, A. Chemical and structural control of the partitioning of Co, Ce, and Pb in marine ferromanganese oxides. Geochim. Cosmochim. Acta 2007, 71, 984–1008. [Google Scholar] [CrossRef]
- Lee, Y.; Tebo, B.M. Cobalt(II) Oxidation by the Marine Manganese(II)-Oxidizing Bacillus sp. Strain SG-1. Appl. Environ. Microbiol. 1994, 60, 2949–2957. [Google Scholar] [CrossRef]
- Manceau, A.; Lanson, M.; Geoffroy, N. Natural speciation of Ni, Zn, Ba, and As in ferromanganese coatings on quartz using X-ray fluorescence, absorption, and diffraction. Geochim. Cosmochim. Acta 2007, 71, 95–128. [Google Scholar] [CrossRef]
- Romano, C.A.; Zhou, M.; Song, Y.; Wysocki, V.H.; Dohnalkova, A.C.; Kovarik, L.; Paša-Tolić, L.; Tebo, B.M. Biogenic manganese oxide nanoparticle formation by a multimeric multicopper oxidase Mnx. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.; Ni, H.; Yang, X.; Li, Q.; Li, L. Decolorization of industrial synthetic dyes using engineered Pseudomonas putida cells with surface-immobilized bacterial laccase. Microb. Cell Factories 2012, 11, 75. [Google Scholar] [CrossRef]
- Su, J.; Deng, L.; Huang, L.; Guo, S.; Liu, F.; He, J. Catalytic oxidation of manganese(II) by multicopper oxidase CueO and characterization of the biogenic Mn oxide. Water Res. 2014, 56, 304–313. [Google Scholar] [CrossRef]
- Caspi, R.; Tebo, B.M.; Haygood, M.G. c-type cytochromes and manganese oxidation in Pseudomonas putida MnB1. Appl. Environ. Microbiol. 1998, 64, 3549–3555. [Google Scholar] [CrossRef]
- Johnson, H.A.; Tebo, B.M. In vitro studies indicate a quinone is involved in bacterial Mn(II) oxidation. Arch. Microbiol. 2007, 189, 59–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, S.K.; Grass, G.; Rensing, C.; Montfort, W.R. Cuprous Oxidase Activity of CueO from Escherichia coli. J. Bacteriol. 2004, 186, 7815–7817. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, H.; Canning, G.; Bancroft, G. XPS study of reductive dissolution of 7Å-birnessite by H3AsO3, with constraints on reaction mechanism. Geochim. Cosmochim. Acta 1998, 62, 2097–2110. [Google Scholar] [CrossRef]
- Yin, H.; Liu, F.; Feng, X.; Liu, M.; Tan, W.; Qiu, G. Co2+-exchange mechanism of birnessite and its application for the removal of Pb2+ and As(III). J. Hazard. Mater. 2011, 196, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. BIOGENIC MANGANESE OXIDES: Properties and Mechanisms of Formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Tebo, B.M.; Johnson, H.A.; McCarthy, J.K.; Templeton, A.S. Geomicrobiology of manganese (II) oxidation. Trends Microbiol. 2005, 13, 421–428. [Google Scholar] [CrossRef]
- Butterfield, C.N.; Soldatova, A.V.; Lee, S.-W.; Spiro, T.G.; Tebo, B.M. Mn (II,III) oxidation and MnO2 mineralization by an expressed bacterial multicopper oxidase. Proc. Natl. Acad. Sci. USA 2013, 110, 11731–11735. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas Adsorption Characterization of Ordered Organic–Inorganic Nanocomposite Materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Benner, S.G.; Blowes, D.W.; Gould, W.D.; Herbert, R.B.; Ptacek, C.J. Geochemistry of a Permeable Reactive Barrier for Metals and Acid Mine Drainage. Environ. Sci. Technol. 1999, 33, 2793–2799. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Z.; Zhang, Z.; Chen, H.; Liu, J.; Ali, M.; Liu, F.; Li, L. Population Structure of Manganese-Oxidizing Bacteria in Stratified Soils and Properties of Manganese Oxide Aggregates under Manganese–Complex Medium Enrichment. PLoS ONE 2013, 8, e73778. [Google Scholar] [CrossRef]
- Mandernack, K.W.; Post, J.; Tebo, B.M. Manganese mineral formation by bacterial spores of the marine Bacillus, strain SG-1: Evidence for the direct oxidation of Mn(II) to Mn(IV). Geochim. Cosmochim. Acta 1995, 59, 4393–4408. [Google Scholar] [CrossRef]
- Webb, S.M.; Dick, G.J.; Bargar, J.R.; Tebo, B.M. Evidence for the presence of Mn(III) intermediates in the bacterial oxidation of Mn(II). Proc. Natl. Acad. Sci. USA 2005, 102, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Omid, H.; Oghabian, M.A.; Ahmadi, R.; Shahbazi, N.; Hosseini, H.R.M.; Shanehsazzadeh, S.; Zangeneh, R.N. Synthesizing and staining manganese oxide nanoparticles for cytotoxicity and cellular uptake investigation. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.-W.; Jin, Y.-H.; Seo, S.-D.; Lee, S.-H.; Kim, D.-W. Highly Reversible Lithium Storage in Bacillus subtilis-Directed Porous Co3O4 Nanostructures. ACS Nano 2010, 5, 443–449. [Google Scholar] [CrossRef]
- Hahn, B.P.; Long, J.W.; Rolison, D.R. Something from Nothing: Enhancing Electrochemical Charge Storage with Cation Vacancies. Acc. Chem. Res. 2013, 46, 1181–1191. [Google Scholar] [CrossRef]
- Sun, B.; Chen, Z.; Kim, H.-S.; Ahn, H.; Wang, G. MnO/C core–shell nanorods as high capacity anode materials for lithium-ion batteries. J. Power Sources 2011, 196, 3346–3349. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Chen, J.; Wang, H.; Liu, S.; Chen, S. Biotemplated MnO/C microtubes from spirogyra with improved electrochemical performance for lithium-ion batterys. Electrochim. Acta 2016, 188, 210–217. [Google Scholar] [CrossRef]
- Huang, X.; Tu, J.; Zhang, B.; Zhang, C.; Li, Y.; Yuan, Y.; Wu, H. Electrochemical properties of NiO–Ni nanocomposite as anode material for lithium ion batteries. J. Power Sources 2006, 161, 541–544. [Google Scholar] [CrossRef]
- Taberna, P.-L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J.-M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat. Mater. 2006, 5, 567–573. [Google Scholar] [CrossRef]
- Hong, L.; Balaya, P.; Maier, J. Li-storage via heterogeneous reaction in aelected binary metal fluorides and oxides. J. Electrochem. Soc. 2004, 151, A1878–A1885. [Google Scholar]
- Xu, G.-L.; Xu, Y.-F.; Sun, H.; Fu, F.; Zheng, X.-M.; Huang, L.; Li, J.-T.; Yang, S.; Sun, S.-G. Facile synthesis of porous MnO/C nanotubes as a high capacity anode material for lithium ion batteries. Chem. Commun. 2012, 48, 8502–8504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Han, P.; Gu, L.; Zhang, L.; Liu, Z.; Kong, Q.; Zhang, C.; Dong, S.; Zhang, Z.; Yao, J.; et al. Synthesis of Nitrogen-Doped MnO/Graphene Nanosheets Hybrid Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2012, 4, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wu, C.; Yu, H.; Xie, J.; Cao, G.; Zhu, T.; Zhao, X.B.; Zeng, Y. Coaxial MnO/C nanotubes as anodes for lithium-ion batteries. Electrochim. Acta 2011, 56, 5844–5848. [Google Scholar] [CrossRef]
- Aurbach, D.; Weissman, I.; Zaban, A.; Dan, P. On the role of water contamination in rechargeable Li batteries. Electrochim. Acta 1999, 45, 1135–1140. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Q.; Tian, J.; Jiang, F. TiO2 Nanobelt@Co9S8 Composites as Promising Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials 2017, 7, 252. [Google Scholar] [CrossRef]
- Ping, H.; Xie, H.; Xiang, M.; Su, B.-L.; Wang, Y.; Zhang, J.; Zhang, F.; Fu, Z. Confined-space synthesis of nanostructured anatase, directed by genetically engineered living organisms for lithium-ion batteries. Chem. Sci. 2016, 7, 6330–6336. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, H.; Zhang, W.; Tao, X.; Gan, Y.; Xia, Y.; Yang, H.; Guo, X. Synthesis of MnO/C composites derived from pollen template for advanced lithium-ion batteries. Electrochim. Acta 2015, 152, 286–293. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Gu, T.; Li, L.; Li, L. Synthesis of MnO/C/NiO-Doped Porous Multiphasic Composites for Lithium-Ion Batteries by Biomineralized Mn Oxides from Engineered Pseudomonas putida Cells. Nanomaterials 2021, 11, 361. https://doi.org/10.3390/nano11020361
Liu J, Gu T, Li L, Li L. Synthesis of MnO/C/NiO-Doped Porous Multiphasic Composites for Lithium-Ion Batteries by Biomineralized Mn Oxides from Engineered Pseudomonas putida Cells. Nanomaterials. 2021; 11(2):361. https://doi.org/10.3390/nano11020361
Chicago/Turabian StyleLiu, Jin, Tong Gu, Li Li, and Lin Li. 2021. "Synthesis of MnO/C/NiO-Doped Porous Multiphasic Composites for Lithium-Ion Batteries by Biomineralized Mn Oxides from Engineered Pseudomonas putida Cells" Nanomaterials 11, no. 2: 361. https://doi.org/10.3390/nano11020361
APA StyleLiu, J., Gu, T., Li, L., & Li, L. (2021). Synthesis of MnO/C/NiO-Doped Porous Multiphasic Composites for Lithium-Ion Batteries by Biomineralized Mn Oxides from Engineered Pseudomonas putida Cells. Nanomaterials, 11(2), 361. https://doi.org/10.3390/nano11020361





