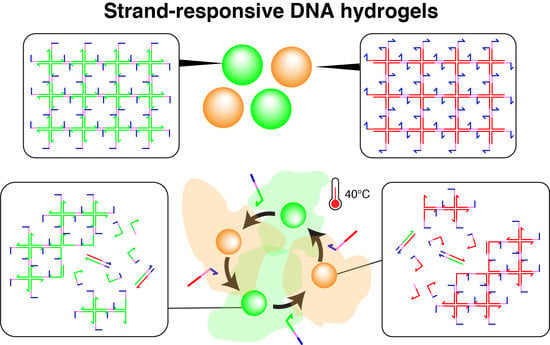
Morphological Manipulation of DNA Gel Microbeads with Biomolecular Stimuli
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. DNA Hydrogel Concept
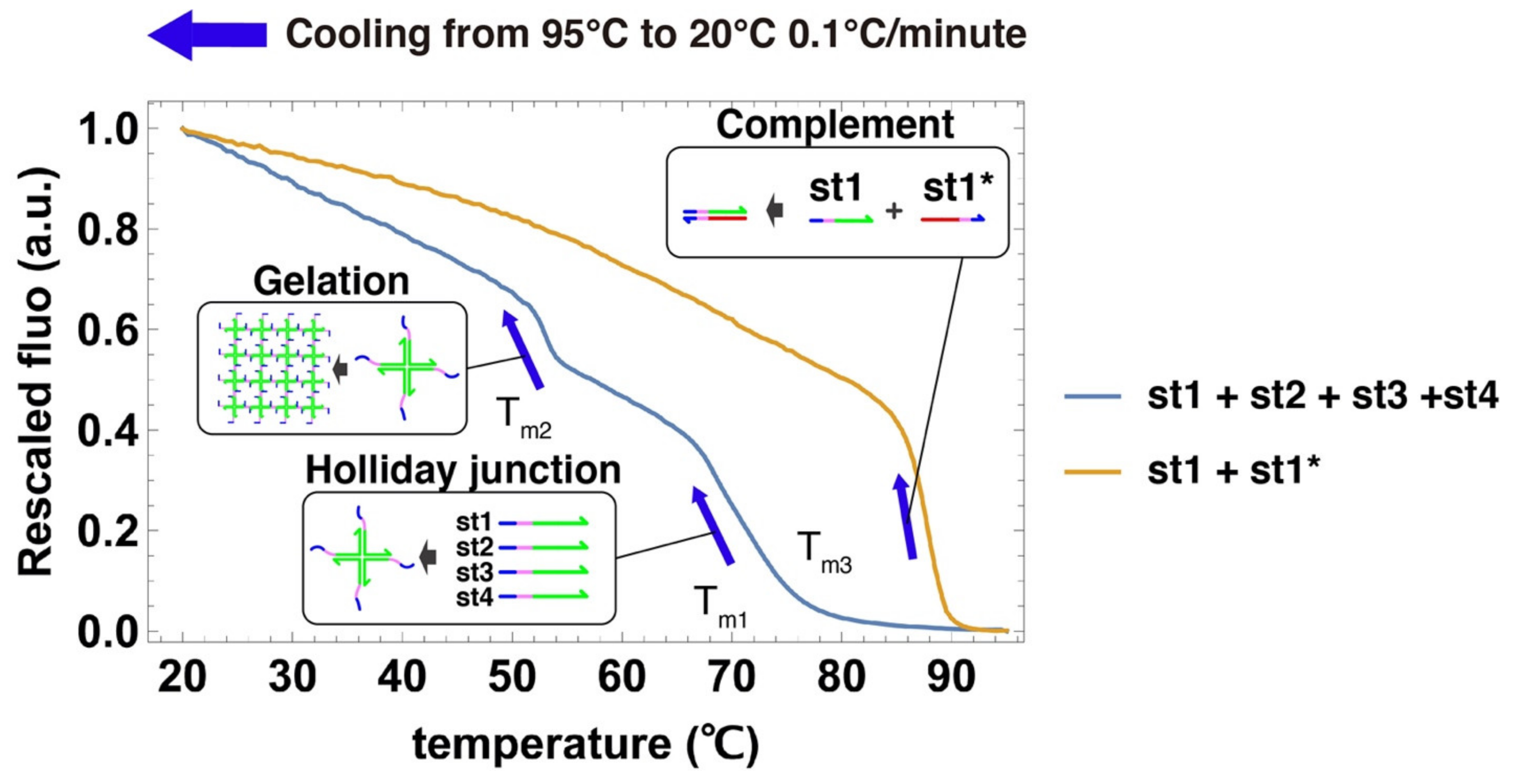
2.3. Cooling Curves of DNA Hydrogel
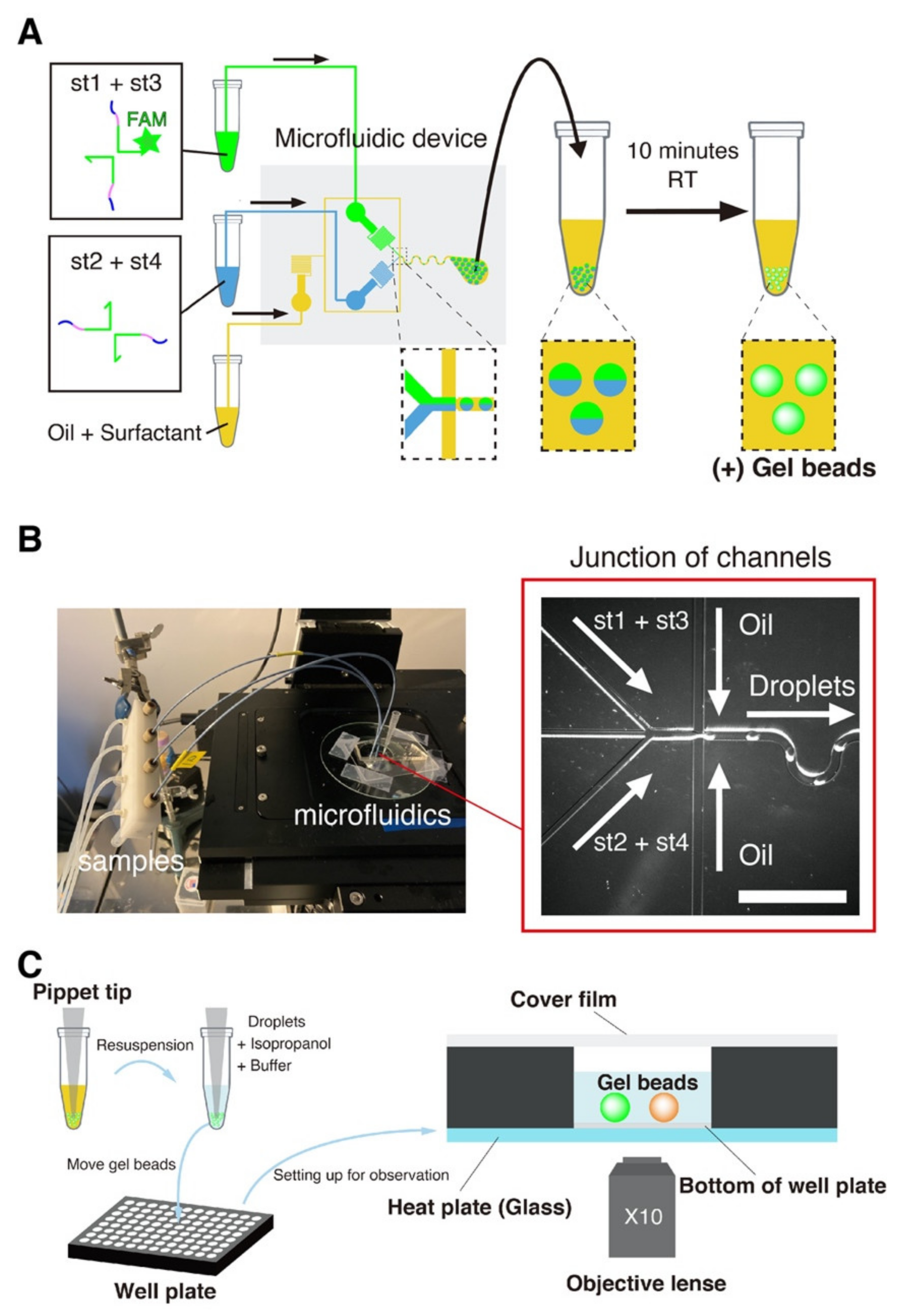
2.4. Beads Preparation with Microfluidic Device
2.5. Beads Assay
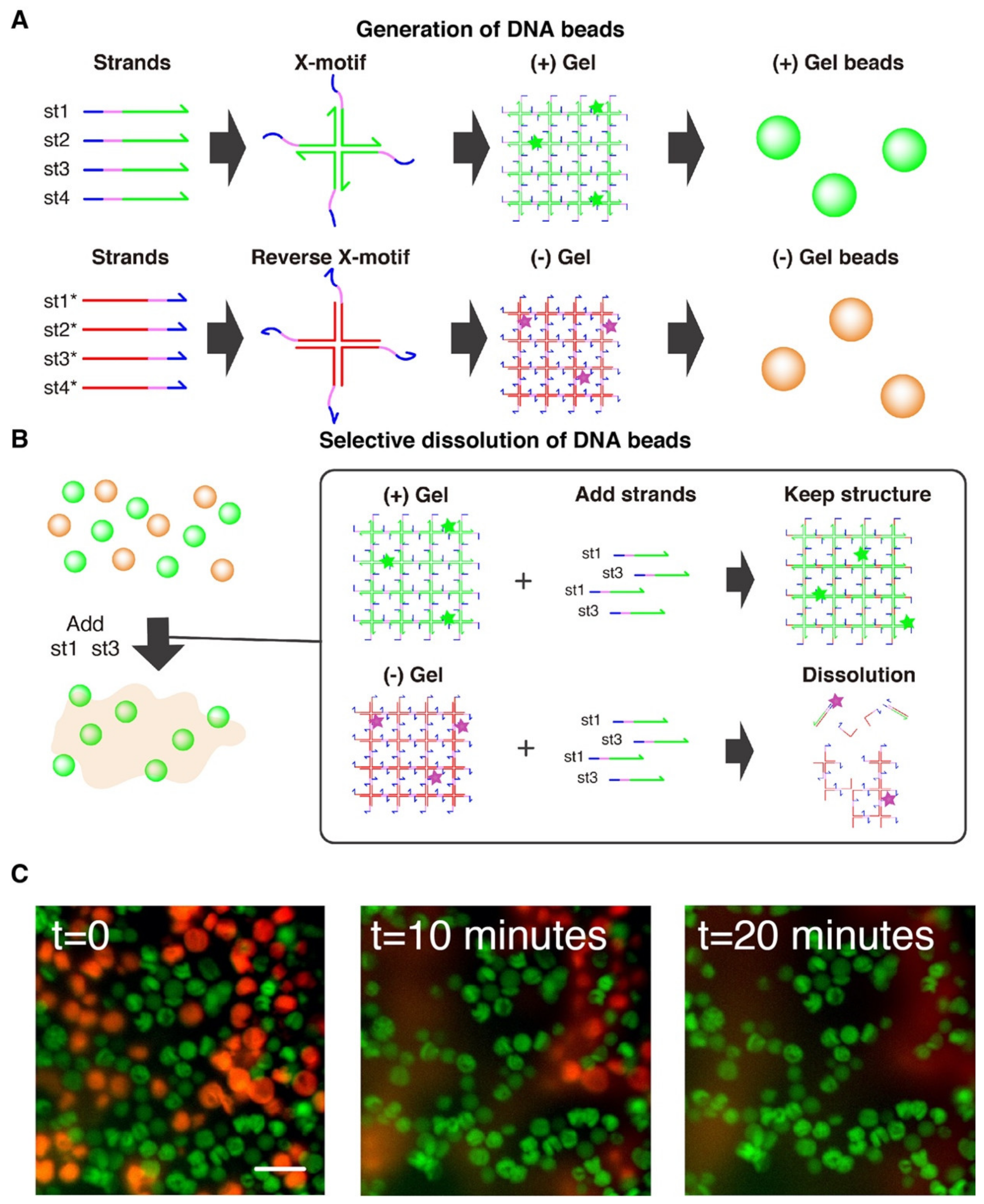
2.6. Selective Dissolution
2.7. Winner-Takes-All Function
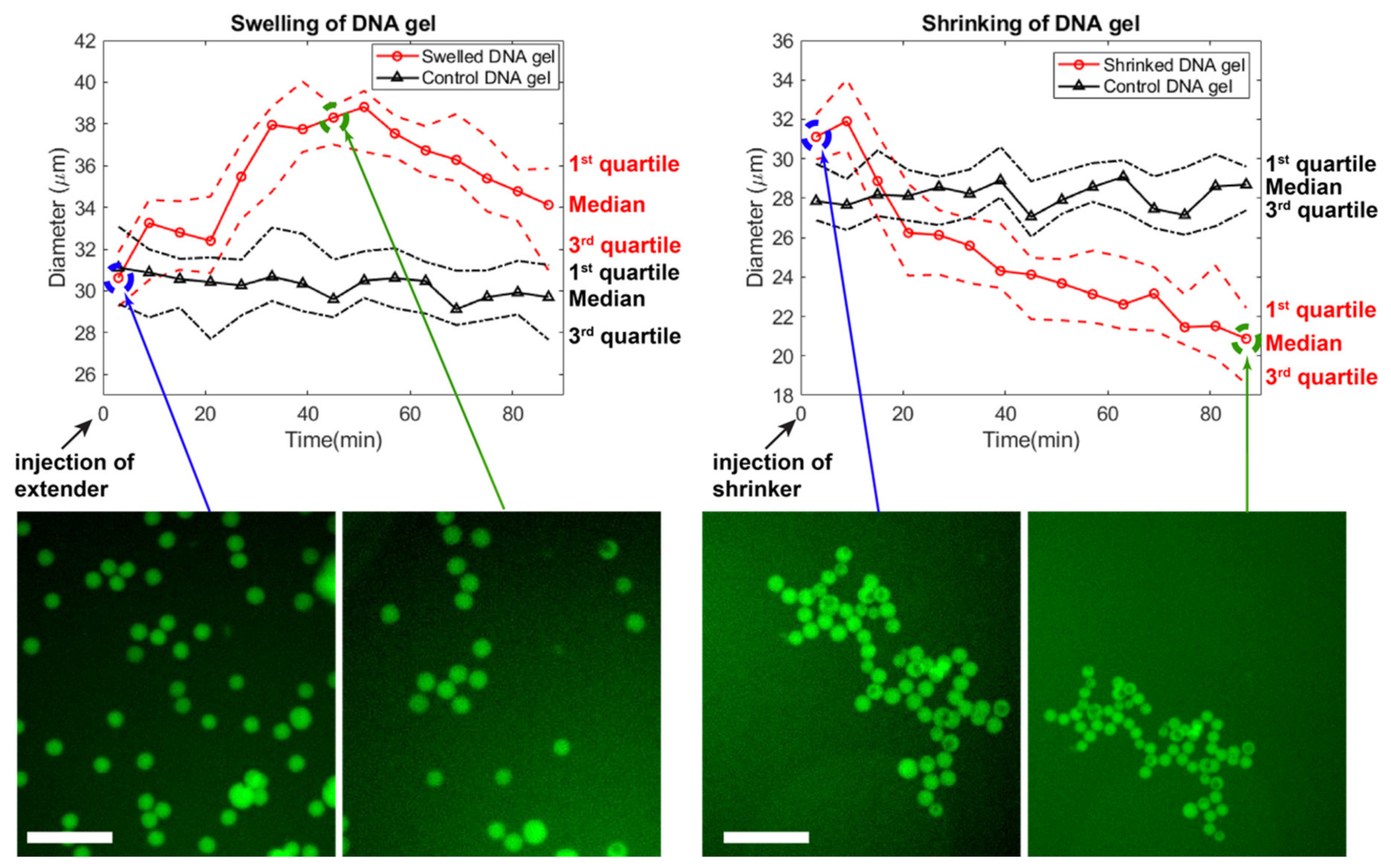
2.8. Swelling and Shrinking
3. Results
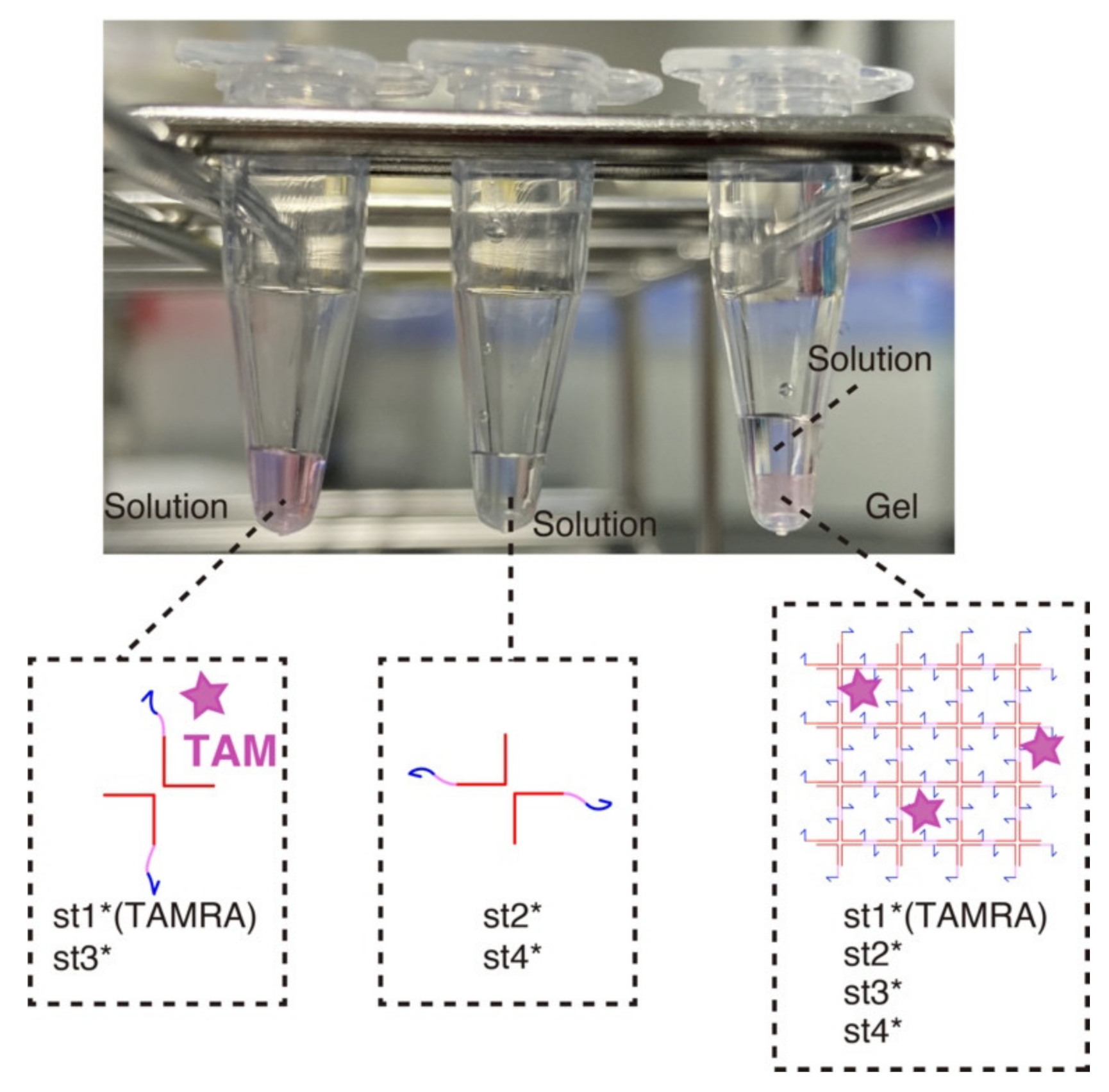
3.1. Hydrogel Formation
3.2. Cooling Curve of DNA Hydrogel
3.3. Selective Dissolution
3.4. Winner-Takes-All
3.5. Swelling and Shrinking of Gels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bulpitt, P.; Aeschlimann, D. New Strategy for Chemical Modification of Hyaluronic Acid: Preparation of Functionalized Derivatives and Their Use in the Formation of Novel Biocompatible Hydrogels. J. Biomed. Mater. Res. 1999, 47, 152–169. [Google Scholar] [CrossRef]
- Molinaro, G.; Leroux, J.; Damas, J.; Adam, A. Biocompatibility of thermosensitive chitosan-based hydrogels: An in vivo experimental approach to injectable biomaterials. Biomaterials 2002, 23, 2717–2722. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, P.; Huang, G.; Wang, L.; Zhou, J.; Lu, T.J.; Xu, F.; Lin, M. Reaction-induced swelling of ionic gels. Soft Matter 2015, 11, 449–455. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, X.; Guo, B.; Ma, P.X. Multifunctional Interpenetrating Polymer Network Hydrogels Based on Methacrylated Alginate for the Delivery of Small Molecule Drugs and Sustained Release of Protein. Biomacromolecules 2014, 15, 3246–3252. [Google Scholar] [CrossRef]
- Hotta, R.; Cheng, L.S.; Graham, H.K.; Nagy, N.; Belkind-Gerson, J.; Mattheolabakis, G.; Amiji, M.M.; Goldstein, A.M. Delivery of enteric neural progenitors with 5-HT4 agonist-loaded nanoparticles and thermosensitive hydrogel enhances cell proliferation and differentiation following transplantation in vivo. Biomaterials 2016, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Allen, W.E.; Wright, M.; Sylwestrak, E.L.; Samusik, N.; Vesuna, S.; Evans, K.; Liu, C.; Ramakrishnan, C.; Liu, J.; et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science 2018, 361, eaat5691. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.X.Y.; Terry, J.M.; Belgrader, P.; Ryvkin, P.; Bent, Z.W.; Wilson, R.; Ziraldo, S.B.; Wheeler, T.D.; McDermott, G.P.; Zhu, J.; et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 2017, 8, 14049. [Google Scholar] [CrossRef]
- Zilionis, R.; Nainys, J.; Veres, A.; Savova, V.; Zemmour, D.; Klein, A.M.; Mazutis, L. Single-cell barcoding and sequencing using droplet microfluidics. Nat. Protoc. 2017, 12, 44–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Liu, M.; Ishida, Y.; Ebina, Y.; Osada, M.; Sasaki, T.; Hansen, J.C.; Takata, M.; Aida, T. Thermoresponsive actuation enabled by permittivity switching in an electrostatically anisotropic hydrogel. Nat. Mater. 2015, 14, 1002–1007. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 1–21. [Google Scholar] [CrossRef]
- Chen, J.-P.; Cheng, T.-H. Thermo-Responsive Chitosan-graft-poly(N-isopropylacrylamide) Injectable Hydrogel for Cultivation of Chondrocytes and Meniscus Cells. Macromol. Biosci. 2006, 6, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.J.; Moore, J.S.; Bauer, J.M.; Yu, Q.; Liu, R.H.; Devadoss, C.; Jo, B.-H. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nat. Cell Biol. 2000, 404, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Hara, Y.; Maeda, S.; Hashimoto, S. A Pendulum-Like Motion of Nanofiber Gel Actuator Synchronized with External Periodic pH Oscillation. Polymers 2011, 3, 405–412. [Google Scholar] [CrossRef]
- Yoshida, R.; Uchida, K.; Kaneko, Y.; Sakai, K.; Kikuchi, A.; Sakurai, Y.; Okano, T. Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nat. Cell Biol. 1995, 374, 240–242. [Google Scholar] [CrossRef]
- Hao, L.; Yegin, C.; Talari, J.V.; Oh, J.K.; Zhang, M.; Sari, M.M.; Zhang, L.; Min, Y.; Akbulut, M.; Bin Jiang, B. Thermo-responsive gels based on supramolecular assembly of an amidoamine and citric acid. Soft Matter 2018, 14, 432–439. [Google Scholar] [CrossRef]
- Zhong, R.; Xiao, M.; Zhu, C.; Shen, X.; Tang, Q.; Zhang, W.; Wang, L.; Song, S.; Qu, X.; Pei, H.; et al. Logic Catalytic Interconversion of G-Molecular Hydrogel. ACS Appl. Mater. Interfaces 2018, 10, 4512–4518. [Google Scholar] [CrossRef]
- Zhong, R.; Tang, Q.; Wang, S.; Zhang, H.; Zhang, F.; Xiao, M.; Man, T.; Qu, X.; Li, L.; Zhang, W.; et al. Self-Assembly of Enzyme-Like Nanofibrous G-Molecular Hydrogel for Printed Flexible Electrochemical Sensors. Adv. Mater. 2018, 30, e1706887. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.I.; Park, S.J.; Kim, I.Y.; Lee, S.H.; Lee, T.S.I.; Kim, S. Behavior in electric fields of smart hydrogels with potential application as bio-inspired actuators. Smart Mater. Struct. 2005, 14, 511–514. [Google Scholar] [CrossRef]
- Wang, E.; Desai, M.S.; Lee, S.-W. Light-Controlled Graphene-Elastin Composite Hydrogel Actuators. Nano Lett. 2013, 13, 2826–2830. [Google Scholar] [CrossRef]
- Keplinger, C.; Sun, J.-Y.; Foo, C.C.; Rothemund, P.; Whitesides, G.M.; Suo, Z. Stretchable, Transparent, Ionic Conductors. Science 2013, 341, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Kidoaki, S.; Matsuda, T. Microelastic gradient gelatinous gels to induce cellular mechanotaxis. J. Biotechnol. 2008, 133, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Kidoaki, S. Elasticity boundary conditions required for cell mechanotaxis on microelastically-patterned gels. Biomaterials 2011, 32, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-W.; Woo, J.; Lee, H.-R.; Sun, J.-Y. A mechanically enhanced electroactive hydrogel for 3D printing using a multileg long chain crosslinker. Smart Mater. Struct. 2019, 28, 095016. [Google Scholar] [CrossRef]
- Cvetkovic, C.; Raman, R.; Chan, V.; Williams, B.J.; Tolish, M.; Bajaj, P.; Sakar, M.S.; Asada, H.H.; Saif, M.T.A.; Bashir, R. Three-dimensionally printed biological machines powered by skeletal muscle. Proc. Natl. Acad. Sci. USA 2014, 111, 10125–10130. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Tang, B.; Xu, W.; Li, J.; Hu, J.; Gu, Z. Quantum-Dot-Tagged Bioresponsive Hydrogel Suspension Array for Multiplex Label-Free DNA Detection. Adv. Funct. Mater. 2010, 20, 976–982. [Google Scholar] [CrossRef]
- Oliveira, J.; Reis, R. Hydrogels from polysaccharide-based materials: Fundamentals and applications in regenerative medicine. In Natural-Based Polymers for Biomedical Applications; Elsevier BV: Amsterdam, The Netherlands, 2008; pp. 485–514. [Google Scholar]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic acid-based hydrogels: From a natural polysaccharide to complex networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef]
- Shibayama, M.; Li, X.; Sakai, T. Precision polymer network science with tetra-PEG gels—A decade history and future. Colloid Polym. Sci. 2019, 297, 1–12. [Google Scholar] [CrossRef]
- Guo, W.; Lu, C.-H.; Qi, X.-J.; Orbach, R.; Fadeev, M.; Yang, H.-H.; Willner, I. Switchable Bifunctional Stimuli-Triggered Poly-N-Isopropylacrylamide/DNA Hydrogels. Angew. Chem. Int. Ed. 2014, 53, 10134–10138. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.; Xing, Y.; Chen, P.; Yang, Y.; Sun, Y.; Zhou, D.; Xu, L.; Fan, Q.; Liu, D. A pH-Triggered, Fast-Responding DNA Hydrogel. Angew. Chem. Int. Ed. 2009, 48, 7660–7663. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Lu, C.-H.; Orbach, R.; Wang, F.; Qi, X.-J.; Cecconello, A.; Seliktar, D.; Willner, I. pH-Stimulated DNA Hydrogels Exhibiting Shape-Memory Properties. Adv. Mater. 2015, 27, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Cheng, E.; Yang, Y.; Chen, P.; Zhang, T.; Sun, Y.; Yang, Z.; Liu, D. Self-Assembled DNA Hydrogels with Designable Thermal and Enzymatic Responsiveness. Adv. Mater. 2010, 23, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nat. Cell Biol. 2006, 440, 297–302. [Google Scholar] [CrossRef]
- Douglas, S.M.; Dietz, H.; Liedl, T.; Högberg, B.; Graf, F.; Shih, W.M. Self-assembly of DNA into nanoscale three-dimensional shapes. Nat. Cell Biol. 2009, 459, 414–418. [Google Scholar] [CrossRef]
- Han, D.; Pal, S.; Nangreave, J.; Deng, Z.; Liu, Y.; Yan, H. DNA Origami with Complex Curvatures in Three-Dimensional Space. Science 2011, 332, 342–346. [Google Scholar] [CrossRef]
- Sato, Y.; Sakamoto, T.; Takinoue, M. Sequence-based engineering of dynamic functions of micrometer-sized DNA droplets. Sci. Adv. 2020, 6, eaba3471. [Google Scholar] [CrossRef]
- Frank-Kamenetskiĭ, M.D.; Anshelevich, V.V.; Lukashin, A.V. Polyelectrolyte model of DNA. Sov. Phys. Uspekhi 1987, 30, 317–330. [Google Scholar] [CrossRef]
- Um, S.H.; Lee, J.B.; Park, N.; Kwon, S.Y.; Umbach, C.C.; Luo, D. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 2006, 5, 797–801. [Google Scholar] [CrossRef]
- Gehring, K.; Leroy, J.-L.; Guéron, M. A tetrameric DNA structure with protonated cytosine-cytosine base pairs. Nat. Cell Biol. 1993, 363, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Maeda, M. DNA-Responsive Hydrogels That Can Shrink or Swell. Biomacromolecules 2005, 6, 2927–2929. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Ye, D.; Zuo, X.; Li, J.; Wang, J.; Liu, H.; Hwang, M.T.; Chao, J.; Su, S.; Wang, L.; et al. DNA Hydrogel with Aptamer-Toehold-Based Recognition, Cloaking, and Decloaking of Circulating Tumor Cells for Live Cell Analysis. Nano Lett. 2017, 17, 5193–5198. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Yeom, S.Y.; Kim, J.; Jung, S.; Jung, S.; Shim, T.S.; Kim, S.K.; Kang, J.Y.; Lee, S.H.; Cho, I.-J.; et al. Hydrogel micropost-based qPCR for multiplex detection of miRNAs associated with Alzheimer’s disease. Biosens. Bioelectron. 2018, 101, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.R.; Yang, D.; Tran, T.N.N.; Lee, K.; Kahn, J.S.; Kiatwuthinon, P.; Yancey, K.G.; Trotsenko, O.; Minko, S.; Luo, D. Thermostable Branched DNA Nanostructures as Modular Primers for Polymerase Chain Reaction. Angew. Chem. Int. Ed. 2013, 52, 8699–8702. [Google Scholar] [CrossRef]
- Li, C.; Faulkner-Jones, A.; Dun, A.R.; Jin, J.; Chen, P.; Xing, Y.; Yang, Z.; Li, Z.; Shu, W.; Liu, D.; et al. Rapid Formation of a Supramolecular Polypeptide-DNA Hydrogel for In Situ Three-Dimensional Multilayer Bioprinting. Angew. Chem. Int. Ed. 2015, 54, 3957–3961. [Google Scholar] [CrossRef]
- English, M.A.; Soenksen, L.R.; Gayet, R.V.; de Puig, H.; Angenent-Mari, N.M.; Mao, A.S.; Nguyen, P.Q.; Collins, J.J. Programmable CRISPR-responsive smart materials. Science 2019, 365, 780–785. [Google Scholar] [CrossRef]
- Xu, S.; Nie, Z.; Seo, M.; Lewis, P.; Kumacheva, E.; Stone, H.A.; Garstecki, P.; Weibel, D.B.; Gitlin, I.; Whitesides, G.M. Generation of Monodisperse Particles by Using Microfluidics: Control over Size, Shape, and Composition. Angew. Chem. 2005, 117, 734–738. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Desbois, L.; Padirac, A.; Kaneda, S.; Genot, A.J.; Rondelez, Y.; Hober, D.; Collard, D.; Fujii, T. A microfluidic device for on-chip agarose microbead generation with ultralow reagent consumption. Biomicrofluidics 2012, 6, 44101. [Google Scholar] [CrossRef]
- Yadavali, S.; Jeong, H.-H.; Lee, S.H.; Issadore, D. Silicon and glass very large-scale microfluidic droplet integration for terascale generation of polymer microparticles. Nat. Commun. 2018, 9, 1222. [Google Scholar] [CrossRef] [PubMed]
- Yelleswarapu, V.; Buser, J.R.; Haber, M.; Baron, J.; Inapuri, E.; Issadore, D. Mobile platform for rapid sub–picogram-per-milliliter, multiplexed, digital droplet detection of proteins. Proc. Natl. Acad. Sci. USA 2019, 116, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- de Rutte, J.M.; di Carlo, D.; di Carlo, D. Scalable High-Throughput Production of Modular Microgels for In Situ Assembly of Microporous Tissue Scaffolds. Adv. Funct. Mater. 2019, 29, 1900071. [Google Scholar] [CrossRef]
- Mealy, J.E.; Chung, J.J.; Jeong, H.-H.; Issadore, D.; Lee, S.H.; Atluri, P.; Burdick, J.A. Injectable Granular Hydrogels with Multifunctional Properties for Biomedical Applications. Adv. Mater. 2018, 30, e1705912. [Google Scholar] [CrossRef]
- Caldwell, A.S.; Campbell, G.T.; Shekiro, K.M.T.; Anseth, K.S. Clickable Microgel Scaffolds as Platforms for 3D Cell Encapsulation. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; di Carlo, D.; Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015, 14, 737–744. [Google Scholar] [CrossRef]
- Wade, R.J.; Bassin, E.J.; Rodell, C.B.; Burdick, J.A. Protease-degradable electrospun fibrous hydrogels. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Highley, C.B.; Song, K.H.; Daly, A.C.; Burdick, J.A. Jammed Microgel Inks for 3D Printing Applications. Adv. Sci. 2019, 6, 1801076. [Google Scholar] [CrossRef]
- Yoshida, S.; Takinoue, M.; Iwase, E.; Onoe, H. Dynamic transformation of self-assembled structures using anisotropic magnetized hydrogel microparticles. J. Appl. Phys. 2016, 120, 084905. [Google Scholar] [CrossRef]
- Hayakawa, M.; Umeyama, S.; Nagai, K.; Onoe, H.; Takinoue, M. Controlled Construction of Stable Network Structure Composed of Honeycomb-Shaped Microhydrogels. Life 2018, 8, 38. [Google Scholar] [CrossRef]
- Yurke, B.; Turberfield, A.J.; Mills, A.P.; Simmel, F.C.; Neumann, J.L. A DNA-fuelled molecular machine made of DNA. Nat. Cell Biol. 2000, 406, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Genot, A.J.; Zhang, D.Y.; Bath, J.; Turberfield, A.J. Remote Toehold: A Mechanism for Flexible Control of DNA Hybridization Kinetics. J. Am. Chem. Soc. 2011, 133, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Genot, A.J.; Bath, J.; Turberfield, A.J. Combinatorial Displacement of DNA Strands: Application to Matrix Multiplication and Weighted Sums. Angew. Chem. Int. Ed. 2012, 52, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Expanding the Rule Set of DNA Circuitry with Associative Toehold Activation. J. Am. Chem. Soc. 2011, 134, 263–271. [Google Scholar] [CrossRef]
- A Mechanism for Gene Conversion in Fungi|Genetics Research|Cambridge Core. Available online: https://www.cambridge.org/core/journals/genetics-research/article/mechanism-for-gene-conversion-in-fungi/E11586A6605C2A54C648BACEABECF954 (accessed on 1 December 2020).
- Elshaarani, T.; Yu, H.; Wang, L.; Feng, J.; Li, C.; Zhou, W.; Khan, A.; Usman, M.; Amin, B.U.; Khan, R. Chitosan reinforced hydrogels with swelling-shrinking behaviors in response to glucose concentration. Int. J. Biol. Macromol. 2020, 161, 109–121. [Google Scholar] [CrossRef]
- Cangialosi, A.; Yoon, C.; Liu, J.; Huang, Q.; Guo, J.; Nguyen, T.D.; Gracias, D.H.; Schulman, R. DNA sequence–directed shape change of photopatterned hydrogels via high-degree swelling. Science 2017, 357, 1126–1130. [Google Scholar] [CrossRef]
- Genot, A.J.; Baccouche, A.; Sieskind, R.; Aubert-Kato, N.; Bredeche, N.; Bartolo, J.F.; Taly, V.; Fujii, T.; Rondelez, Y. High-resolution mapping of bifurcations in nonlinear biochemical circuits. Nat. Chem. 2016, 8, 760–767. [Google Scholar] [CrossRef]
- Baccouche, A.; Okumura, S.; Sieskind, R.; Henry, E.; Aubert-Kato, N.; Bredeche, N.; Bartolo, J.-F.; Taly, V.; Rondelez, Y.; Fujii, T.; et al. Massively parallel and multiparameter titration of biochemical assays with droplet microfluidics. Nat. Protoc. 2017, 12, 1912–1932. [Google Scholar] [CrossRef]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in Poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef]
- Kandatsu, D.; Cervantes-Salguero, K.; Kawamata, I.; Hamada, S.; Nomura, S.-I.M.; Fujimoto, K.; Murata, S. Reversible Gel-Sol Transition of a Photo-Responsive DNA Gel. ChemBioChem 2016, 17, 1118–1121. [Google Scholar] [CrossRef]
- Hiraide, M.; Ishikawa, K.; Kawaguchi, H. Water-in-oil emulsion containing oxine for the collection of traces of copper(II) in water. Anal. Bioanal. Chem. 1996, 356, 155–158. [Google Scholar] [CrossRef]
- Schmitt, M.; Limage, S.; Denoyel, R.; Antoni, M. Effect of SPAN80 on the structure of emulsified aqueous suspensions. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 521, 121–132. [Google Scholar] [CrossRef]
- Xing, Z.; Caciagli, A.; Cao, T.; Stoev, I.; Zupkauskas, M.; O’Neill, T.; Wenzel, T.; Lamboll, R.; Liu, D.; Eiser, E. Microrheology of DNA hydrogels. Proc. Natl. Acad. Sci. USA 2018, 115, 8137–8142. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982, 99, 237–247. [Google Scholar] [CrossRef]
- Cherry, K.M.; Qian, L. Scaling up molecular pattern recognition with DNA-based winner-take-all neural networks. Nat. Cell Biol. 2018, 559, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Gaweł, K.; Barriet, D.; Sletmoen, M.; Stokke, B.T. Responsive Hydrogels for Label-Free Signal Transduction within Biosensors. Sensors 2010, 10, 4381–4409. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, X.; Zhao, H.; Liu, H. Long non-coding RNAs: Potential new biomarkers for predicting tumor invasion and metastasis. Mol. Cancer 2016, 15, 1–15. [Google Scholar] [CrossRef]
- Klingenberg, M.; Matsuda, A.; Diederichs, S.; Patel, T. Non-coding RNA in hepatocellular carcinoma: Mechanisms, biomarkers and therapeutic targets. J. Hepatol. 2017, 67, 603–618. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, C.; Wu, M. New insights into long noncoding RNAs and their roles in glioma. Mol. Cancer 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Charles, A.; Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. The Structure of a Typical Antibody Molecule. In Immunobiology: The Immune System in Health and Disease, 5th ed.; NCBI: Bethesda, MD, USA, 2001. [Google Scholar]
- Miyata, T.; Asami, A.N.; Uragami, T. Preparation of an Antigen-Sensitive Hydrogel Using Antigen−Antibody Bindings. Macromolecules 1999, 32, 2082–2084. [Google Scholar] [CrossRef]
- Charles, P.T.; Goldman, E.R.; Rangasammy, J.G.; Schauer, C.L.; Chen, M.-S.; Taitt, C.R. Fabrication and characterization of 3D hydrogel microarrays to measure antigenicity and antibody functionality for biosensor applications. Biosens. Bioelectron. 2004, 20, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Asami, N.; Uragami, T. A reversibly antigen-responsive hydrogel. Nat. Cell Biol. 1999, 399, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, R.F.-X.; Sart, S.; Champetier, T.; Baroud, C.N. Individual Control and Quantification of 3D Spheroids in a High-Density Microfluidic Droplet Array. Cell Rep. 2020, 31, 107670. [Google Scholar] [CrossRef] [PubMed]
- Sart, S.; Tomasi, R.F.-X.; Amselem, G.; Baroud, C.N. Multiscale cytometry and regulation of 3D cell cultures on a chip. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]


| st1 | 5′-CACTCTTCTGGATCCAGTTTGTTATCGCAGGAGCGTCGGTATTCAAA-3′ |
| st2 | 5′-CACTCTTCTGGATCCAGTTTGAATACCGACGCCACGACCTAATCTTA-3′ |
| st3 | 5′-CACTCTTCTGGATCCAGTAAGATTAGGTCGTGATGGTGAAATGTAAA-3′ |
| st4 | 5′-CACTCTTCTGGATCCAGTTTACATTTCACCATTCCTGCGATAACAAA-3′ |
| st1* | 5′-TTTGAATACCGACGCTCCTGCGATAACAAACTGGATCCAGAAGAGTG-3′ |
| st2* | 5′-TAAGATTAGGTCGTGGCGTCGGTATTCAAACTGGATCCAGAAGAGTG-3′ |
| st3* | 5′-TTTACATTTCACCATCACGACCTAATCTTACTGGATCCAGAAGAGTG-3′ |
| st4* | 5′-TTTGTTATCGCAGGAATGGTGAAATGTAAACTGGATCCAGAAGAGTG-3′ |
| st1 [FAM] | 5′-CTGGATCCAGTTTGTTATCGCAGGAGCGTCGGTATTCAAA[FAM]-3′ |
| st1* [TAM] | 5′-TTTGAATACCGACGCTCCTGCGATAACAAACTGGATCCAG[TAM]-3′ |
| Bridge | 5′-CTGGATCCAGAAGAGTGTACACTCACTCAGATGAGACAGTGAGTGTAATGCTAGCAT-3′ |
| Bridge extender | 5′-AGTGAGCTGTCTCATCTGAGTGAGTGTA-3′ |
| Bridge shrinker | 5′-TACACTCACTCAGATGAGACAGCTCACT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okumura, S.; Hapsianto, B.N.; Lobato-Dauzier, N.; Ohno, Y.; Benner, S.; Torii, Y.; Tanabe, Y.; Takada, K.; Baccouche, A.; Shinohara, M.; et al. Morphological Manipulation of DNA Gel Microbeads with Biomolecular Stimuli. Nanomaterials 2021, 11, 293. https://doi.org/10.3390/nano11020293
Okumura S, Hapsianto BN, Lobato-Dauzier N, Ohno Y, Benner S, Torii Y, Tanabe Y, Takada K, Baccouche A, Shinohara M, et al. Morphological Manipulation of DNA Gel Microbeads with Biomolecular Stimuli. Nanomaterials. 2021; 11(2):293. https://doi.org/10.3390/nano11020293
Chicago/Turabian StyleOkumura, Shu, Benediktus Nixon Hapsianto, Nicolas Lobato-Dauzier, Yuto Ohno, Seiju Benner, Yosuke Torii, Yuuka Tanabe, Kazuki Takada, Alexandre Baccouche, Marie Shinohara, and et al. 2021. "Morphological Manipulation of DNA Gel Microbeads with Biomolecular Stimuli" Nanomaterials 11, no. 2: 293. https://doi.org/10.3390/nano11020293
APA StyleOkumura, S., Hapsianto, B. N., Lobato-Dauzier, N., Ohno, Y., Benner, S., Torii, Y., Tanabe, Y., Takada, K., Baccouche, A., Shinohara, M., Kim, S. H., Fujii, T., & Genot, A. (2021). Morphological Manipulation of DNA Gel Microbeads with Biomolecular Stimuli. Nanomaterials, 11(2), 293. https://doi.org/10.3390/nano11020293