Preparation of TiO2/WO3/C/N Composite Nanofibers by Electrospinning Using Precursors Soluble in Water and Their Photocatalytic Activity in Visible Light
Abstract
1. Introduction
2. Materials and Methods
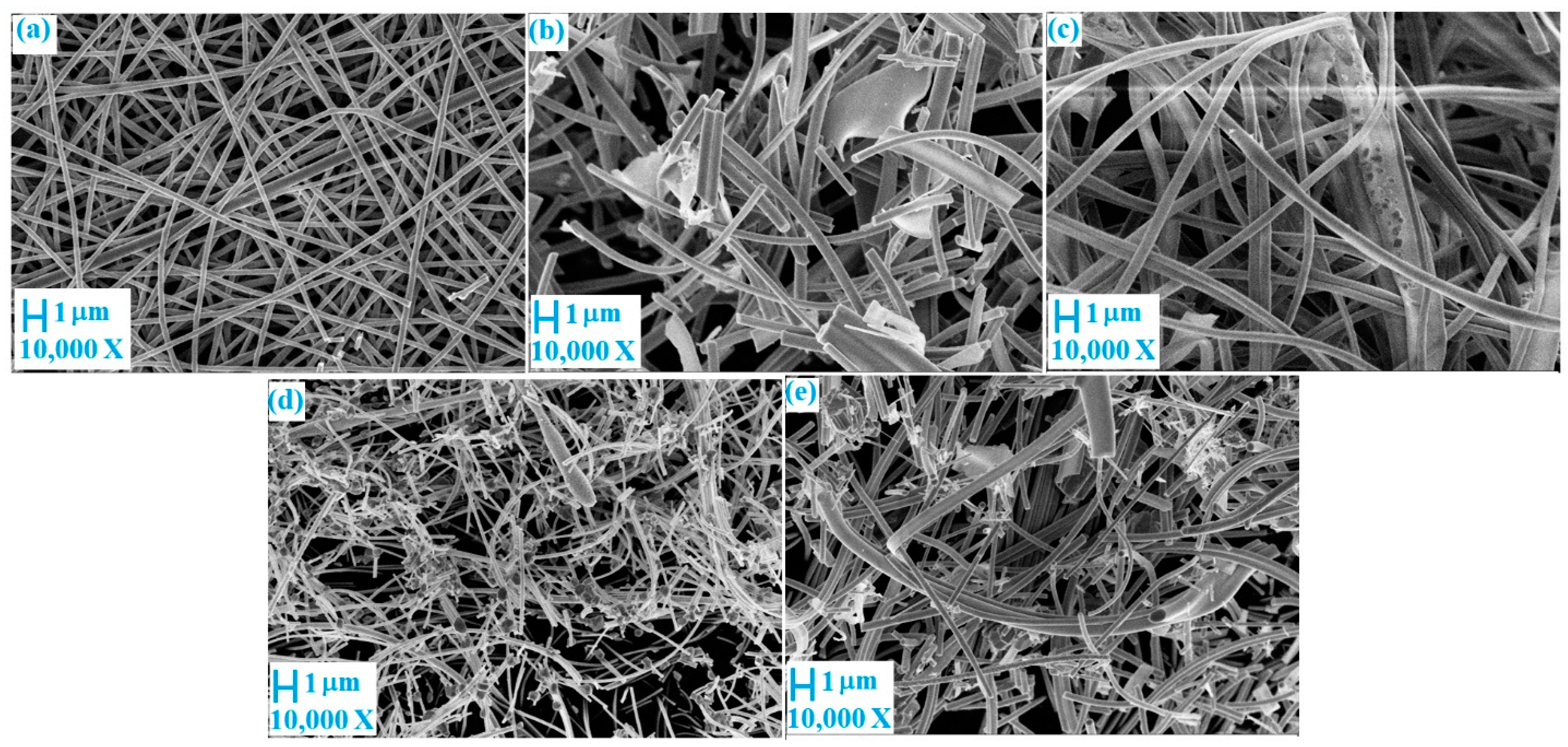
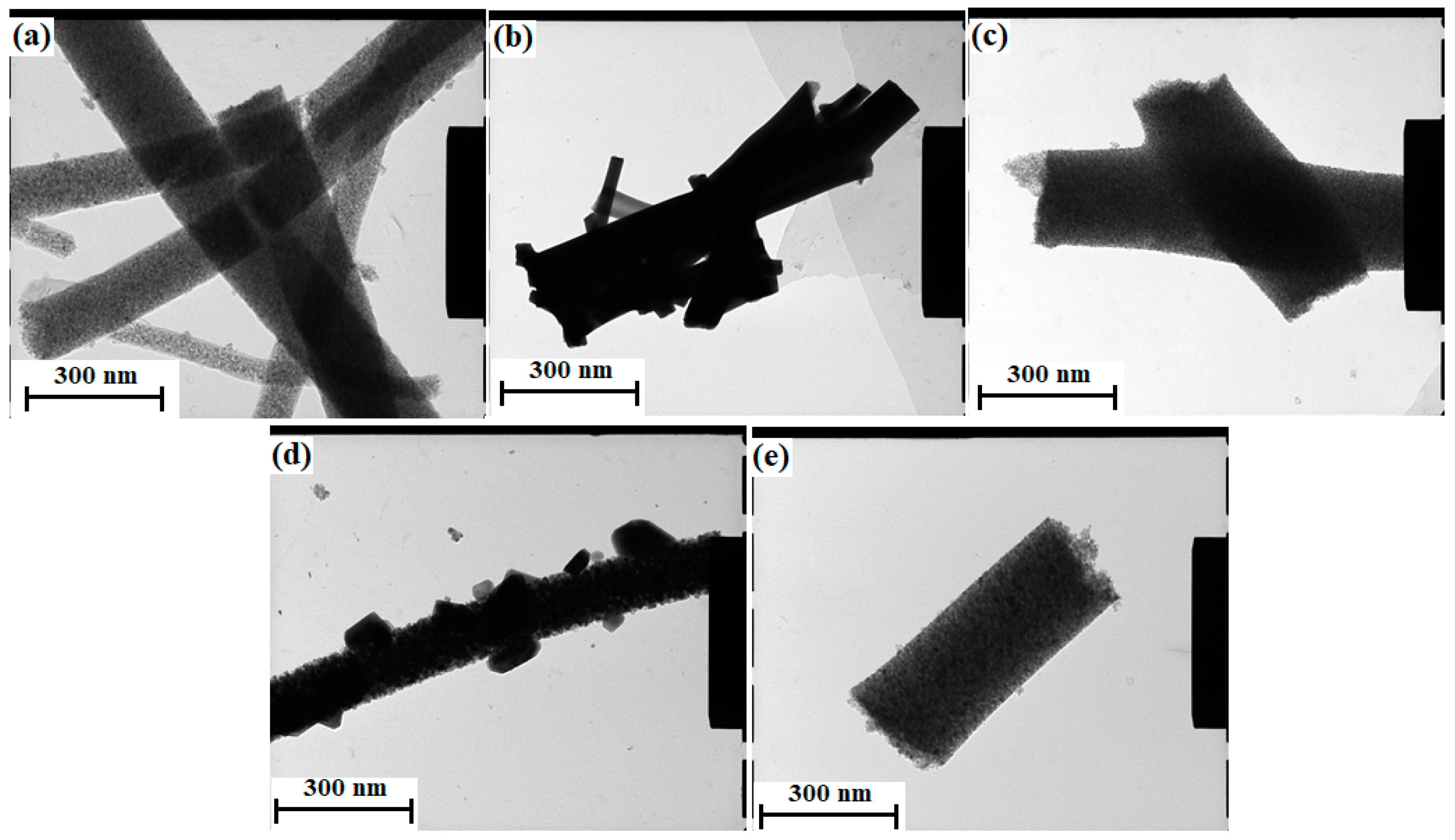
2.1. Synthesis of N Doped TiO2/WO3/C Nanofibers
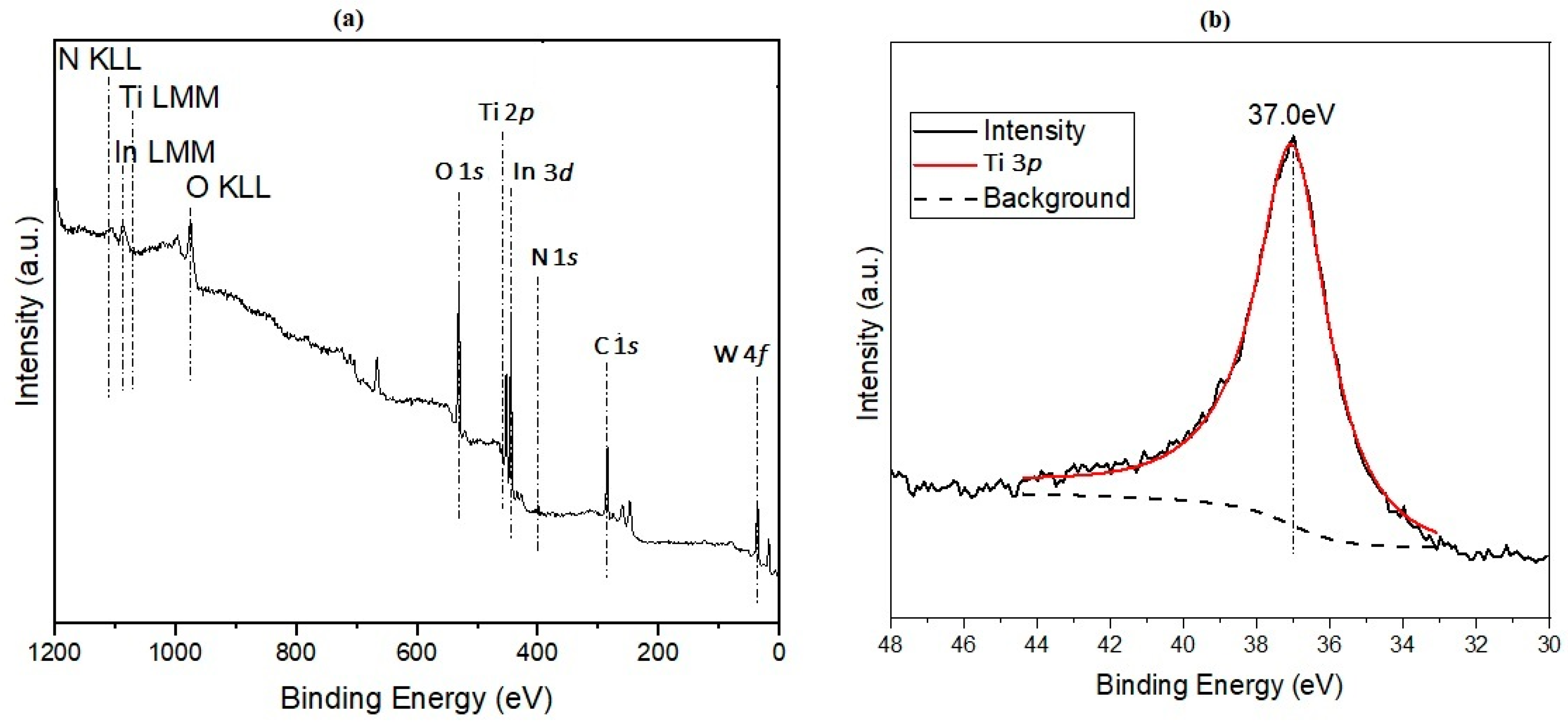
2.2. Characterization of the Nanofibers
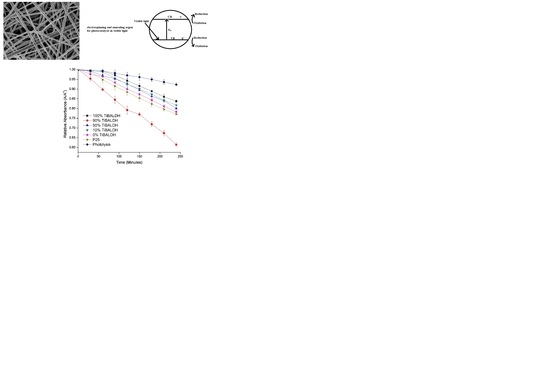
2.3. Photocatalysis in Visible Light
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chowdhury, M.I.H.; Hossain, M.S.; Azad, M.A.S.; Islam, M.Z.; Dewan, M.A. Photocatalytic Degradation of Methyl Orange Under UV Using ZnO as Catalyst. Int. J. Sci. Eng. Res. 2018, 9, 1646–1649. [Google Scholar]
- Soares, L.; Alves, A. Photocatalytic properties of TiO2 and TiO2/WO3 films applied as semiconductors in heterogeneous photocatalysis. Mater. Lett. 2018, 211, 339–342. [Google Scholar] [CrossRef]
- Anderson, A.L.; Binions, R. A preferential precursor for photocatalytically active titanium dioxide thin films: Titanium bis-ammonium lactato dihydroxide as an alternative to titanium tetra iso-propoxide. Polyhedron 2016, 118, 81–90. [Google Scholar] [CrossRef]
- Justh, N.; Mikula, G.J.; Bakos, L.P.; Nagy, B.; László, K.; Parditka, B.; Erdélyi, Z.; Takáts, V.; Mizsei, J.; Szilágyi, I.M. Photocatalytic properties of TiO2@polymer and TiO2@carbon aerogel composites prepared by atomic layer deposition. Carbon N. Y. 2019, 147, 476–482. [Google Scholar] [CrossRef]
- Paula, L.F.; Hofer, M.; Lacerda, V.P.; Bahnemann, D.W.; Patrocinio, A.O.T. Unraveling the photocatalytic properties of TiO2/WO3 mixed oxides. Photochem. Photobiol. Sci. 2019, 18, 2469–2483. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, M.; Yu, W.; Zhang, Q.; Xie, H.; Sun, Z.; Shao, Q.; Guo, X.; Hao, L.; Zheng, Y.; et al. Heterostructured TiO2/WO3 Nanocomposites for Photocatalytic Degradation of Toluene under Visible Light. J. Electrochem. Soc. 2017, 164, 1086–1090. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, P.; Fan, J.; Hu, J.; Shao, G. Constructing 2D layered MoS 2 nanosheets-modified Z-scheme TiO2/WO3 nanofibers ternary nanojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 430, 466–474. [Google Scholar] [CrossRef]
- Tryba, B.; Piszcz, M.; Morawski, A.W. Photocatalytic activity of TiO2—WO3 Composites. Int. J. Photoenergy 2009, 1–7. [Google Scholar] [CrossRef]
- Chakornpradit, P.; Phiriyawirut, M.; Meeyoo, V. Preparation of TiO2/WO3 Composite Nanofibers by Electrospinning. Key Eng. Mater. 2017, 751, 296–301. [Google Scholar] [CrossRef]
- Nagy, D.; Firkala, T.; Drotár, E.; Szegedi, Á.; László, K.; Szilágyi, I.M. Photocatalytic WO3/TiO2 nanowires: WO3 polymorphs influencing the atomic layer deposition of TiO2. RSC Adv. 2016, 6, 95369–95377. [Google Scholar] [CrossRef]
- Viswanathan, B.; Krishanmurthy, K.R. Nitrogen incorporation in TiO2: Does it make a visible light photo-active material? Int. J. Photoenergy 2012, 1–10. [Google Scholar] [CrossRef]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 3, 3–16. [Google Scholar]
- Park, H.; Kim HIl Moon, G.H.; Choi, W. Photoinduced charge transfer processes in solar photocatalysis based on modified TiO2. Energy Environ. Sci. 2016, 9, 411–433. [Google Scholar]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Dai, W.L.; Li, H.; Fan, K. One-pot synthesis of twist-like helix tungsten-nitrogen-codoped titania photocatalysts with highly improved visible light activity in the abatement of phenol. Appl. Catal. B Environ. 2008, 82, 233–243. [Google Scholar] [CrossRef]
- Sajjad AK, L.; Shamaila, S.; Zhang, J. Study of new states in visible light active W, N co-doped TiO2 photo catalyst. Mater. Res. Bull. 2012, 47, 3083–3089. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.; Lee, J.H.; Patel, R. Visible Light-based Photocatalytic Degradation by Transition Metal Oxide. Membr. J. 2019, 29, 299–307. [Google Scholar] [CrossRef]
- Bai, S.; Liu, H.; Sun, J.; Tian, Y.; Chen, S.; Song, J.; Luo, R.; Li, D.; Chen, A.; Liu, C.C. Improvement of TiO2 photocatalytic properties under visible light by WO3/TiO2 and MoO3/TiO2 composites. Appl. Surf. Sci. 2015, 338, 61–68. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. 2017 Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Nagy, D. Review on one-dimensional nanostructures prepared by electrospinning and atomic layer deposition. J. Phys. Conf. Ser. 2014, 559, 012010. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, P.; Hu, J.; Pan, J.; Fan, J.; Shao, G. One-dimensional Z-scheme TiO2/WO3/Pt heterostructures for enhanced hydrogen generation. Appl. Surf. Sci. 2017, 391, 211–217. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Yang, X.; Ye, Q.; Huang, K.; Hou, C.; Zhao, Z.; You, J.; Li, Y. Fabrication of TiO2/WO3Composite Nanofibers by Electrospinning and Photocatalystic Performance of the Resultant Fabrics. Ind. Eng. Chem. Res. 2016, 55, 80–85. [Google Scholar] [CrossRef]
- Balta, Z.; Bilgin Simsek, E.; Berek, D. Solvothermal synthesis of WO3/TiO2/carbon fiber composite photocatalysts for enhanced performance under sunlight illumination. Photochem. Photobiol. 2019, 95, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, L.; Zhang, P.; Liang, C.; Shao, G. Construction of solid-state Z-scheme carbon-modified TiO2/WO3 nanofibers with enhanced photocatalytic hydrogen production. J. Power Sources 2016, 328, 28–36. [Google Scholar] [CrossRef]
- Choi, T.; Kim, J.S.; Kim, J.H. Transparent nitrogen doped TiO2/WO3 composite films for self-cleaning glass applications with improved photodegradation activity. Adv. Powder Technol. 2016, 27, 347–353. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jo, W.K. Heterojunction-based two-dimensional N-doped TiO2/WO3 composite architectures for photocatalytic treatment of hazardous organic vapor. J. Hazard. Mater. 2016, 314, 22–31. [Google Scholar] [CrossRef]
- Gao, B.; Ma, Y.; Cao, Y.; Yang, W.; Yao, J. Great enhancement of photocatalytic activity of nitrogen-doped titania by coupling with tungsten oxide. J. Phys. Chem. B 2006, 110, 14391–14397. [Google Scholar] [CrossRef]
- Odhiambo, V.O.; Ongarbayeva, A.; Kéri, O.; Simon, L.; Szilágyi, I.M. Synthesis of TiO2/WO3 composite nanofibers by a water-based electrospinning process and their application in photocatalysis. Nanomaterials 2020, 10, 882. [Google Scholar] [CrossRef]
- Kéri, O.; Bárdos, P.; Boyadjiev, S.; Igricz, T.; Nagy, Z.K.; Szilágyi, I.M. Thermal properties of electrospun polyvinylpyrrolidone/titanium tetraisopropoxide composite nanofibers. J. Therm. Anal. Calorim. 2019, 137, 1249–1254. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Fu, P.; Luan, Y.; Dai, X. Preparation of activated carbon fibers supported TiO2 photocatalyst and evaluation of its photocatalytic reactivity. J. Mol. Catal. A Chem. 2004, 221, 81–88. [Google Scholar] [CrossRef]
- Potlog, T.; Dumitriu, P.; Dobromir, M.; Luca, D. XRD and XPS Analysis of TiO2 Thin Films Annealed in Different Environments. J. Mater. Sci. Eng. B 2014, 4, 163–170. [Google Scholar]
- Thummavichai, K.; Wang, N.; Xu, F.; Rance, G.; Xia, Y.; Zhu, Y. In situ investigations of the phase change behaviour of tungsten oxide nanostructures. R. Soc. Open Sci. 2018, 5, 171932. [Google Scholar] [CrossRef] [PubMed]
- Branca, C.; D’Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan and chitosan/clay films. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Baganizi, D.R.; Nyairo, E.; Duncan, S.A.; Singh, S.R.; Dennis, V.A. Interleukin-10 conjugation to carboxylated PVP-coated silver nanoparticles for improved stability and therapeutic efficacy. Nanomaterials 2017, 7, 165. [Google Scholar] [CrossRef]
- Vijaya, N.; Selvasekarapandian, S.; Hirankumar, G.; Karthikeyan, S.; Nithya, H.; Ramya, C.S.; Prabu, M. Structural, vibrational, thermal, and conductivity studies on proton-conducting polymer electrolyte based on poly (N-vinylpyrrolidone). Ionics 2012, 18, 91–99. [Google Scholar] [CrossRef]
- Mondal, K.; Bhattacharyya, S.; Sharma, A. Photocatalytic Degradation of Naphthalene by Electrospun Mesoporous Carbon-Doped Anatase TiO2 Nanofiber Mats. Ind. Eng. Chem. Res. 2014, 53, 18900–18909. [Google Scholar] [CrossRef]
- Kumar, A.; Jose, R.; Fujihara, K.; Wang, J.; Ramakrishna, S. Structural and optical properties of electrospun TiO2 nanofibers. Chem. Mater. 2007, 19, 6536–6542. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kürti, J.; Koltai, J.; Kavan, L. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 2012, 14, 14567–14572. [Google Scholar] [CrossRef]
- Zou, Y.S.; Zhang, Y.C.; Lou, D.; Wang, H.P.; Gu, L.; Dong, Y.H.; Dou, K.; Song, X.F.; Zeng, H.B. Structural and optical properties of WO3 films deposited by pulsed laser deposition. J. Alloys Compd. 2014, 583, 465–470. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C J. Carbon Res. 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Liu, R.; Ye, H.; Xiong, X.; Liu, H. Fabrication of TiO2/ZnO composite nanofibers by electrospinning and their photocatalytic property. Mater. Chem. Phys. 2010, 121, 432–439. [Google Scholar] [CrossRef]




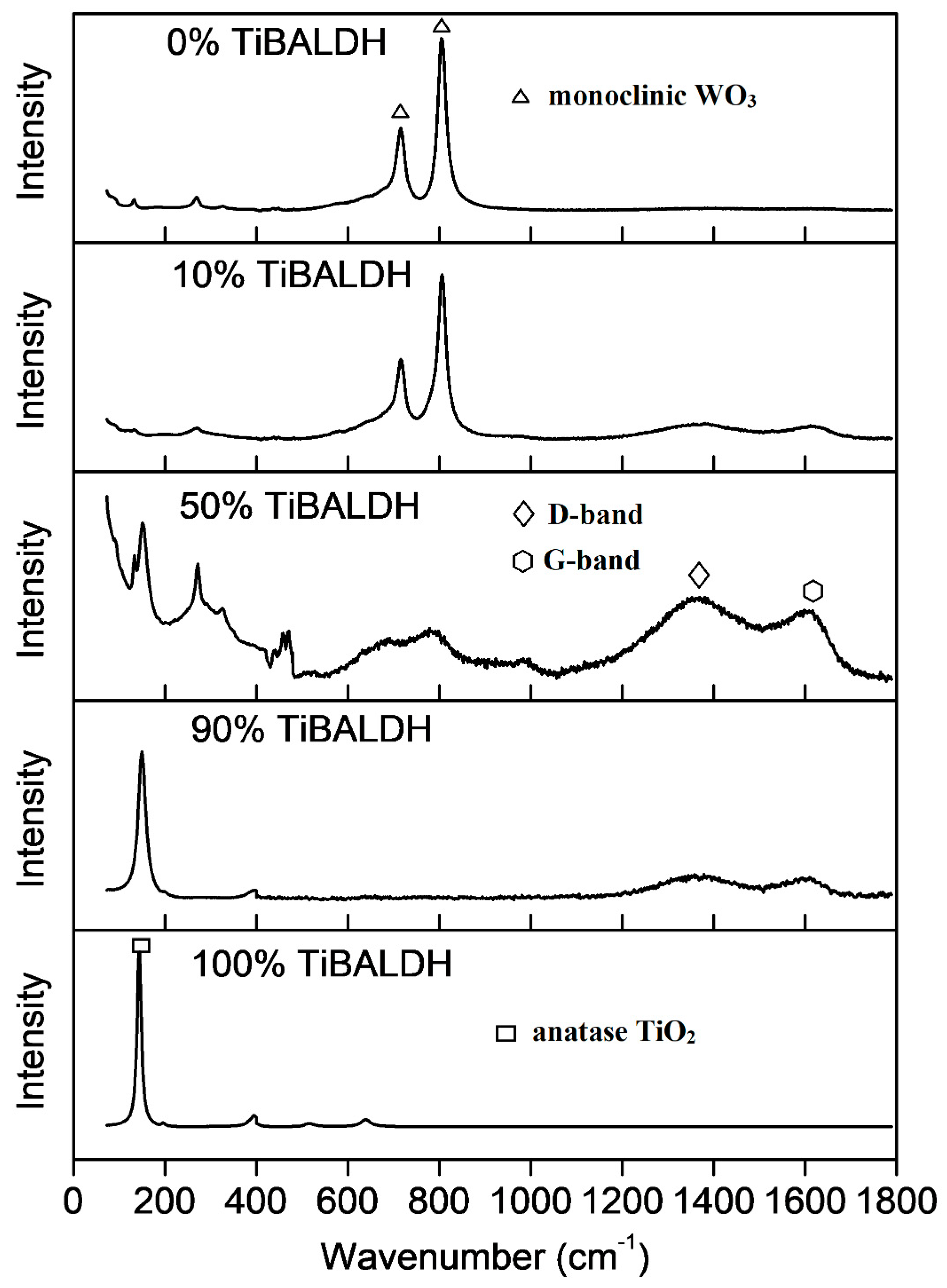
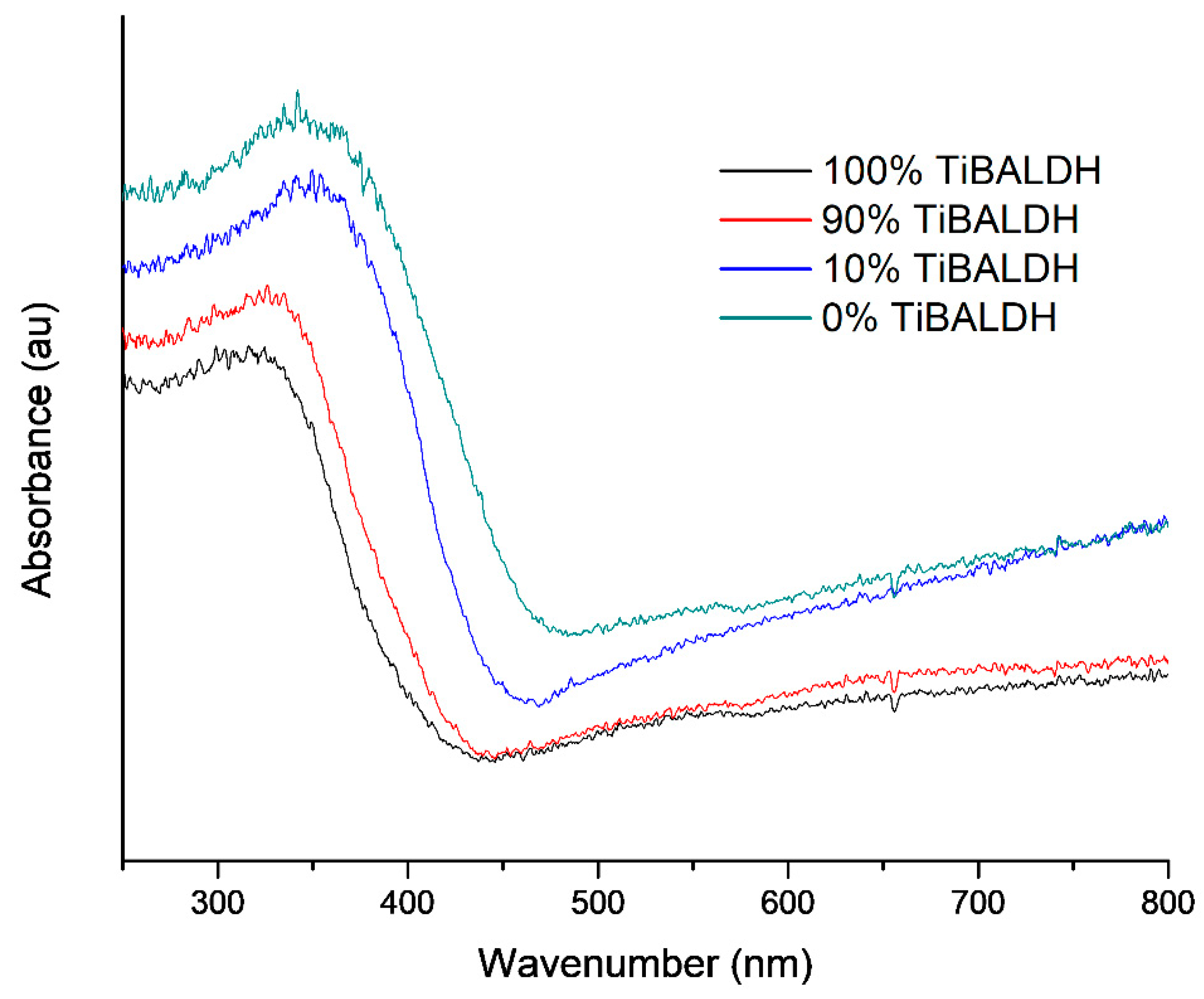
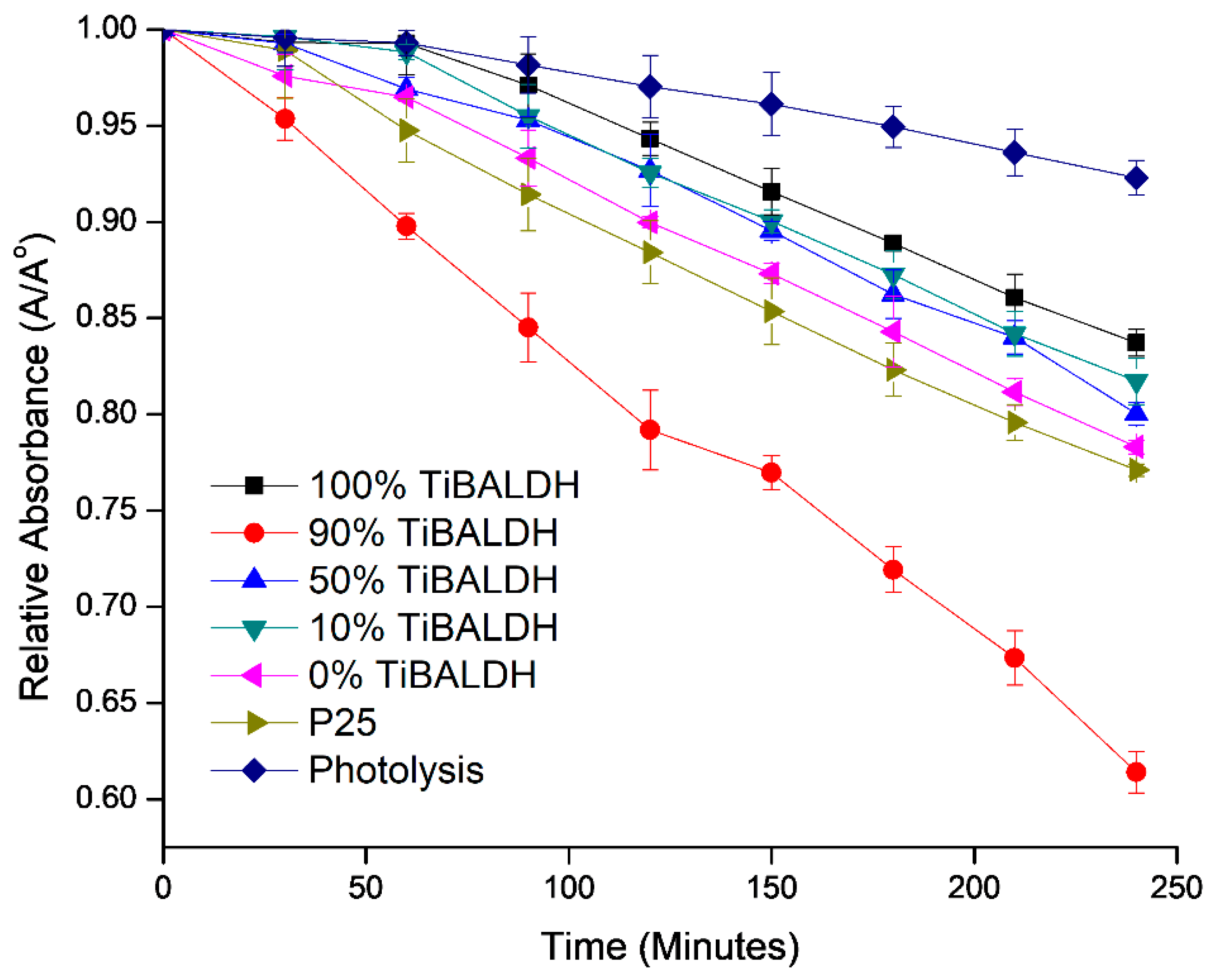
| Sample | C | O | Ti | W | N | |
|---|---|---|---|---|---|---|
| At% | ||||||
| 100% TiBALDH | XPS | 35.3 | 52.4 | 10.3 | 2.0 | |
| EDX | 30.1 | 47.2 | 18.2 | 4.5 | ||
| 90% TiBALDH | XPS | 24.9 | 57.6 | 11.7 | 3.5 | 2.3 |
| EDX | 40.4 | 44.2 | 6.3 | 1.0 | 8.1 | |
| 50% TiBALDH | XPS | 50.7 | 38.0 | 1.5 | 4.8 | 5.0 |
| EDX | 22.1 | 51.8 | 9.6 | 14.5 | 2.0 | |
| 10% TiBALDH | XPS | 28.7 | 54.7 | 2.8 | 12.6 | 1.2 |
| EDX | 21.1 | 41.8 | 1.3 | 34.7 | 1.1 | |
| 0% TiBALDH | XPS | 30.0 | 52.5 | 15.9 | 1.6 | |
| EDX | 14.2 | 42.5 | 41.7 | 1.6 | ||
| Fibers | 100% TiBALDH | 90% TiBALDH | 10% TiBALDH | 0% TiBALDH |
|---|---|---|---|---|
| Band gap (eV) | 2.9 | 2.7 | 2.6 | 2.4 |
| Sample | Kapp (min−1) | r2 |
|---|---|---|
| 100% TiBALDH | 0.0009 | 97.8 |
| 90% TiBALDH | 0.002 | 99.0 |
| 50% TiBALDH | 0.001 | 98.5 |
| 10% TiBALDH | 0.001 | 99.2 |
| 0% TiBALDH | 0.0011 | 99.2 |
| P25 | 0.0012 | 99.9 |
| Bare methylene blue | 0.0004 | 98.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odhiambo, V.O.; Mustafa, C.R.M.; Thong, L.B.; Kónya, Z.; Cserháti, C.; Erdélyi, Z.; Lukác, I.E.; Szilágyi, I.M. Preparation of TiO2/WO3/C/N Composite Nanofibers by Electrospinning Using Precursors Soluble in Water and Their Photocatalytic Activity in Visible Light. Nanomaterials 2021, 11, 351. https://doi.org/10.3390/nano11020351
Odhiambo VO, Mustafa CRM, Thong LB, Kónya Z, Cserháti C, Erdélyi Z, Lukác IE, Szilágyi IM. Preparation of TiO2/WO3/C/N Composite Nanofibers by Electrospinning Using Precursors Soluble in Water and Their Photocatalytic Activity in Visible Light. Nanomaterials. 2021; 11(2):351. https://doi.org/10.3390/nano11020351
Chicago/Turabian StyleOdhiambo, Vincent Otieno, Chra Rasool M. Mustafa, Le Ba Thong, Zoltán Kónya, Csaba Cserháti, Zoltán Erdélyi, István Endre Lukác, and Imre Miklós Szilágyi. 2021. "Preparation of TiO2/WO3/C/N Composite Nanofibers by Electrospinning Using Precursors Soluble in Water and Their Photocatalytic Activity in Visible Light" Nanomaterials 11, no. 2: 351. https://doi.org/10.3390/nano11020351
APA StyleOdhiambo, V. O., Mustafa, C. R. M., Thong, L. B., Kónya, Z., Cserháti, C., Erdélyi, Z., Lukác, I. E., & Szilágyi, I. M. (2021). Preparation of TiO2/WO3/C/N Composite Nanofibers by Electrospinning Using Precursors Soluble in Water and Their Photocatalytic Activity in Visible Light. Nanomaterials, 11(2), 351. https://doi.org/10.3390/nano11020351