Effect of Au Nanoparticles and Scattering Layer in Dye-Sensitized Solar Cells Based on Freestanding TiO2 Nanotube Arrays
Abstract
1. Introduction
2. Materials and Methods
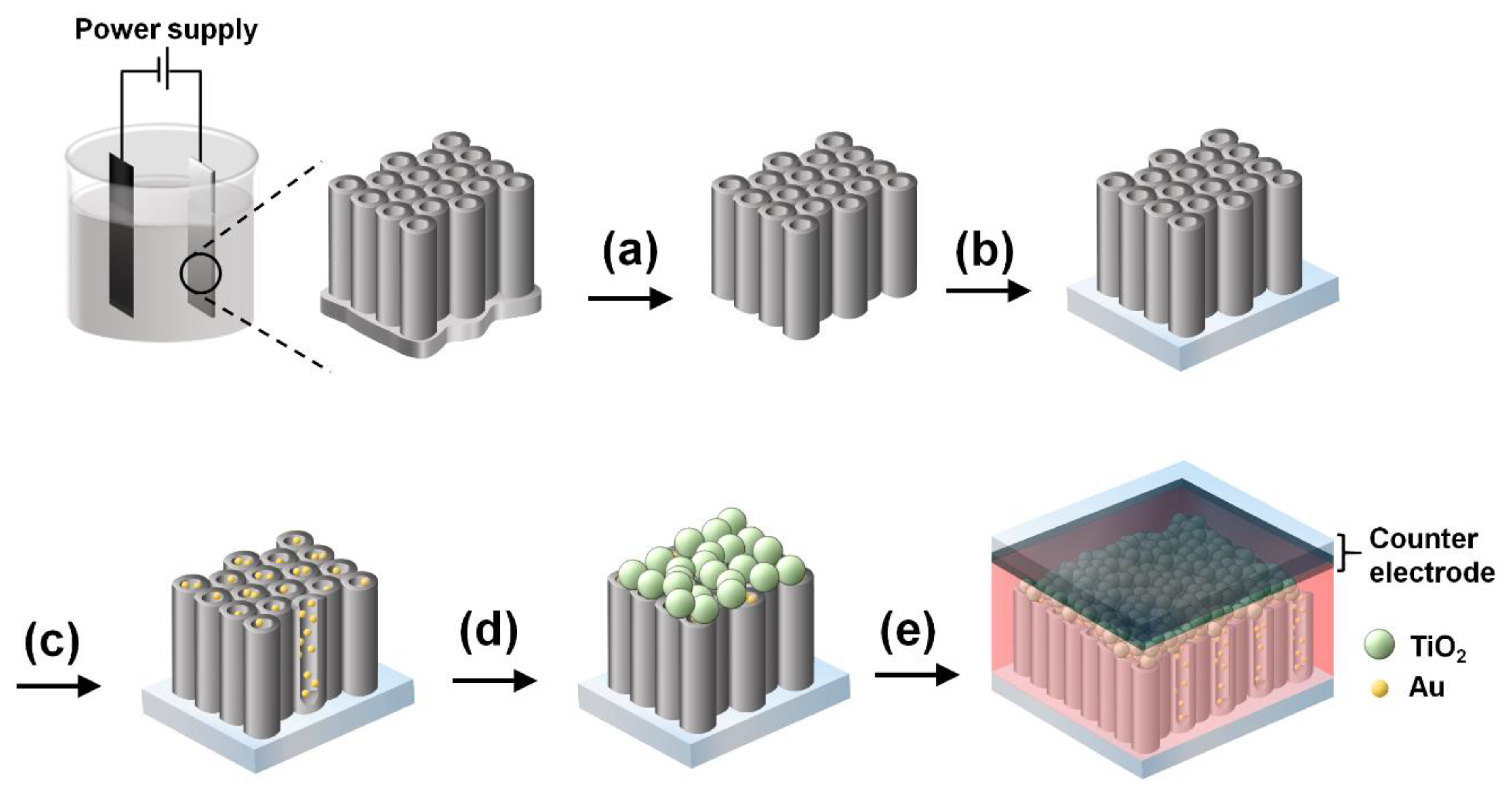
2.1. Preparation of f-TNTAs
2.2. Preparation of Au NPs into the f-TNTAs
2.3. Fabrication of DSSC f-TNTAs with a Scattering Layer
2.4. Fabrication of DSSCs with Au NPs Decorated into f-TNTAs
2.5. Characterization
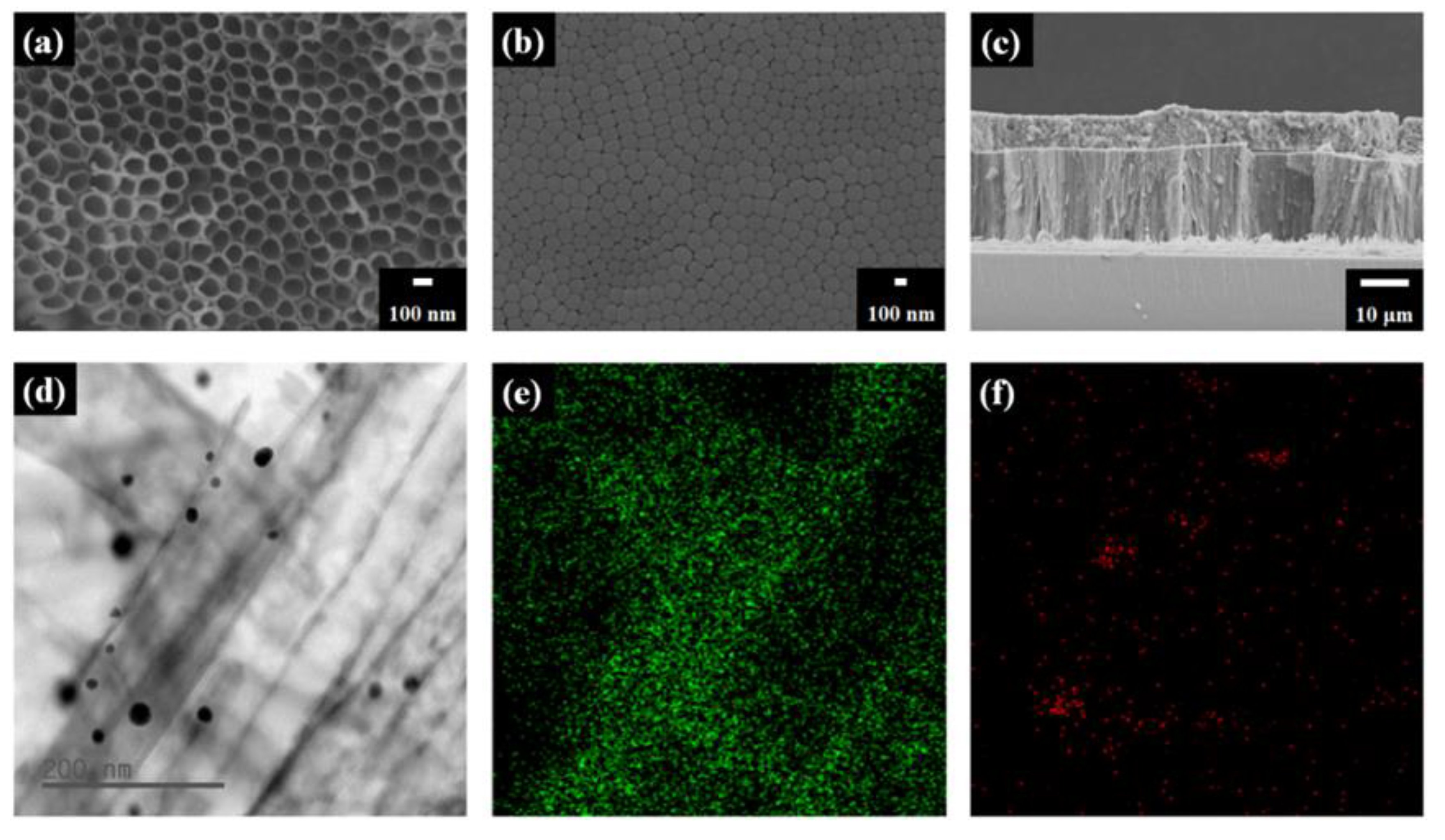
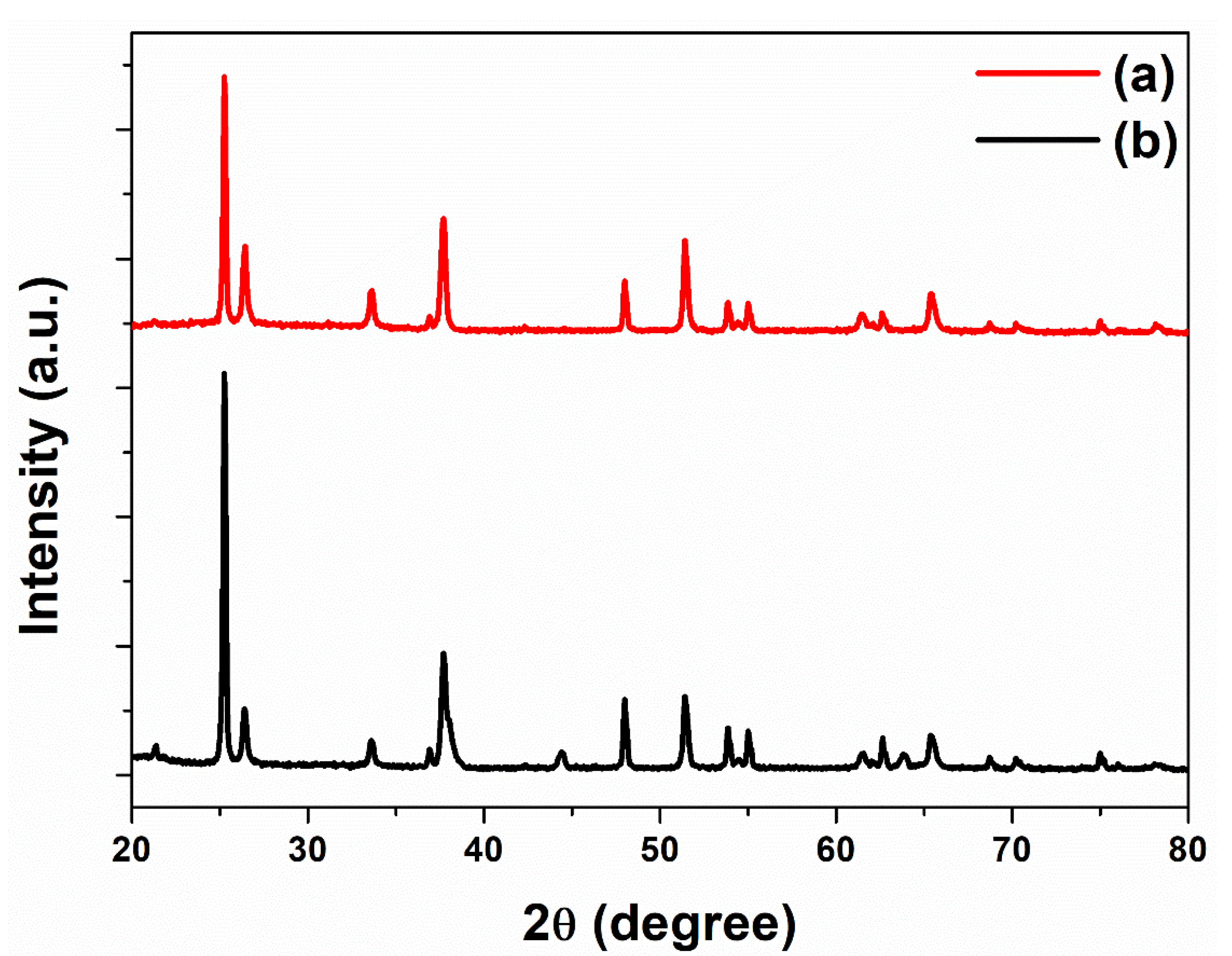
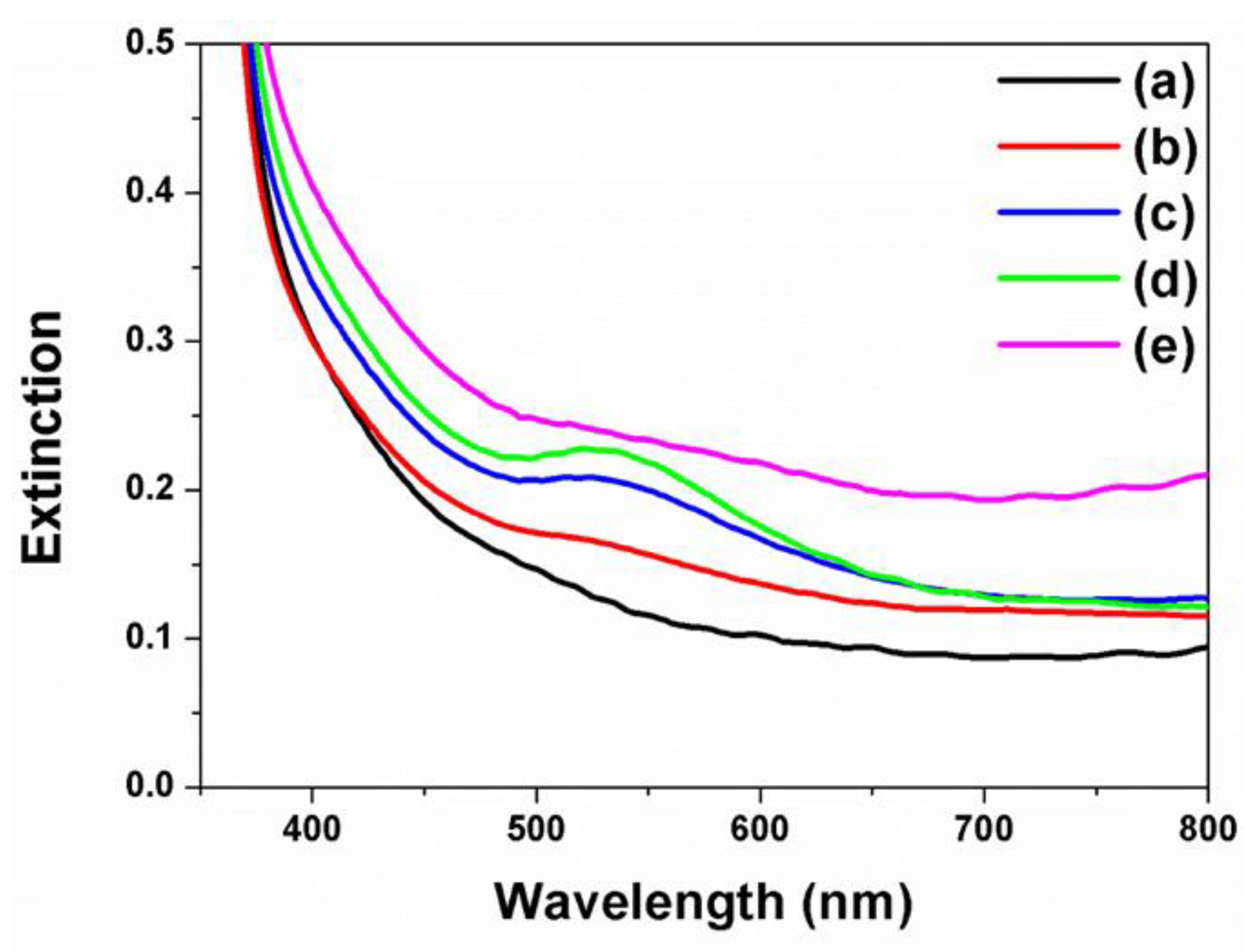
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Murakami, T.N.; Comte, P.; Liska, P.; Grätzel, C.; Nazeeruddin, M.K.; Grätzel, M. Fabrication of thin film dye sensitized solar cells with solar to electric power conversion efficiency over 10%. Thin Solid Film. 2008, 516, 4613–4619. [Google Scholar] [CrossRef]
- Bach, U.; Lupo, D.; Comte, P.; Moser, J.-E.; Weissörtel, F.; Salbeck, J.; Spreitzer, H.; Grätzel, M. Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 1998, 395, 583–585. [Google Scholar] [CrossRef]
- Ardo, S.; Meyer, G.J. Photodriven heterogeneous charge transfer with transition-metal compounds anchored to TiO2 semiconductor surfaces. Chem. Soc. Rev. 2009, 38, 115–164. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Grätzel, M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
- Benkstein, K.D.; Kopidakis, N.; Van de Lagemaat, J.; Frank, A.J. Influence of the percolation network geometry on electron transport in dye-sensitized titanium dioxide solar cells. J. Phys. Chem. B 2003, 107, 7759–7767. [Google Scholar] [CrossRef]
- Van de Lagemaat, J.; Benkstein, K.D.; Frank, A.J. Relation between particle coordination number and porosity in nanoparticle films: Implications to dye-sensitized solar cells. J. Phys. Chem. B 2001, 105, 12433–12436. [Google Scholar] [CrossRef]
- De Jongh, P.; Vanmaekelbergh, D. Trap-Limited Electronic Transport in Assemblies of Nanometer-Size TiO2 Particles. Phys. Rev. Lett. 1996, 77, 3427–3430. [Google Scholar] [CrossRef]
- Cao, F.; Oskam, G.; Meyer, G.J.; Searson, P.C. Electron transport in porous nanocrystalline TiO2 photoelectrochemical cells. J. Phys. Chem. 1996, 100, 17021–17027. [Google Scholar] [CrossRef]
- Zwilling, V.; Darque-Ceretti, E.; Boutry-Forveille, A.; David, D.; Perrin, M.-Y.; Aucouturier, M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf. Interface Anal. 1999, 27, 629–637. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Varghese, O.K.; Gong, D.; Paulose, M.; Grimes, C.A.; Dickey, E.C. Crystallization and high-temperature structural stability of titanium oxide nanotube arrays. J. Mater. Res. 2003, 18, 156–165. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Aldabergerova, S.; Schmuki, P. Smooth anodic TiO2 nanotubes. Angew. Chem. Int. Ed. 2005, 44, 7463–7465. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Rho, C.; Min, J.-H.; Suh, J.S. Barrier layer effect on the electron transport of the dye-sensitized solar cells based on TiO2 nanotube arrays. J. Phys. Chem. C 2012, 116, 7213–7218. [Google Scholar] [CrossRef]
- Jennings, J.R.; Ghicov, A.; Peter, L.M.; Schmuki, P.; Walker, A.B. Dye-sensitized solar cells based on oriented TiO2 nanotube arrays: Transport, trapping, and transfer of electrons. J. Am. Chem. Soc. 2008, 130, 13364–13372. [Google Scholar] [CrossRef]
- Juan, M.L.; Righini, M.; Quidant, R. Plasmon nano-optical tweezers. Nat. Photonics 2011, 5, 349. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef]
- Choi, H.; Chen, W.T.; Kamat, P.V. Know thy nano neighbor. Plasmonic versus electron charging effects of metal nanoparticles in dye-sensitized solar cells. ACS Nano 2012, 6, 4418–4427. [Google Scholar] [CrossRef]
- Song, D.H.; Kim, H.-S.; Suh, J.S.; Jun, B.-H.; Rho, W.-Y. Multi-shaped Ag nanoparticles in the plasmonic layer of dye-sensitized solar cells for increased power conversion efficiency. Nanomaterials 2017, 7, 136. [Google Scholar] [CrossRef]
- Rho, W.-Y.; Yang, H.-Y.; Kim, H.-S.; Son, B.S.; Suh, J.S.; Jun, B.-H. Recent advances in plasmonic dye-sensitized solar cells. J. Solid State Chem. 2018, 258, 271–282. [Google Scholar] [CrossRef]
- Gangishetty, M.K.; Lee, K.E.; Scott, R.W.; Kelly, T.L. Plasmonic enhancement of dye sensitized solar cells in the red-to-near-infrared region using triangular core-shell Ag@SiO2 nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 11044–11051. [Google Scholar] [CrossRef]
- Tan, H.; Santbergen, R.; Smets, A.H.; Zeman, M. Plasmonic light trapping in thin-film silicon solar cells with improved self-assembled silver nanoparticles. Nano Lett. 2012, 12, 4070–4076. [Google Scholar] [CrossRef]
- Choi, H.; Lee, J.P.; Ko, S.J.; Jung, J.W.; Park, H.; Yoo, S.; Park, O.; Jeong, J.R.; Park, S.; Kim, J.Y. Multipositional silica-coated silver nanoparticles for high-performance polymer solar cells. Nano Lett. 2013, 13, 2204–2208. [Google Scholar] [CrossRef]
- Hore, S.; Vetter, C.; Kern, R.; Smit, H.; Hinsch, A. Influence of scattering layers on efficiency of dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 1176–1188. [Google Scholar] [CrossRef]
- Yang, L.; Lin, Y.; Jia, J.; Xiao, X.; Li, X.; Zhou, X. Light harvesting enhancement for dye-sensitized solar cells by novel anode containing cauliflower-like TiO2 spheres. J. Power Sources 2008, 182, 370–376. [Google Scholar] [CrossRef]
- Zhang, Z.; Ito, S.; O’Regan, B.; Kuang, D.; Zakeeruddin, S.M.; Liska, P.; Charvet, R.; Comte, P.; Nazeeruddin, M.K.; Péchy, P.; et al. The electronic role of the TiO2 light-scattering layer in dye-sensitized solar cells. Z. Phys. Chem. 2007, 221, 319–327. [Google Scholar] [CrossRef]
- Hu, L.; Dai, S.; Weng, J.; Xiao, S.; Sui, Y.; Huang, Y.; Chen, S.; Kong, F.; Pan, X.; Liang, L. Microstructure design of nanoporous TiO2 photoelectrodes for dye-sensitized solar cell modules. J. Phys. Chem. B 2007, 111, 358–362. [Google Scholar] [CrossRef]
- Nakayama, K.; Kubo, T.; Nishikitani, Y. Electrophoretically deposited TiO2 nanotube light-scattering layers of dye-sensitized solar cells. Jpn. J. Appl. Phys. 2008, 47, 6610. [Google Scholar] [CrossRef]
- Qian, J.; Liu, P.; Xiao, Y.; Jiang, Y.; Cao, Y.; Ai, X.; Yang, H. TiO2-coated multilayered SnO2 hollow microspheres for dye-sensitized solar cells. Adv. Mater. 2009, 21, 3663–3667. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, M.H.; Kim, H.J.; Lim, G.; Choi, Y.S.; Park, N.G.; Kim, K.; Lee, W.I. Formation of highly efficient dye-sensitized solar cells by hierarchical pore generation with nanoporous TiO2 spheres. Adv. Mater. 2009, 21, 3668–3673. [Google Scholar] [CrossRef]
- Han, S.-H.; Rho, W.-Y.; Jun, B.-H. Au-Nanoparticle-embedded open-ended freestanding TiO2 nanotube arrays in dye-sensitized solar cells for better electron generation and electron transport. ACS Omega 2019, 4, 20346–20352. [Google Scholar] [CrossRef]
- HyeokáPark, J.; GuáKang, M. Growth, detachment and transfer of highly-ordered TiO2 nanotube arrays: Use in dye-sensitized solar cells. Chem. Commun. 2008, 25, 2867–2869. [Google Scholar]
- Chen, Q.; Xu, D. Large-scale, noncurling, and free-standing crystallized TiO2 nanotube arrays for dye-sensitized solar cells. J. Phys. Chem. C 2009, 113, 6310–6314. [Google Scholar] [CrossRef]
- Li, L.-L.; Chen, Y.-J.; Wu, H.-P.; Wang, N.S.; Diau, E.W.-G. Detachment and transfer of ordered TiO2 nanotube arrays for front-illuminated dye-sensitized solar cells. Energy Environ. Sci. 2011, 4, 3420–3425. [Google Scholar] [CrossRef]
- Rho, W.-Y.; Lee, K.-H.; Han, S.-H.; Kim, H.-Y.; Jun, B.-H. Au-embedded and carbon-doped freestanding TiO2 nanotube arrays in dye-sensitized solar cells for better energy conversion efficiency. Micromachines 2019, 10, 805. [Google Scholar] [CrossRef]
- Benoit, A.; Paramasivam, I.; Nah, Y.-C.; Roy, P.; Schmuki, P. Decoration of TiO2 nanotube layers with WO3 nanocrystals for high-electrochromic activity. Electrochem. Commun. 2009, 11, 728–732. [Google Scholar] [CrossRef]
- Paramasivam, I.; Macak, J.; Ghicov, A.; Schmuki, P. Enhanced photochromism of Ag loaded self-organized TiO2 nanotube layers. Chem. Phys. Lett. 2007, 445, 233–237. [Google Scholar] [CrossRef]
- Rho, C.; Suh, J.S. Filling TiO2 nanoparticles in the channels of TiO2 nanotube membranes to enhance the efficiency of dye-sensitized solar cells. Chem. Phys. Lett. 2011, 513, 108–111. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV−Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Munoz, G.A.; Balderas-Lopez, J.A.; Ortega-Lopez, J.; Pescador-Rojas, J.A.; Salazar, J.S. Thermal diffusivity measurement for urchin-like gold nanofluids with different solvents, sizes and concentrations/shapes. Nanoscale Res. Lett. 2012, 7, 667. [Google Scholar] [CrossRef] [PubMed]
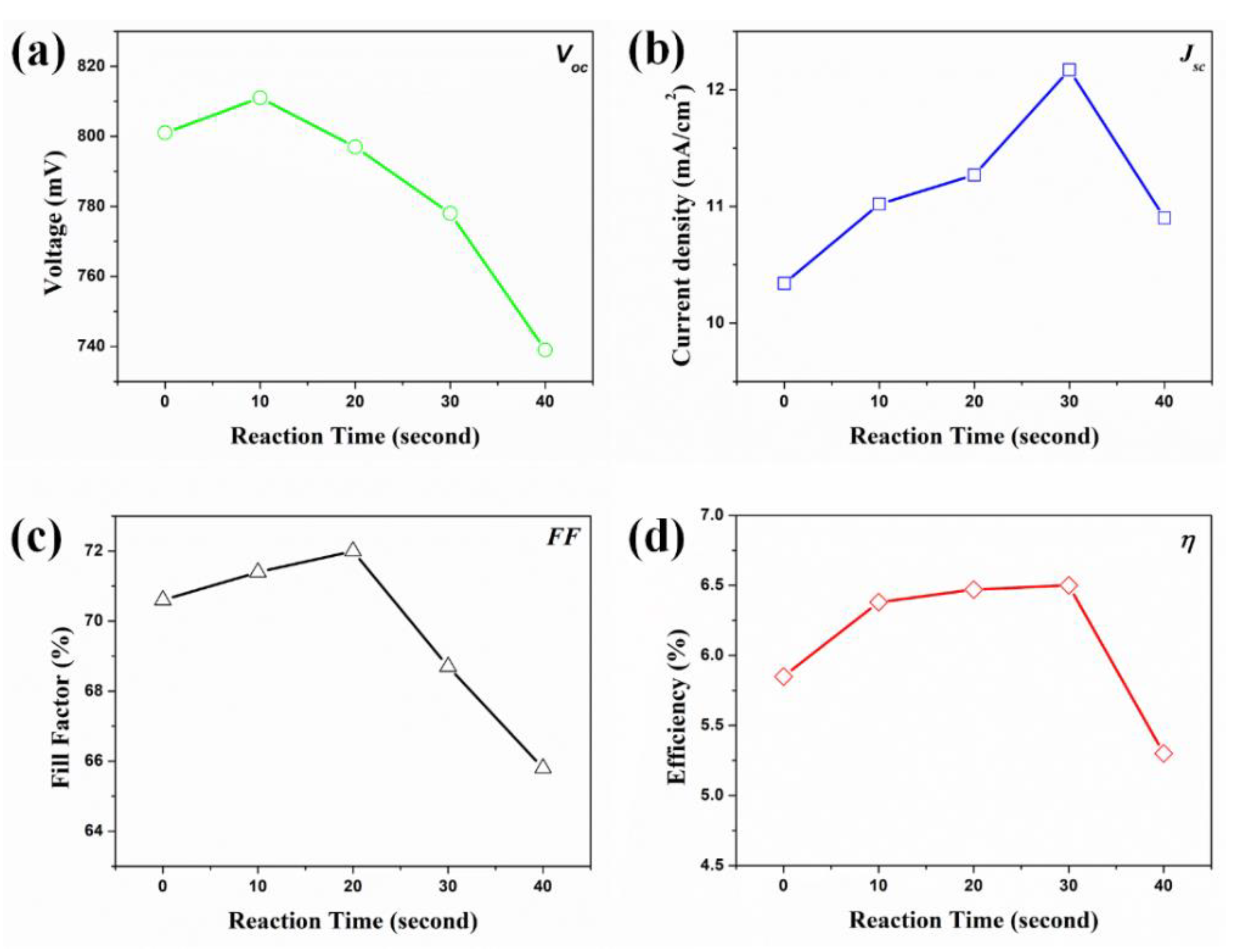
| DSSCs Based on the f-TNTAs with Au NPs | Jsc (mA/cm2) | Voc (mV) | FF (%) | η (%) | Dye Loading (nmol/cm2) | |
|---|---|---|---|---|---|---|
| (a) | for 0 s | 10.34 | 801 | 70.6 | 5.85 ± 0.31 | 143 |
| (b) | for 10 s | 11.02 | 811 | 71.4 | 6.38 ± 0.47 | 145 |
| (c) | for 20 s | 11.27 | 797 | 72.0 | 6.47 ± 0.43 | 147 |
| (d) | for 30 s | 12.17 | 778 | 68.7 | 6.50 ± 0.51 | 148 |
| (e) | for 40 s | 10.90 | 739 | 65.8 | 5.30 ± 0.94 | 162 |
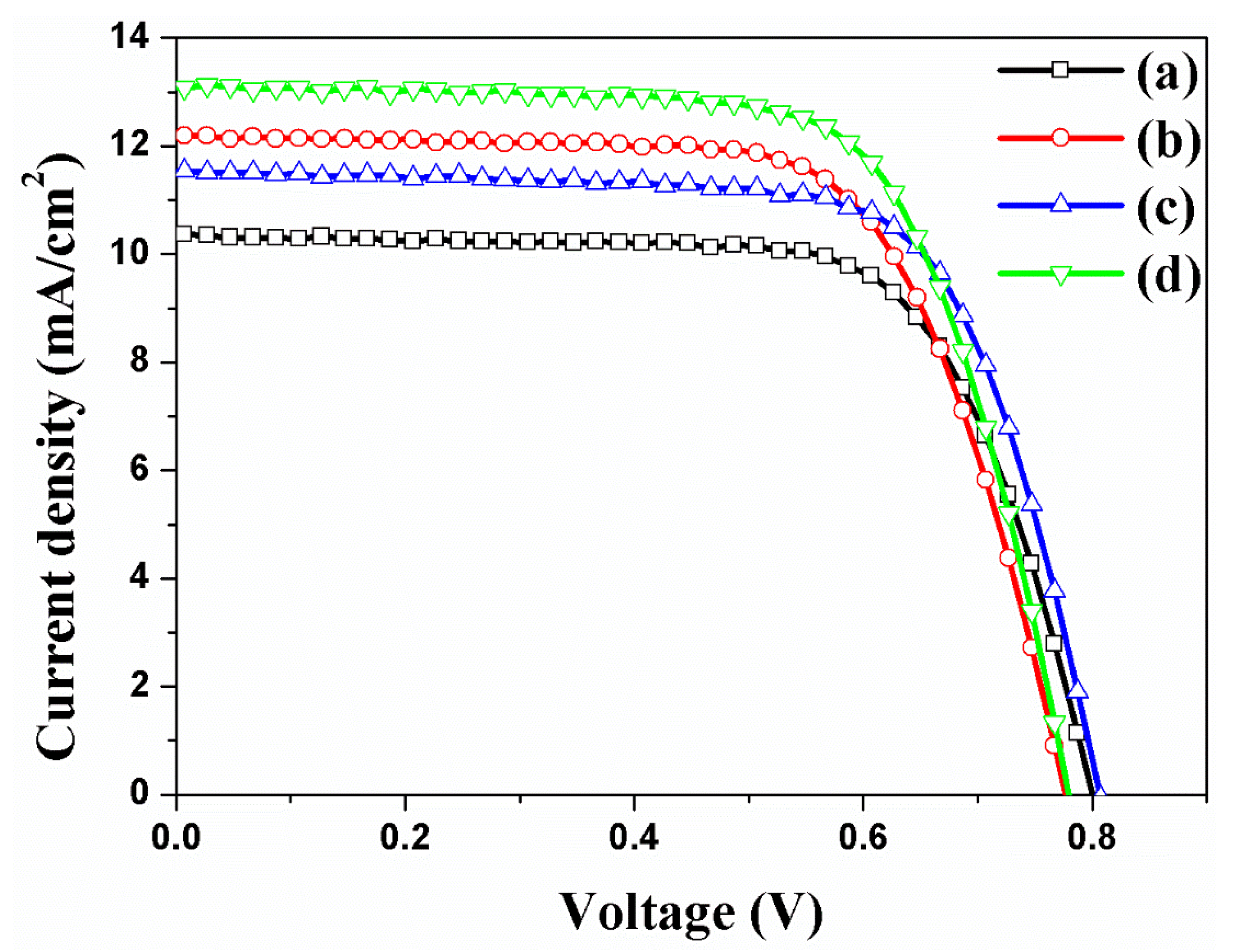
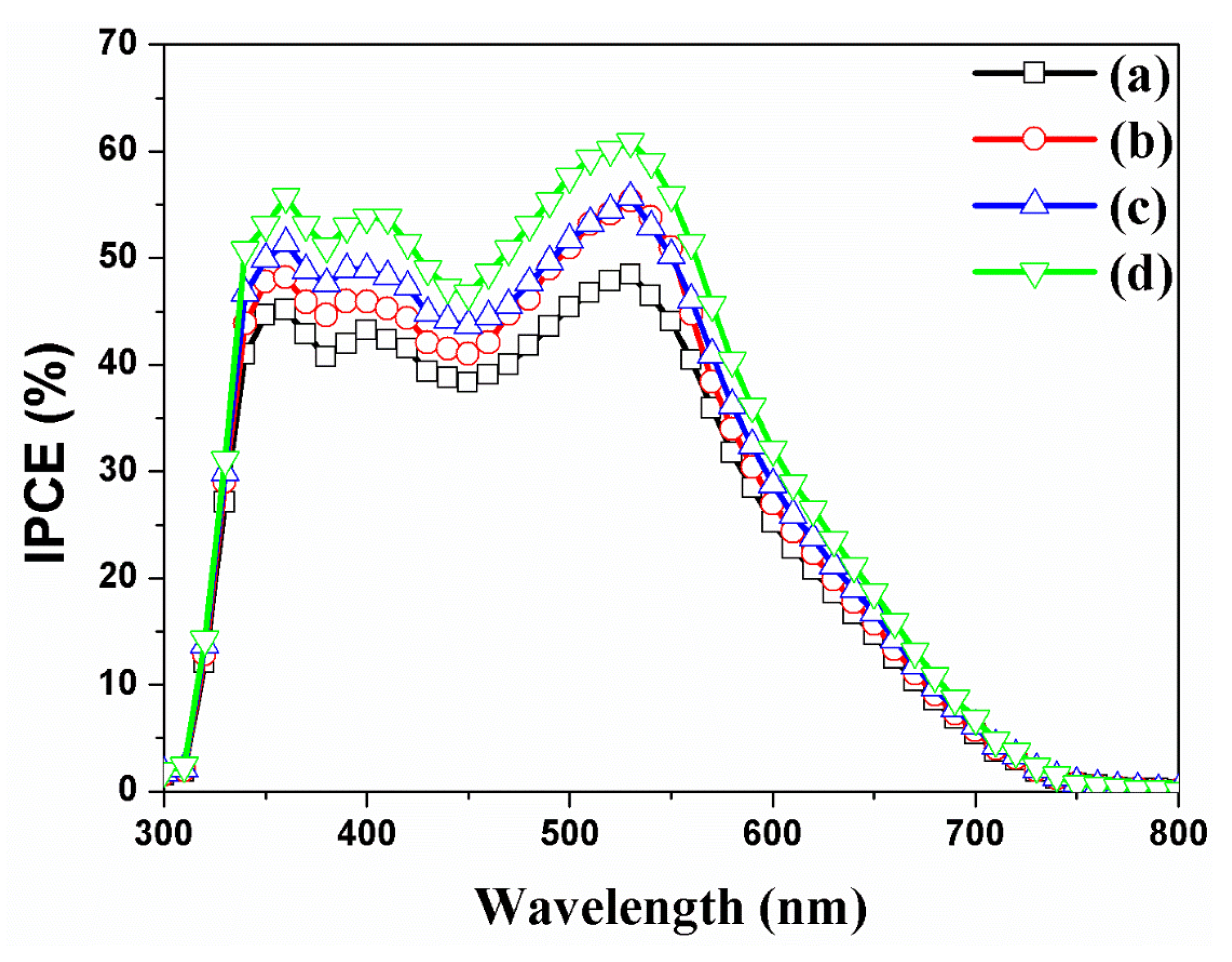
| DSSCs Based on the f-TNTAs | Jsc (mA/cm2) | Voc (mV) | FF (%) | η (%) | Dye Loading (nmol/cm2) | |
|---|---|---|---|---|---|---|
| (a) | without both Au NPs and a scattering layer | 10.34 | 801 | 70.6 | 5.85 ± 0.31 | 143 |
| (b) | with Au NPs | 12.17 | 778 | 68.7 | 6.50 ± 0.51 | 148 |
| (c) | with a scattering layer | 11.56 | 807 | 70.8 | 6.61 ± 0.37 | 153 |
| (d) | with Au NPs and a scattering layer | 13.10 | 780 | 69.7 | 7.12 ± 0.55 | 154 |
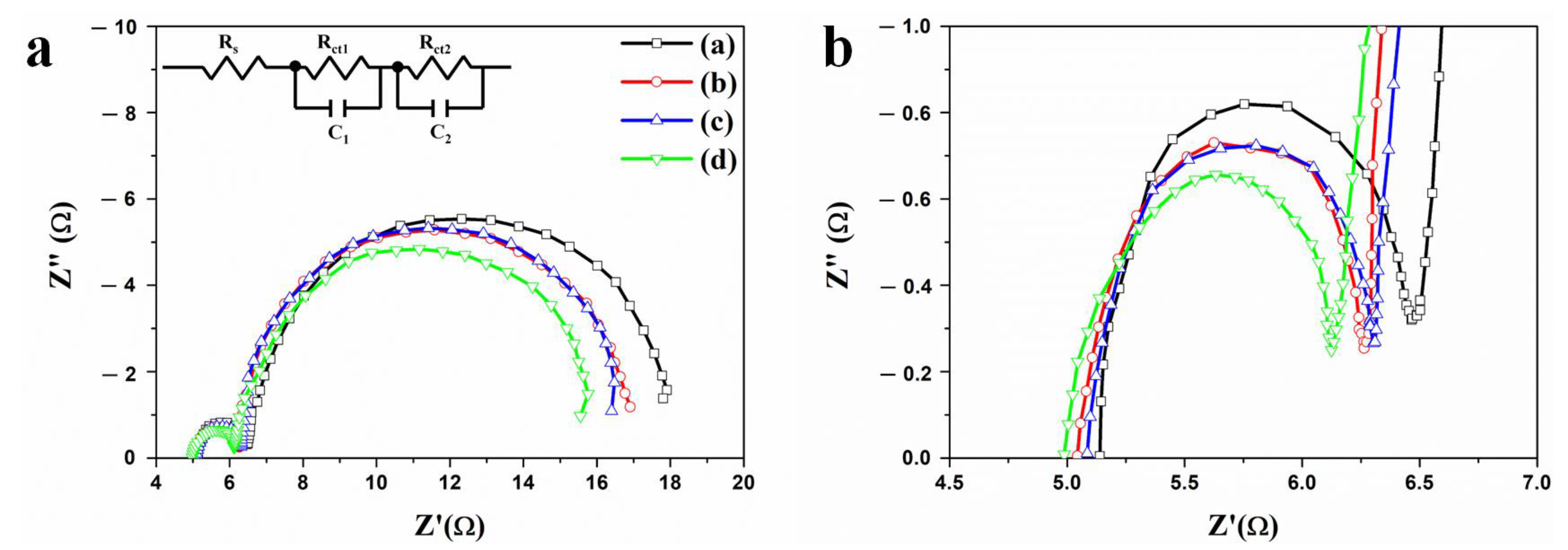
| DSSCs Based on the f-TNTAs | Rs (Ω) | R1 (Ω) | R2 (Ω) | |
|---|---|---|---|---|
| (a) | without Au NPs and a scattering layer | 5.099 | 1.257 | 12.77 |
| (b) | with Au NPs | 5.039 | 1.088 | 11.68 |
| (c) | with a scattering layer | 5.059 | 1.098 | 11.75 |
| (d) | with Au NPs and a scattering layer | 4.942 | 1.071 | 10.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-H.; Han, S.-H.; Chuquer, A.; Yang, H.-Y.; Kim, J.; Pham, X.-H.; Yun, W.-J.; Jun, B.-H.; Rho, W.-Y. Effect of Au Nanoparticles and Scattering Layer in Dye-Sensitized Solar Cells Based on Freestanding TiO2 Nanotube Arrays. Nanomaterials 2021, 11, 328. https://doi.org/10.3390/nano11020328
Lee K-H, Han S-H, Chuquer A, Yang H-Y, Kim J, Pham X-H, Yun W-J, Jun B-H, Rho W-Y. Effect of Au Nanoparticles and Scattering Layer in Dye-Sensitized Solar Cells Based on Freestanding TiO2 Nanotube Arrays. Nanomaterials. 2021; 11(2):328. https://doi.org/10.3390/nano11020328
Chicago/Turabian StyleLee, Kang-Hun, Seung-Hee Han, Ana Chuquer, Hwa-Young Yang, Jaehi Kim, Xuan-Hung Pham, Won-Ju Yun, Bong-Hyun Jun, and Won-Yeop Rho. 2021. "Effect of Au Nanoparticles and Scattering Layer in Dye-Sensitized Solar Cells Based on Freestanding TiO2 Nanotube Arrays" Nanomaterials 11, no. 2: 328. https://doi.org/10.3390/nano11020328
APA StyleLee, K.-H., Han, S.-H., Chuquer, A., Yang, H.-Y., Kim, J., Pham, X.-H., Yun, W.-J., Jun, B.-H., & Rho, W.-Y. (2021). Effect of Au Nanoparticles and Scattering Layer in Dye-Sensitized Solar Cells Based on Freestanding TiO2 Nanotube Arrays. Nanomaterials, 11(2), 328. https://doi.org/10.3390/nano11020328








