Morphology Controlled Synthesis of γ-Al2O3 Nano-Crystallites in Al@Al2O3 Core–Shell Micro-Architectures by Interfacial Hydrothermal Reactions of Al Metal Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
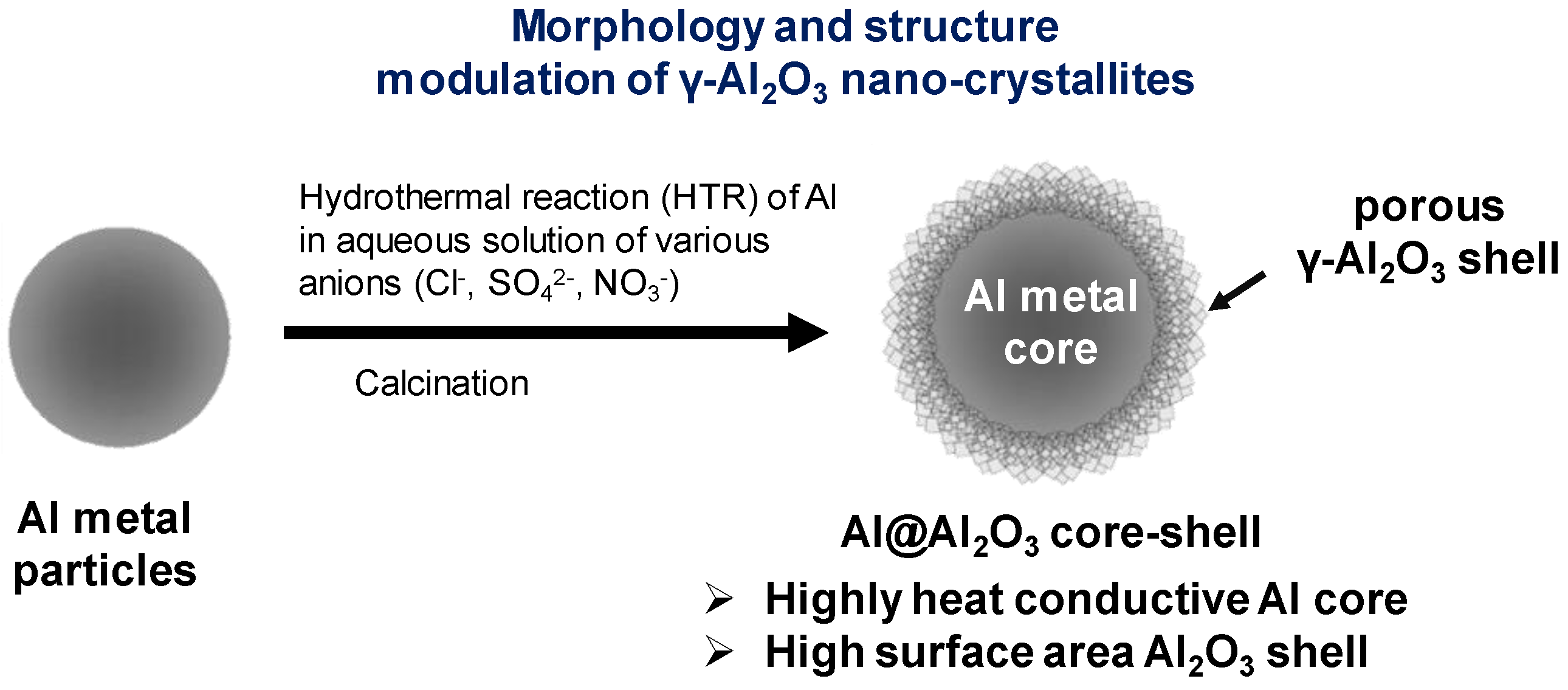
2.2. Morphology Controlled Synthesis of Al@Al2O3 Core–Shell Microstructures
2.3. Characterizations
3. Results
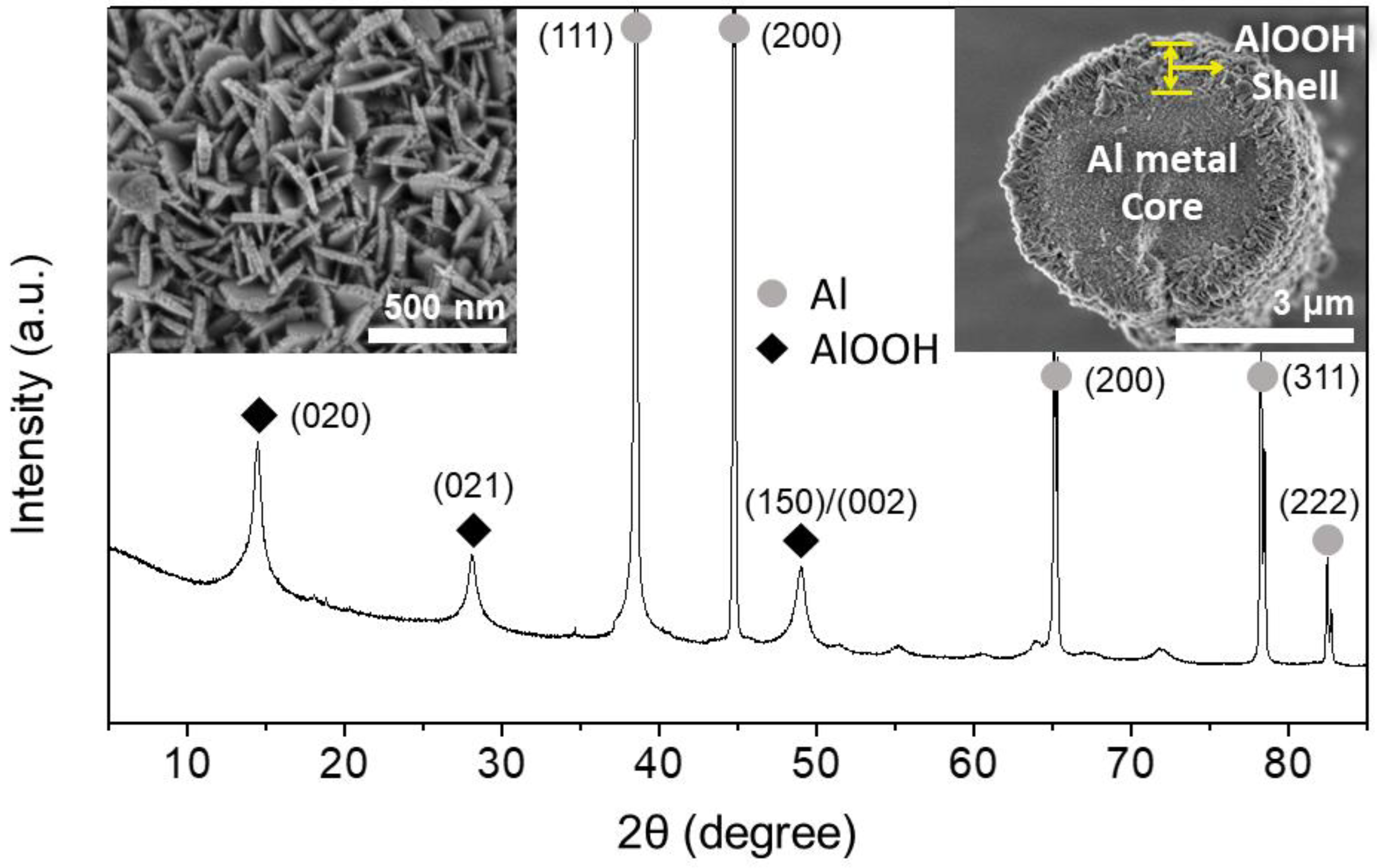
3.1. Morphology and Structural Properties of Al@Al2O3 Core–Shell Prepared by HTR in D.I.-H2O
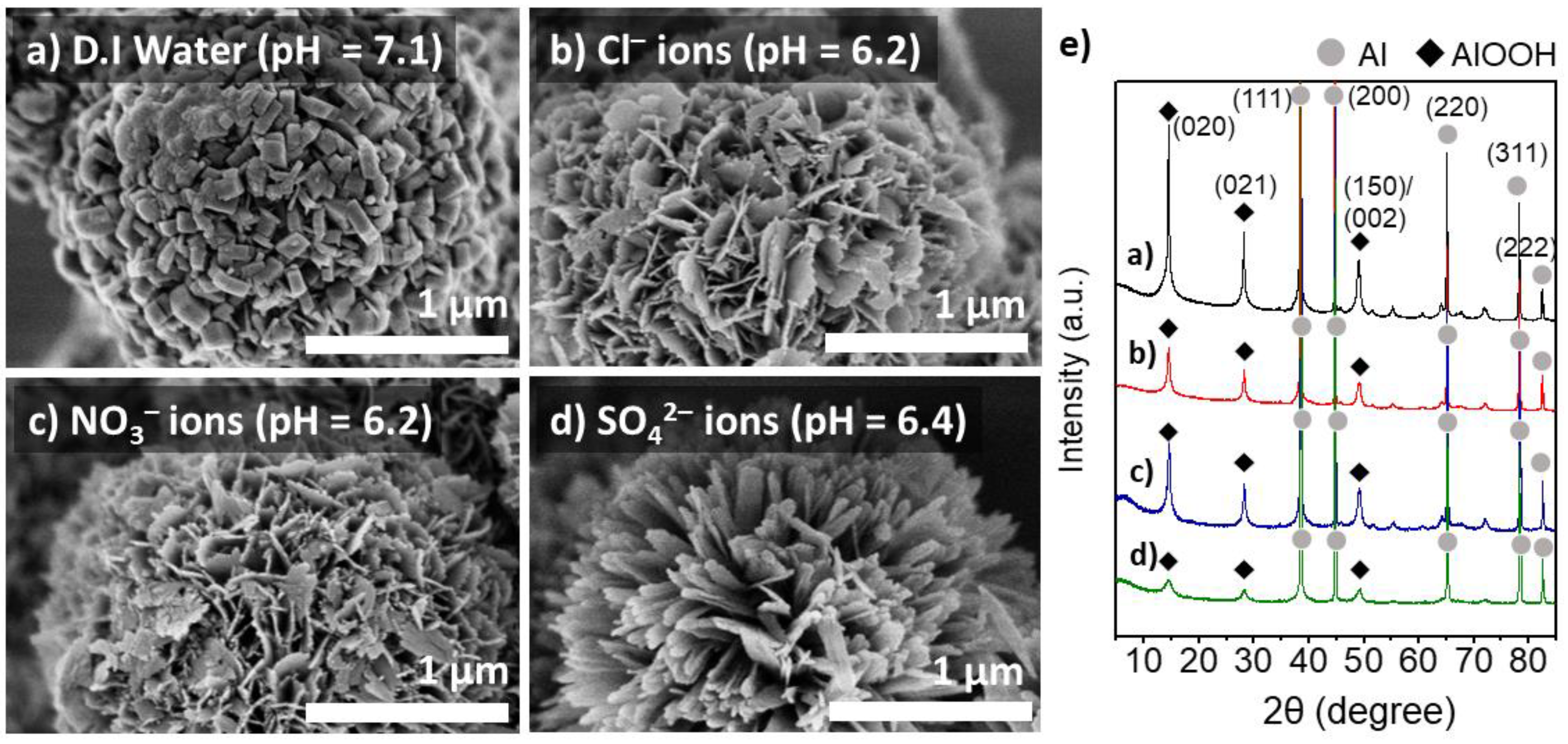
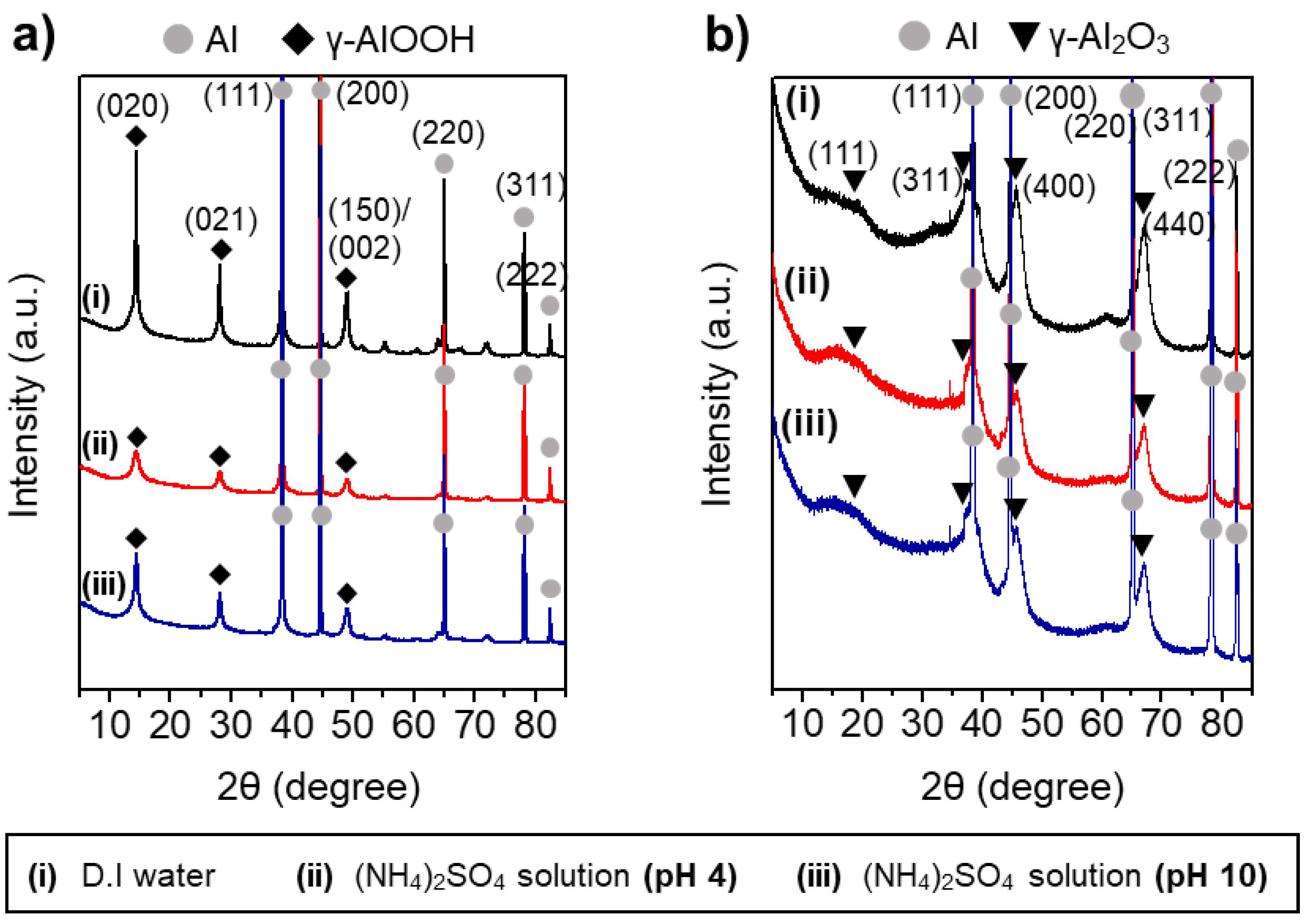
3.2. The Effects of Heteroatom Anions on the Interfacial Growth of Boehmites Crystallites
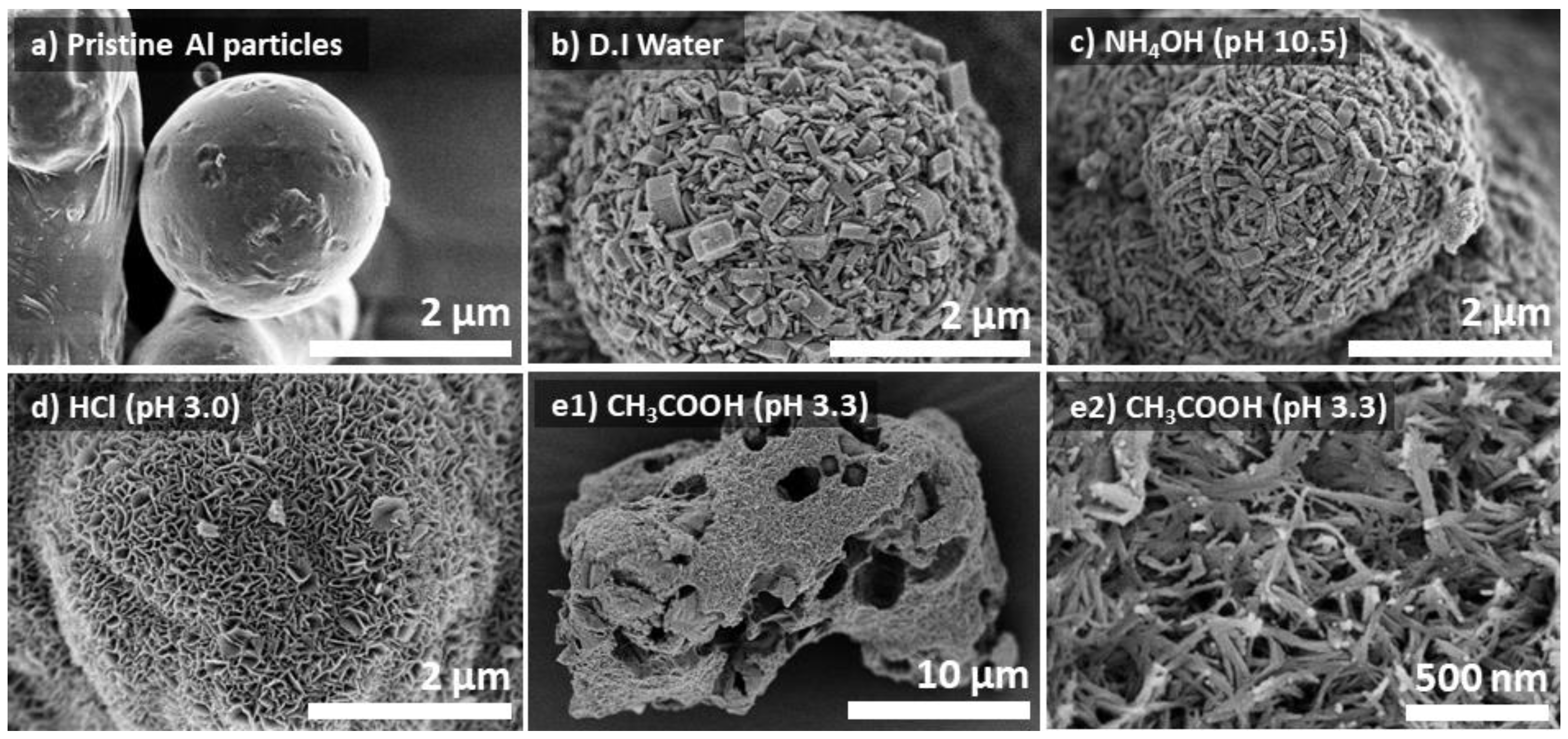
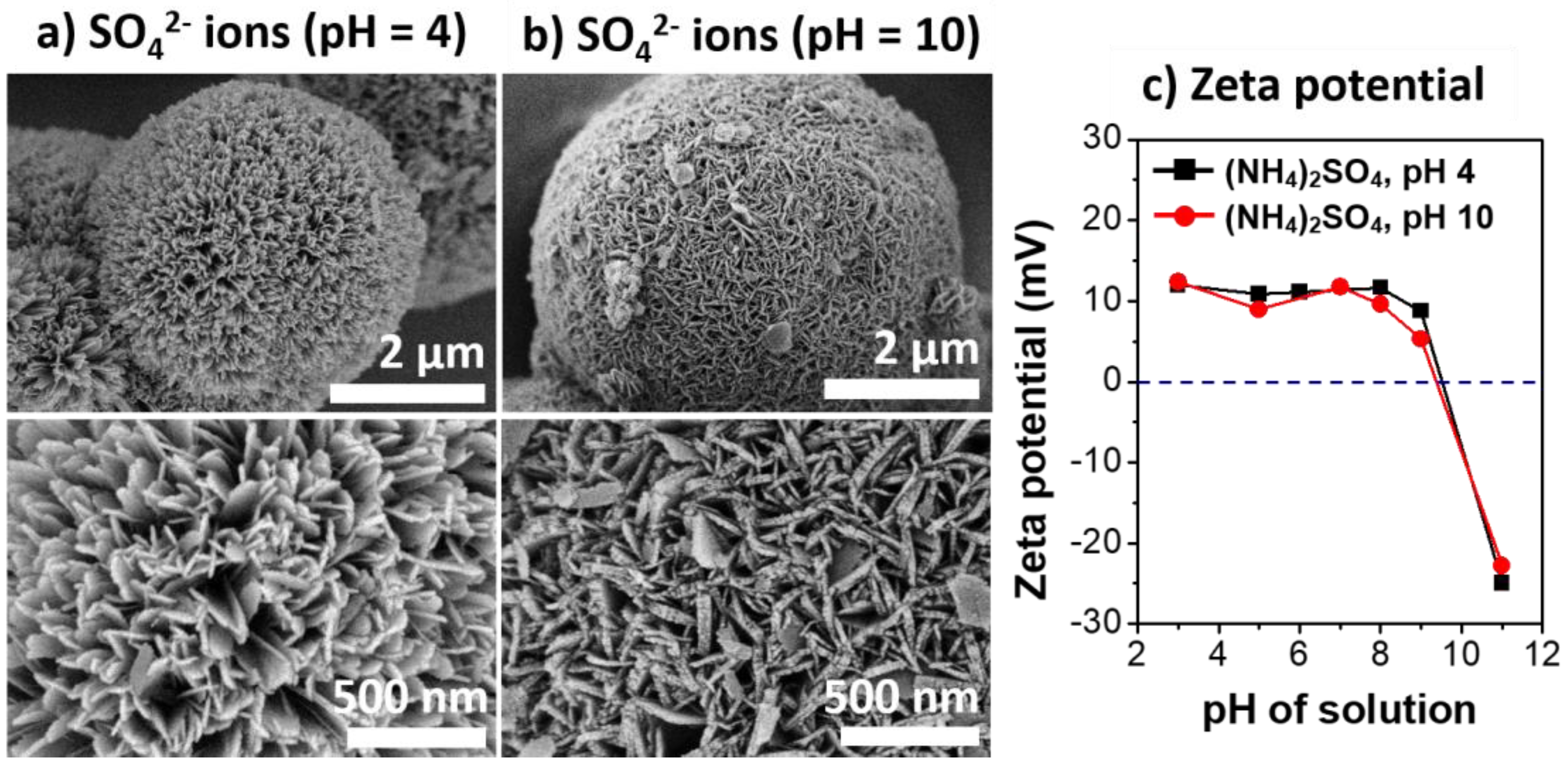
3.3. The Effects of Solution pH on the Properties of Al@AlOOH Core–Shell Composites
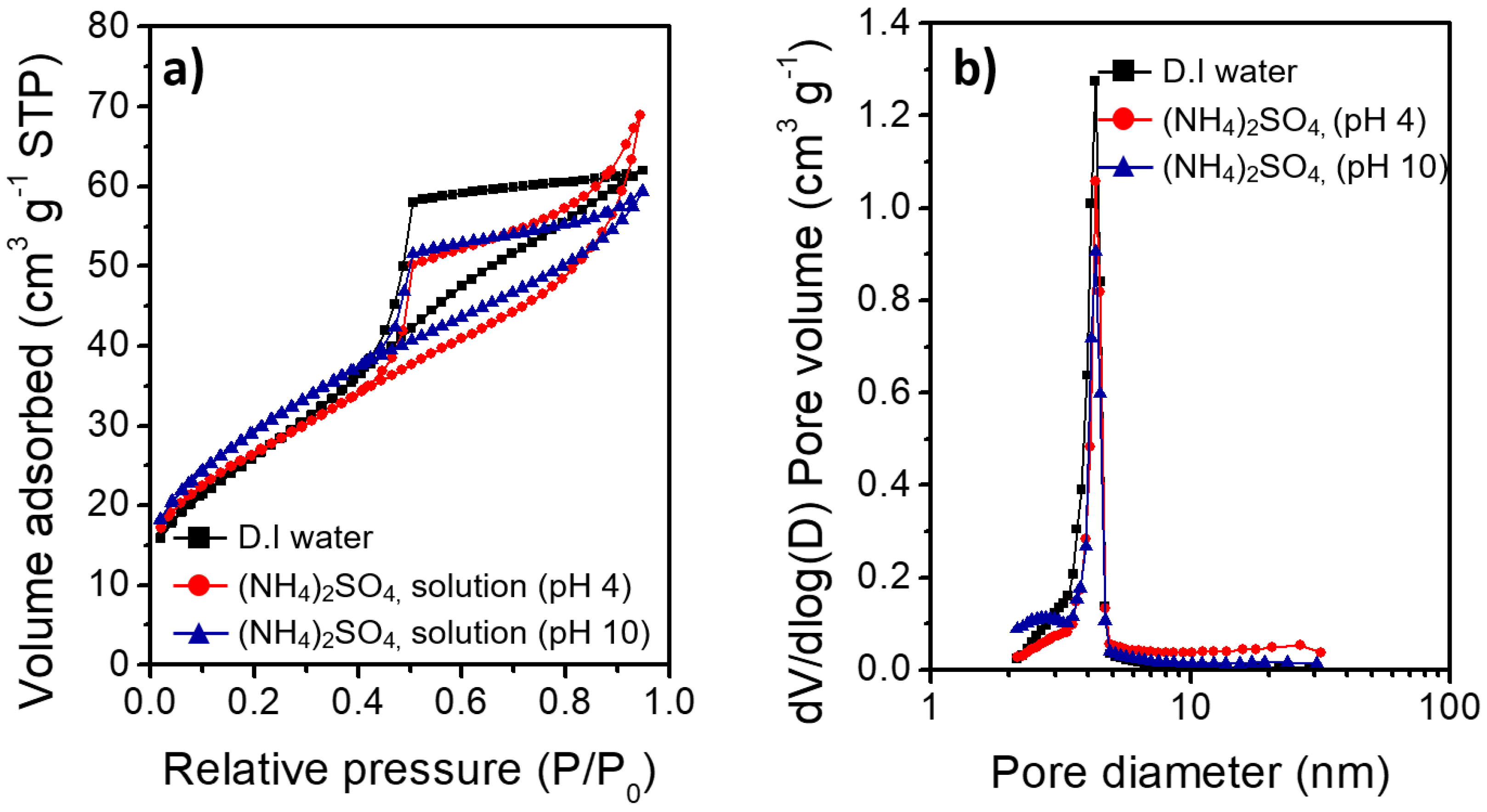
3.4. Physicochemical Properties of the Al@Al2O3 Core–Shell Microstructures
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekker, F.H.M.; Bliek, A.; Kapteijn, F.; Moulijn, J.A. Analysis of mass and heat transfer in transient experiments over heterogeneous catalysts. Chem. Eng. Sci. 1995, 50, 3573–3580. [Google Scholar] [CrossRef]
- Vogt, C.; Monai, M.; Sterk, E.B.; Palle, J.; Melcherts, A.E.M.; Zijlstra, B.; Groeneveld, E.; Berben, P.H.; Boereboom, J.M.; Hensen, E.J.M.; et al. Understanding carbon dioxide activation and carbon-carbon coupling over nickel. Nat. Commun. 2017, 10, 5330. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Zhou, Z.-Y.; Sun, S.-G. Platinum metal catalysts of high-index surfaces: From single-crystal planes to electrochemically shape-controlled nanoparticles. J. Phys. Chem. C 2008, 112, 19801–19817. [Google Scholar] [CrossRef]
- Faure, R.; Rossignol, F.; Chartier, T.; Bonhomme, C.; Maître, A.; Etchegoyen, G.; Gallo, P.D.; Gary, D. Alumina foam catalyst support for industrial steam reforming processes. J. Eur. Ceram. 2011, 31, 303–312. [Google Scholar] [CrossRef]
- Yarulina, I.; Kapteijn, F.; Gascon, J. The importance of heat effects in the methanol to hydrocarbons reaction over ZSM-5: On the role of mesoporosity on catalyst performance. Catal. Sci. Technol. 2016, 6, 5320. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Lee, K.-Y.; La, H.; Kim, H.-J.; Yang, J.-I.; Jung, H. Ni catalyst wash-coated on metal monolith with enhanced heat-transfer capability for steam reforming. J. Power Sources 2007, 171, 499–505. [Google Scholar] [CrossRef]
- Richardson, J.T.; Remue, D.; Hung, J.-K. Properties of ceramic foam catalyst supports mass and heat transfer. Appl. Catal. A 2016, 6, 5320. [Google Scholar] [CrossRef]
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineer’s Handbook, 8th ed.; McGraw-Hill: New York, NY, USA, 2008; pp. 199–206. [Google Scholar]
- Kim, C.S. Thermophysical Properties of Stainless Steels; Report No. ANL-75-55; Argonne National Laboratory: Argonne, IL, USA, 1975; pp. 11–29.
- Kim, J.; Lee, D. Core-shell metal-ceramic microstructures: Mechanism of hydrothermal formation and properties as catalyst materials. Chem. Mater. 2016, 28, 2786–2794. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Lee, D. A new design and synthesis approach of supported metal catalysts via interfacial hydrothermal-oxidation/reductive-exolution chemistry of Al metal surface. App. Cat. A. 2020, 594, 117461. [Google Scholar] [CrossRef]
- Lee, H.C.; Potapova, Y.; Lee, D. A core-shell structured, metal-ceramic composite-supported Ru catalyst for methane steam reforming. J. Power Sources 2012, 216, 256–260. [Google Scholar] [CrossRef]
- Rah, I.J.; Kim, T.W.; Kim, J.; Lee, D.; Park, E.D. Selective CO oxidation in the hydrogen stream over Ru/Al@Al2O3 catalysts. Catal. Today 2020, 352, 148–156. [Google Scholar] [CrossRef]
- He, T.; Xiang, L.; Zhu, S. Different nanostructures of boehmite fabricated by hydrothermal process: Effects of pH and anions. CrystEngComm 2009, 11, 1338–1342. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, W.; Page, K.L.; Pearce, C.I.; Bowden, M.E.; Graham, T.R.; Shen, Z.; Li, P.; Wang, Z.; Kerisit, S.; et al. Size and morphology controlled synthesis of boehmite nanoplates and crystal growth mechanisms. Cryst. Growth Des. 2018, 18, 3596–3606. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M.; Varyani, A.S. Analysis and the understanding of fluoride removal mechanisms by an electrocoagulation/flotation (ECF) process. Desalination 2011, 275, 102–106. [Google Scholar] [CrossRef]
- Hudson, L.K.; Misra, C.; Perrotta, A.J.; Wefers, K.; Williams, F.S. Aluminum oxide in Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: Weinheim, Germany, 2003; Volume 2, pp. 342–343. [Google Scholar]
- Xia, Y.; Jiao, X.; Liu, Y.; Chen, D.; Zhang, L.; Qin, Z. Study of the formation mechanism of boehmite with different morphology upon surface hydroxyls and adsorption of chloride ions. J. Phys. Chem. C 2013, 117, 15279–15286. [Google Scholar] [CrossRef]
- He, T.; Xiang, L.; Zhu, S. Hydrothermal preparation of boehmite nanorods by selective adsorption of sulfate. Langmuir 2008, 24, 8284–8289. [Google Scholar] [CrossRef] [PubMed]
- Raybaud, P.; Digne, M.; Iftimie, R.; Wellens, W.; Euzen, P.; Toulhoat, H. Morphology and surface properties of boehmite (γ-AlOOH): A density functional theory study. J. Catal. 2001, 201, 236–246. [Google Scholar] [CrossRef]
- Jiao, W.; Wu, X.; Xue, T.; Li, G.; Wang, W.; Wang, Y.M.; Tang, Y.; He, M.-Y. Morphological controlled growth of nanosized boehmite with enhanced aspect ratios in an organic additive-free cationic-anionic double hydrolysis method. Cryst. Growth Des. 2016, 16, 5166–5173. [Google Scholar] [CrossRef]
| Title 1 | Materials | Thermal Conductivity (W m−1 K−1) | Specific Heat Capacity (J mol−1 K−1) | Reference |
|---|---|---|---|---|
| Al2O3 | 36 | 78 | [9] | |
| Metal Oxides | SiO2 | 1.3 | 44 | |
| TiO2 | 8.4 | 57 | ||
| Al | 273 | 24 | ||
| Metal | Au | 315 | 25 | |
| Stainless steel | 13 | 28 | [10] |
| Synthesis Condition | BET Surface Area (m2 g−1) | Average Pore Size (nm) | Total Pore Volume (cm3 g−1) |
|---|---|---|---|
| D.I. Water | 98 | 3.9 | 0.096 |
| (NH4)2SO4 (pH = 4) | 95 | 4.9 | 0.110 |
| (NH4)2SO4 (pH = 10) | 106 | 4.0 | 0.092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Lee, D. Morphology Controlled Synthesis of γ-Al2O3 Nano-Crystallites in Al@Al2O3 Core–Shell Micro-Architectures by Interfacial Hydrothermal Reactions of Al Metal Substrates. Nanomaterials 2021, 11, 310. https://doi.org/10.3390/nano11020310
Han D, Lee D. Morphology Controlled Synthesis of γ-Al2O3 Nano-Crystallites in Al@Al2O3 Core–Shell Micro-Architectures by Interfacial Hydrothermal Reactions of Al Metal Substrates. Nanomaterials. 2021; 11(2):310. https://doi.org/10.3390/nano11020310
Chicago/Turabian StyleHan, Dohyeon, and Doohwan Lee. 2021. "Morphology Controlled Synthesis of γ-Al2O3 Nano-Crystallites in Al@Al2O3 Core–Shell Micro-Architectures by Interfacial Hydrothermal Reactions of Al Metal Substrates" Nanomaterials 11, no. 2: 310. https://doi.org/10.3390/nano11020310
APA StyleHan, D., & Lee, D. (2021). Morphology Controlled Synthesis of γ-Al2O3 Nano-Crystallites in Al@Al2O3 Core–Shell Micro-Architectures by Interfacial Hydrothermal Reactions of Al Metal Substrates. Nanomaterials, 11(2), 310. https://doi.org/10.3390/nano11020310






