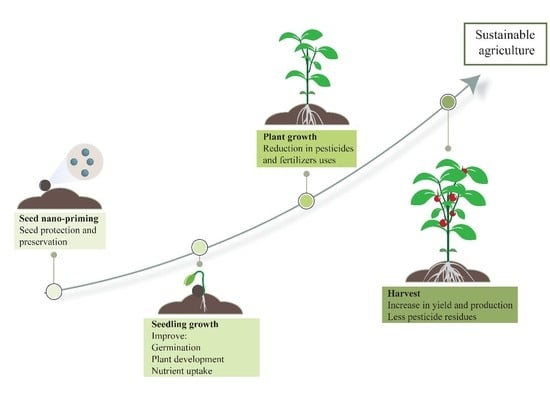
Nanotechnology Potential in Seed Priming for Sustainable Agriculture
Abstract
1. Introduction
2. Seed Priming and Nanoparticles: Definition and Potential Applications
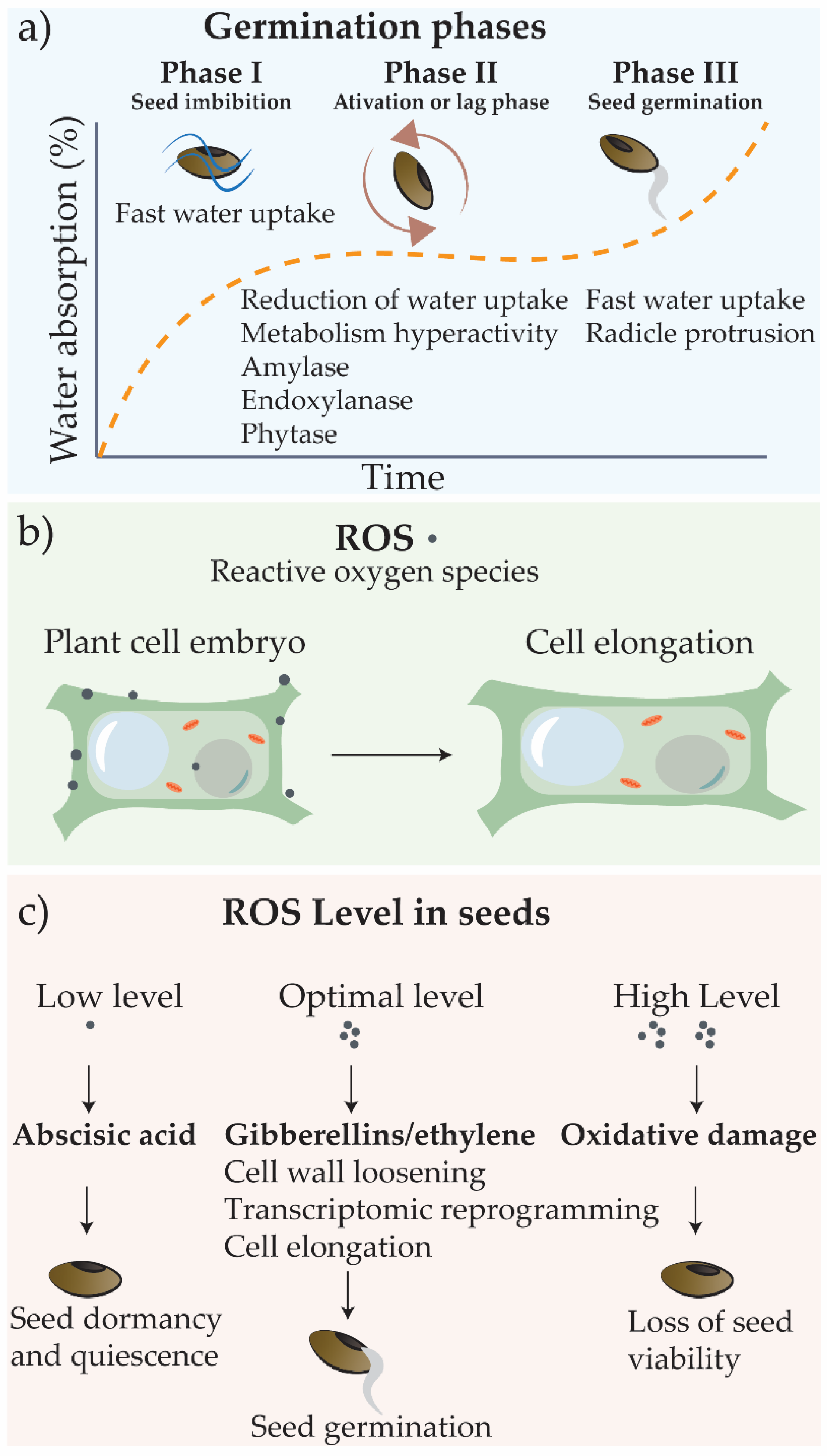
2.1. Germination and Principles of Seed Priming
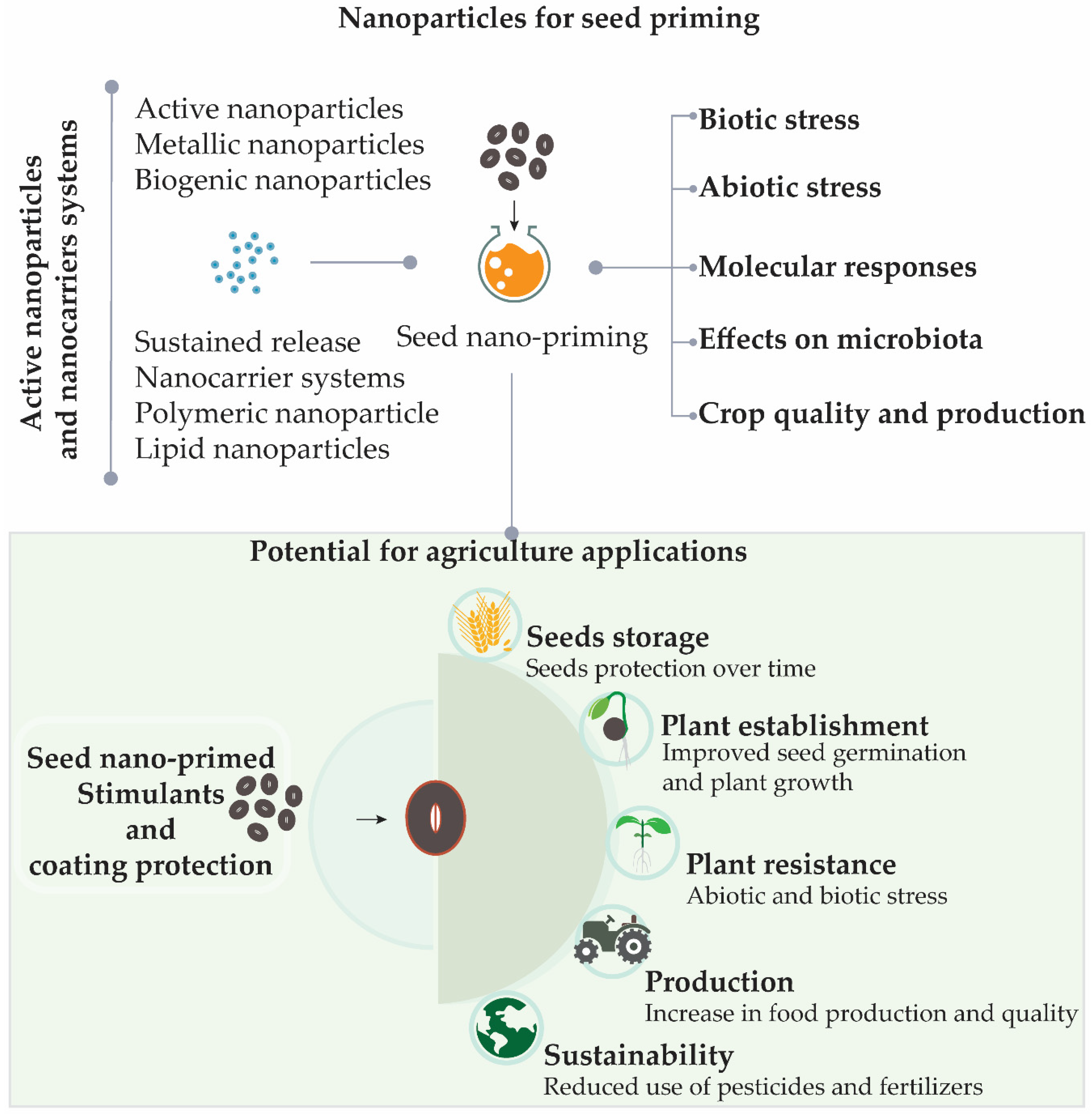
2.2. Nanoparticles for Seed Priming
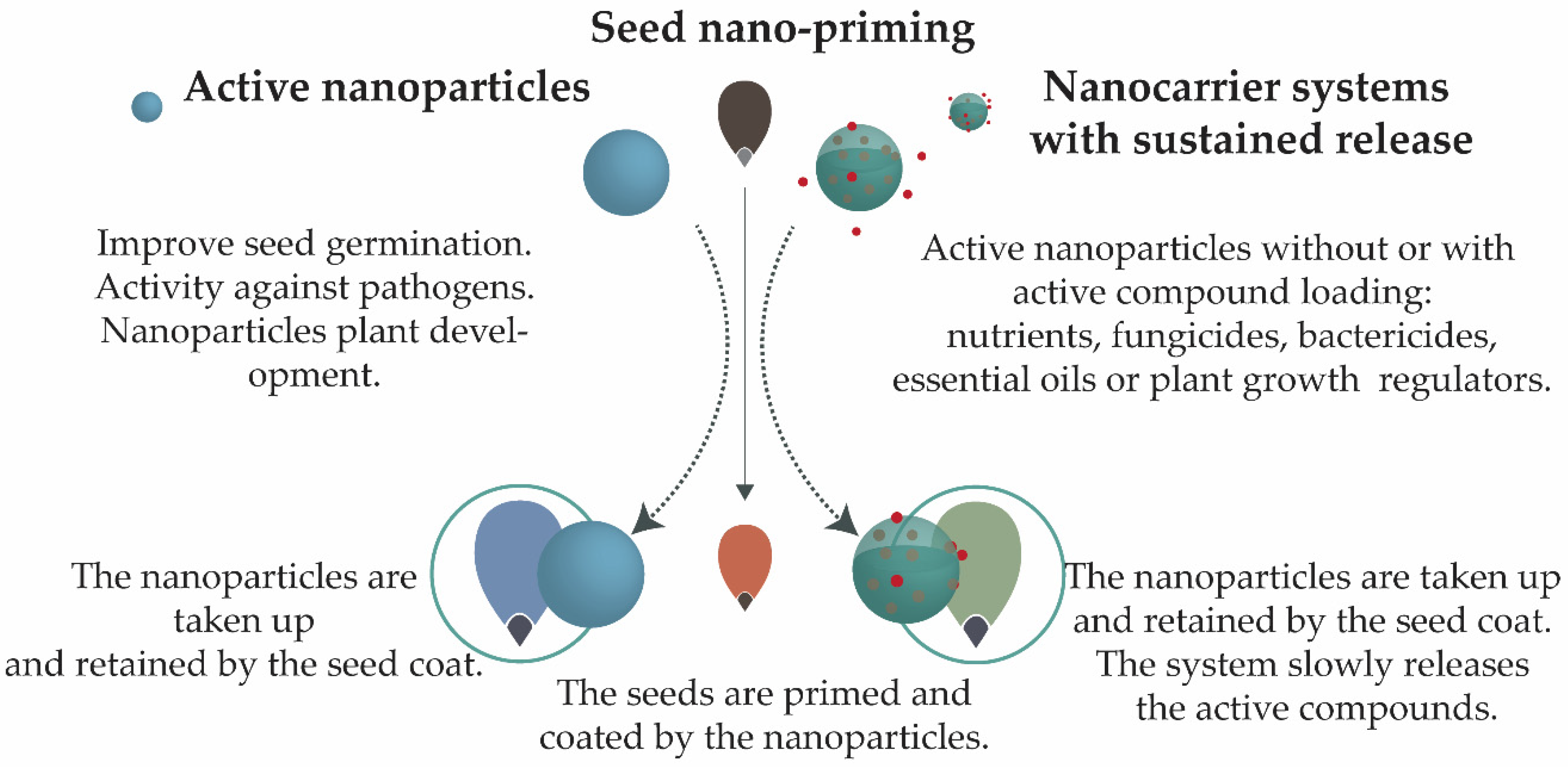
2.3. Active Nanoparticles and Nanocarriers Systems
2.3.1. Active Nanoparticles
2.3.2. Sustained Release Nanocarrier Systems
2.4. Seed Nano-Priming and Effects on Plant Metabolism under Abiotic and Biotic Stresses
2.4.1. Biotic Stress
2.4.2. Abiotic Stress
2.5. Molecular Responses Induced by Seed Priming During Germination, Abiotic and Biotic Stress
2.6. Effects on Microbiota
2.7. Improving Crop Quality and Production
3. Concerns
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De La Torre-Roche, R.; Cantu, J.; Tamez, C.; Zuverza-Mena, N.; Hamdi, H.; Adisa, I.O.; Elmer, W.; Gardea-Torresdey, J.; White, J.C. Seed Biofortification by Engineered Nanomaterials: A Pathway To Alleviate Malnutrition? J. Agric. Food Chem. 2020, 68, 12189–12202. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-Enabled Strategies to Enhance Crop Nutrition and Protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and Challenges for Nanotechnology in the Agri-Tech Revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-Biotechnology in Agriculture: Use of Nanomaterials to Promote Plant Growth and Stress Tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.R.; Chen, H.; Cui, H. Nanotechnology Applications and Implications of Agrochemicals toward Sustainable Agriculture and Food Systems. J. Agric. Food Chem. 2018, 66, 6451–6456. [Google Scholar] [CrossRef] [PubMed]
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in Agriculture: Which Innovation Potential Does It Have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef]
- Panpatte, D.G.; Jhala, Y.K.; Shelat, H.N.; Vyas, R.V. Nanoparticles: The Next Generation Technology for Sustainable Agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 289–300. ISBN 978-81-322-2642-0. [Google Scholar]
- Camara, M.C.; Campos, E.V.R.; Monteiro, R.A.; Pereira, A.E.S.; de Freitas Proença, P.L.; Fraceto, L.F. Development of Stimuli-Responsive Nano-Based Pesticides: Emerging Opportunities for Agriculture. J. Nanobiotechnol. 2019, 17, 100. [Google Scholar] [CrossRef]
- Shakiba, S.; Astete, C.E.; Paudel, S.; Sabliov, C.M.; Rodrigues, D.F.; Louie, S.M. Emerging Investigator Series: Polymeric Nanocarriers for Agricultural Applications: Synthesis, Characterization, and Environmental and Biological Interactions. Environ. Sci. Nano 2020, 7, 37–67. [Google Scholar] [CrossRef]
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N.; et al. Biostimulant-Treated Seedlings under Sustainable Agriculture: A Global Perspective Facing Climate Change. Agronomy 2021, 11, 14. [Google Scholar] [CrossRef]
- Chau, N.H.; Doan, Q.H.; Chu, T.H.; Nguyen, T.T.; Dao Trong, H.; Ngo, Q.B. Effects of Different Nanoscale Microelement-Containing Formulations for Presowing Seed Treatment on Growth of Soybean Seedlings. J. Chem. 2019, 2019, 8060316. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at Multi-Locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Luque, A. Interaction of Nanomaterials with Plants: What Do We Need for Real Applications in Agriculture? Front. Environ. Sci. 2017, 5. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming Technology for Enhancing Germination and Starch Metabolism of Aged Rice Seeds Using Phytosynthesized Silver Nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef] [PubMed]
- Pelegrino, M.T.; Kohatsu, M.Y.; Seabra, A.B.; Monteiro, L.R.; Gomes, D.G.; Oliveira, H.C.; Rolim, W.R.; de Jesus, T.A.; Batista, B.L.; Lange, C.N. Effects of Copper Oxide Nanoparticles on Growth of Lettuce (Lactuca sativa L.) Seedlings and Possible Implications of Nitric Oxide in Their Antioxidative Defense. Environ. Monit. Assess. 2020, 192, 232. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.L.; Mui, J.; Song, B.; Sani, E.S.; Eisenman, S.W.; Sheffield, J.B.; Kim, B. Effects, Uptake, and Translocation of Aluminum Oxide Nanoparticles in Lettuce: A Comparison Study to Phytotoxic Aluminum Ions. Sci. Total Environ. 2020, 719, 137393. [Google Scholar] [CrossRef] [PubMed]
- Falco, W.F.; Scherer, M.D.; Oliveira, S.L.; Wender, H.; Colbeck, I.; Lawson, T.; Caires, A.R.L. Phytotoxicity of Silver Nanoparticles on Vicia Faba: Evaluation of Particle Size Effects on Photosynthetic Performance and Leaf Gas Exchange. Sci. Total Environ. 2020, 701, 134816. [Google Scholar] [CrossRef]
- Abbasi Khalaki, M.; Moameri, M.; Asgari Lajayer, B.; Astatkie, T. Influence of Nano-Priming on Seed Germination and Plant Growth of Forage and Medicinal Plants. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Green-Synthesized Nanoparticles Enhanced Seedling Growth, Yield, and Quality of Onion (Allium cepa L.). ACS Sustain. Chem. Eng. 2019, 7, 14580–14590. [Google Scholar] [CrossRef]
- Hu, P.; An, J.; Faulkner, M.M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle Charge and Size Control Foliar Delivery Efficiency to Plant Cells and Organelles. ACS Nano 2020. [Google Scholar] [CrossRef]
- Bombo, A.B.; Pereira, A.E.S.; Lusa, M.G.; de Medeiros Oliveira, E.; de Oliveira, J.L.; Campos, E.V.R.; de Jesus, M.B.; Oliveira, H.C.; Fraceto, L.F.; Mayer, J.L.S. A Mechanistic View of Interactions of a Nanoherbicide with Target Organism. J. Agric. Food Chem. 2019, 67, 4453–4462. [Google Scholar] [CrossRef]
- Palocci, C.; Valletta, A.; Chronopoulou, L.; Donati, L.; Bramosanti, M.; Brasili, E.; Baldan, B.; Pasqua, G. Endocytic Pathways Involved in PLGA Nanoparticle Uptake by Grapevine Cells and Role of Cell Wall and Membrane in Size Selection. Plant Cell Rep. 2017, 36, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Valletta, A.; Chronopoulou, L.; Palocci, C.; Baldan, B.; Donati, L.; Pasqua, G. Poly(Lactic-Co-Glycolic) Acid Nanoparticles Uptake by Vitis Vinifera and Grapevine-Pathogenic Fungi. J. Nanoparticle Res. 2014, 16, 2744. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Avellan, A.; Bland, G.D.; Tappero, R.V.; Acerbo, A.S.; Unrine, J.M.; Giraldo, J.P.; Lowry, G.V. Nanoparticle Surface Charge Influences Translocation and Leaf Distribution in Vascular Plants with Contrasting Anatomy. Environ. Sci. Nano 2019, 6, 2508–2519. [Google Scholar] [CrossRef]
- Avellan, A.; Schwab, F.; Masion, A.; Chaurand, P.; Borschneck, D.; Vidal, V.; Rose, J.; Santaella, C.; Levard, C. Nanoparticle Uptake in Plants: Gold Nanomaterial Localized in Roots of Arabidopsis Thaliana by X-Ray Computed Nanotomography and Hyperspectral Imaging. Environ. Sci. Technol. 2017, 51, 8682–8691. [Google Scholar] [CrossRef] [PubMed]
- Avellan, A.; Yun, J.; Zhang, Y.; Spielman-Sun, E.; Unrine, J.M.; Thieme, J.; Li, J.; Lombi, E.; Bland, G.; Lowry, G.V. Nanoparticle Size and Coating Chemistry Control Foliar Uptake Pathways, Translocation, and Leaf-to-Rhizosphere Transport in Wheat. ACS Nano 2019, 13, 5291–5305. [Google Scholar] [CrossRef]
- An, J.; Hu, P.; Li, F.; Wu, H.; Shen, Y.; White, J.C.; Tian, X.; Li, Z.; Giraldo, J.P. Emerging Investigator Series: Molecular Mechanisms of Plant Salinity Stress Tolerance Improvement by Seed Priming with Cerium Oxide Nanoparticles. Environ. Sci. Nano 2020, 7, 2214–2228. [Google Scholar] [CrossRef]
- Salama, D.M. Effect of Zinc Oxide Nanoparticles on the Growth, Genomic DNA, Production and the Quality of Common Dry Bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 2019, 11, 101083. [Google Scholar] [CrossRef]
- Ye, Y.; Cota-Ruiz, K.; Hernández-Viezcas, J.A.; Valdés, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese Nanoparticles Control Salinity-Modulated Molecular Responses in Capsicum annuum L. through Priming: A Sustainable Approach for Agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed Priming with Silicon Nanoparticles Improved the Biomass and Yield While Reduced the Oxidative Stress and Cadmium Concentration in Wheat Grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef]
- Kasote, D.M.; Lee, J.H.J.; Jayaprakasha, G.K.; Patil, B.S. Seed Priming with Iron Oxide Nanoparticles Modulate Antioxidant Potential and Defense-Linked Hormones in Watermelon Seedlings. ACS Sustain. Chem. Eng. 2019, 7, 5142–5151. [Google Scholar] [CrossRef]
- Pirzada, T.; de Farias, B.V.; Mathew, R.; Guenther, R.H.; Byrd, M.V.; Sit, T.L.; Pal, L.; Opperman, C.H.; Khan, S.A. Recent Advances in Biodegradable Matrices for Active Ingredient Release in Crop Protection: Towards Attaining Sustainability in Agriculture. Curr. Opin. Colloid Interface Sci. 2020, 48, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Savassa, S.M.; Duran, N.M.; Rodrigues, E.S.; de Almeida, E.; van Gestel, C.A.M.; Bompadre, T.F.V.; de Carvalho, H.W.P. Effects of ZnO Nanoparticles on Phaseolus Vulgaris Germination and Seedling Development Determined by X-Ray Spectroscopy. ACS Appl. Nano Mater. 2018, 1, 6414–6426. [Google Scholar] [CrossRef]
- Mahakham, W.; Theerakulpisut, P.; Maensiri, S.; Phumying, S.; Sarmah, A.K. Environmentally Benign Synthesis of Phytochemicals-Capped Gold Nanoparticles as Nanopriming Agent for Promoting Maize Seed Germination. Sci. Total Environ. 2016, 573, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, U.; Luo, X.; Wang, Q.; Shu, K. Are There Unidentified Factors Involved in the Germination of Nanoprimed Seeds? Front. Plant Sci. 2020, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination—Still a Mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Nonogaki, H. Seed Dormancy and Germination—Emerging Mechanisms and New Hypotheses. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.A. Seed Priming to Alleviate Salinity Stress in Germinating Seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Oracz, K.; Karpiński, S. Phytohormones Signaling Pathways and ROS Involvement in Seed Germination. Front. Plant Sci. 2016, 7, 864. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and Oxidative Burst: Roots in Plant Development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef]
- Bailly, C. The Signalling Role of ROS in the Regulation of Seed Germination and Dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef]
- Wu, M.; Wu, J.; Gan, Y. The New Insight of Auxin Functions: Transition from Seed Dormancy to Germination and Floral Opening in Plants. Plant Growth Regul. 2020, 91, 169–174. [Google Scholar] [CrossRef]
- Shuai, H.; Meng, Y.; Luo, X.; Chen, F.; Qi, Y.; Yang, W.; Shu, K. The Roles of Auxin in Seed Dormancy and Germination. Yi Chuan Hered. 2016, 38, 314–322. [Google Scholar] [CrossRef]
- Bourioug, M.; Ezzaza, K.; Bouabid, R.; Alaoui-Mhamdi, M.; Bungau, S.; Bourgeade, P.; Alaoui-Sossé, L.; Alaoui-Sossé, B.; Aleya, L. Influence of Hydro- and Osmo-Priming on Sunflower Seeds to Break Dormancy and Improve Crop Performance under Water Stress. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed Priming for Abiotic Stress Tolerance: An Overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Thomas, T.T.D.; Puthur, J.T. UV Radiation Priming: A Means of Amplifying the Inherent Potential for Abiotic Stress Tolerance in Crop Plants. Environ. Exp. Bot. 2017, 138, 57–66. [Google Scholar] [CrossRef]
- Carrillo-Reche, J.; Vallejo-Marín, M.; Quilliam, R.S. Quantifying the Potential of ‘on-Farm’ Seed Priming to Increase Crop Performance in Developing Countries. A Meta-Analysis. Agron. Sustain. Dev. 2018, 38, 64. [Google Scholar] [CrossRef]
- Lemmens, E.; Deleu, L.J.; De Brier, N.; De Man, W.L.; De Proft, M.; Prinsen, E.; Delcour, J.A. The Impact of Hydro-Priming and Osmo-Priming on Seedling Characteristics, Plant Hormone Concentrations, Activity of Selected Hydrolytic Enzymes, and Cell Wall and Phytate Hydrolysis in Sprouted Wheat (Triticum aestivum L.). ACS Omega 2019, 4, 22089–22100. [Google Scholar] [CrossRef]
- Noorhosseini, S.A.; Jokar, N.K.; Damalas, C.A. Improving Seed Germination and Early Growth of Garden Cress (Lepidium sativum) and Basil (Ocimum basilicum) with Hydro-Priming. J. Plant Growth Regul. 2018, 37, 323–334. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Iqbal, S.; Afzal, I.; Ibrahim, A.M.H.; Bakhtavar, M.A.; Jahanzaib, M.B.H.; Maqbool, M.M. Mitigation of Salinity Stress in Wheat (Triticum aestivum L.) Seedlings through Physiological Seed Enhancements. J. Plant Nutr. 2019, 42, 1192–1204. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone Priming: Regulator for Heavy Metal Stress in Plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Shukla, P.; Chaurasia, P.; Younis, K.; Qadri, O.S.; Faridi, S.A.; Srivastava, G. Nanotechnology in Sustainable Agriculture: Studies from Seed Priming to Post-Harvest Management. Nanotechnol. Environ. Eng. 2019, 4, 11. [Google Scholar] [CrossRef]
- Duran, N.M.; Savassa, S.M.; Lima, R.G.D.; de Almeida, E.; Linhares, F.S.; van Gestel, C.A.M.; Pereira de Carvalho, H.W. X-Ray Spectroscopy Uncovering the Effects of Cu Based Nanoparticle Concentration and Structure on Phaseolus vulgaris Germination and Seedling Development. J. Agric. Food Chem. 2017, 65, 7874–7884. [Google Scholar] [CrossRef] [PubMed]
- Falsini, S.; Clemente, I.; Papini, A.; Tani, C.; Schiff, S.; Salvatici, M.C.; Petruccelli, R.; Benelli, C.; Giordano, C.; Gonnelli, C.; et al. When Sustainable Nanochemistry Meets Agriculture: Lignin Nanocapsules for Bioactive Compound Delivery to Plantlets. ACS Sustain. Chem. Eng. 2019, 7, 19935–19942. [Google Scholar] [CrossRef]
- Montanha, G.S.; Rodrigues, E.S.; Marques, J.P.R.; de Almeida, E.; Colzato, M.; Pereira de Carvalho, H.W. Zinc Nanocoated Seeds: An Alternative to Boost Soybean Seed Germination and Seedling Development. SN Appl. Sci. 2020, 2, 857. [Google Scholar] [CrossRef]
- Gross, M.S.; Bean, T.G.; Hladik, M.L.; Rattner, B.A.; Kuivila, K.M. Uptake, Metabolism, and Elimination of Fungicides from Coated Wheat Seeds in Japanese Quail (Coturnix japonica). J. Agric. Food Chem. 2020, 68, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon Nanotubes Are Able To Penetrate Plant Seed Coat and Dramatically Affect Seed Germination and Plant Growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Kim, B.-S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon Nanotubes as Plant Growth Regulators: Effects on Tomato Growth, Reproductive System, and Soil Microbial Community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Dervishi, E.; Chen, J.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of Carbon Nanotube Exposure to Seeds of Valuable Crops. ACS Appl. Mater. Interfaces 2013, 5, 7965–7973. [Google Scholar] [CrossRef]
- Villagarcia, H.; Dervishi, E.; de Silva, K.; Biris, A.S.; Khodakovskaya, M.V. Surface Chemistry of Carbon Nanotubes Impacts the Growth and Expression of Water Channel Protein in Tomato Plants. Small 2012, 8, 2328–2334. [Google Scholar] [CrossRef]
- Siddaiah, C.N.; Prasanth, K.V.H.; Satyanarayana, N.R.; Mudili, V.; Gupta, V.K.; Kalagatur, N.K.; Satyavati, T.; Dai, X.-F.; Chen, J.-Y.; Mocan, A.; et al. Chitosan Nanoparticles Having Higher Degree of Acetylation Induce Resistance against Pearl Millet Downy Mildew through Nitric Oxide Generation. Sci. Rep. 2018, 8, 2485. [Google Scholar] [CrossRef]
- Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S.K.; Sun, Y.; Hu, J.; Yin, H. Effects of Chitosan Nanoparticles on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2019, 126, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Abdelfattah, K.E. The Possible Roles of Priming with ZnO Nanoparticles in Mitigation of Salinity Stress in Lupine (Lupinus termis) Plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Itroutwar, P.D.; Govindaraju, K.; Tamilselvan, S.; Kannan, M.; Raja, K.; Subramanian, K.S. Seaweed-Based Biogenic ZnO Nanoparticles for Improving Agro-Morphological Characteristics of Rice (Oryza sativa L.). J. Plant Growth Regul. 2019, 39. [Google Scholar] [CrossRef]
- Maswada, H.F.; Djanaguiraman, M.; Prasad, P.V.V. Seed Treatment with Nano-Iron (III) Oxide Enhances Germination, Seeding Growth and Salinity Tolerance of Sorghum. J. Agron. Crop Sci. 2018, 204, 577–587. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia ur Rehman, M.; Waris, A.A. Zinc and Iron Oxide Nanoparticles Improved the Plant Growth and Reduced the Oxidative Stress and Cadmium Concentration in Wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Dileep Kumar, G.; Raja, K.; Natarajan, N.; Govindaraju, K.; Subramanian, K.S. Invigouration Treatment of Metal and Metal Oxide Nanoparticles for Improving the Seed Quality of Aged Chilli Seeds (Capsicum annum L.). Mater. Chem. Phys. 2020, 242, 122492. [Google Scholar] [CrossRef]
- Ahuja, R.; Sidhu, A.; Bala, A. Synthesis and Evaluation of Iron (Ii) Sulfide Aqua Nanoparticles (FeS-NPs) against Fusarium Verticillioides Causing Sheath Rot and Seed Discoloration of Rice. Eur. J. Plant Pathol. 2019, 155, 163–171. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.M.M.; Hasaneen, M.N.A.; Omer, A.M. Impact of Engineered Nanomaterials Either Alone or Loaded with NPK on Growth and Productivity of French Bean Plants: Seed Priming vs Foliar Application. S. Afr. J. Bot. 2019, 125, 102–108. [Google Scholar] [CrossRef]
- Divya, K.; Vijayan, S.; Nair, S.J.; Jisha, M.S. Optimization of Chitosan Nanoparticle Synthesis and Its Potential Application as Germination Elicitor of Oryza sativa L. Int. J. Biol. Macromol. 2019, 124, 1053–1059. [Google Scholar] [CrossRef]
- Rahman, M.S.; Chakraborty, A.; Mazumdar, S.; Nandi, N.C.; Bhuiyan, M.N.I.; Alauddin, S.M.; Khan, I.A.; Hossain, M.J. Effects of Poly (Vinylpyrrolidone) Protected Platinum Nanoparticles on Seed Germination and Growth Performance of Pisum Sativum. Nano Struct. Nano Objects 2020, 21, 100408. [Google Scholar] [CrossRef]
- Kannaujia, R.; Srivastava, C.M.; Prasad, V.; Singh, B.N.; Pandey, V. Phyllanthus Emblica Fruit Extract Stabilized Biogenic Silver Nanoparticles as a Growth Promoter of Wheat Varieties by Reducing ROS Toxicity. Plant Physiol. Biochem. 2019, 142, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, M.; Muthukumar, S. Chitosan Guar Nanoparticle Preparation and Its in Vitro Antimicrobial Activity towards Phytopathogens of Rice. Int. J. Biol. Macromol. 2020, 153, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.P.; Jaiswal, J.P.; Kumar, A. Seed Priming with Iron Oxide Nanoparticles Triggers Iron Acquisition and Biofortification in Wheat (Triticum aestivum L.) Grains. J. Plant Growth Regul. 2019, 38, 122–131. [Google Scholar] [CrossRef]
- Guha, T.; Ravikumar, K.V.G.; Mukherjee, A.; Mukherjee, A.; Kundu, R. Nanopriming with Zero Valent Iron (NZVI) Enhances Germination and Growth in Aromatic Rice Cultivar (Oryza sativa Cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Kaur, S.; Dharamvir, K.; Nayyar, H.; Verma, G. Multi-Walled Carbon Nanotubes Applied through Seed-Priming Influence Early Germination, Root Hair, Growth and Yield of Bread Wheat (Triticum aestivum L.): Multiwalled Carbon Nanotube Influence on Bread Wheat. J. Sci. Food Agric. 2018, 98, 3148–3160. [Google Scholar] [CrossRef]
- Das, C.K.; Jangir, H.; Kumar, J.; Verma, S.; Mahapatra, S.S.; Philip, D.; Srivastava, G.; Das, M. Nano-Pyrite Seed Dressing: A Sustainable Design for NPK Equivalent Rice Production. Nanotechnol. Environ. Eng. 2018, 3, 14. [Google Scholar] [CrossRef]
- Bravo Cadena, M.; Preston, G.M.; Van der Hoorn, R.A.L.; Flanagan, N.A.; Townley, H.E.; Thompson, I.P. Enhancing Cinnamon Essential Oil Activity by Nanoparticle Encapsulation to Control Seed Pathogens. Ind. Crops Prod. 2018, 124, 755–764. [Google Scholar] [CrossRef]
- Yasmeen, F.; Raja, N.I.; Razzaq, A.; Komatsu, S. Proteomic and Physiological Analyses of Wheat Seeds Exposed to Copper and Iron Nanoparticles. Biochim. Biophys. Acta BBA Proteins Proteom. 2017, 1865, 28–42. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Mao, J.; Chen, B. Effects of Biochar Nanoparticles on Seed Germination and Seedling Growth. Environ. Pollut. 2020, 256, 113409. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Murugan, I.; Selvaraj, M. Chitosan Nanoparticles Loaded with Thiamine Stimulate Growth and Enhances Protection against Wilt Disease in Chickpea. Carbohydr. Polym. 2019, 212, 169–177. [Google Scholar] [CrossRef]
- Pereira, A.E.S.; Oliveira, H.C.; Fraceto, L.F. Polymeric Nanoparticles as an Alternative for Application of Gibberellic Acid in Sustainable Agriculture: A Field Study. Sci. Rep. 2019, 9, 7135. [Google Scholar] [CrossRef] [PubMed]
- Spagnoletti, F.N.; Spedalieri, C.; Kronberg, F.; Giacometti, R. Extracellular Biosynthesis of Bactericidal Ag/AgCl Nanoparticles for Crop Protection Using the Fungus Macrophomina Phaseolina. J. Environ. Manag. 2019, 231, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, M.; Rajini, S.B.; Udayashankar, A.C.; Niranjana, S.R.; Lund, O.S.; Shetty, H.S.; Prakash, H.S. Biofabricated Zinc Oxide Nanoparticles as an Eco-Friendly Alternative for Growth Promotion and Management of Downy Mildew of Pearl Millet. Crop Prot. 2019, 121, 103–112. [Google Scholar] [CrossRef]
- Saharan, V.; Kumaraswamy, R.V.; Choudhary, R.C.; Kumari, S.; Pal, A.; Raliya, R.; Biswas, P. Cu-Chitosan Nanoparticle Mediated Sustainable Approach To Enhance Seedling Growth in Maize by Mobilizing Reserved Food. J. Agric. Food Chem. 2016, 64, 6148–6155. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Zinc Encapsulated Chitosan Nanoparticle to Promote Maize Crop Yield. Int. J. Biol. Macromol. 2019, 127, 126–135. [Google Scholar] [CrossRef]
- Shcherbakova, E.N.; Shcherbakov, A.V.; Andronov, E.E.; Gonchar, L.N.; Kalenskaya, S.M.; Chebotar, V.K. Combined Pre-Seed Treatment with Microbial Inoculants and Mo Nanoparticles Changes Composition of Root Exudates and Rhizosphere Microbiome Structure of Chickpea (Cicer arietinum L.) Plants. Symbiosis 2017, 73, 57–69. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Tuteja, S.K.; Kim, K.-H. Nanovehicles for Plant Modifications towards Pest- and Disease-Resistance Traits. Trends Plant Sci. 2020, 25, 198–212. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.R.; Germano-Costa, T.; Lima, R.; Vechia, J.F.D.; Soares, S.T.; de Andrade, D.J.; Gonçalves, K.C.; do Nascimento, J.; Polanczyk, R.A.; et al. Association of Zein Nanoparticles with Botanical Compounds for Effective Pest Control Systems. Pest Manag. Sci. 2019, 75, 1855–1865. [Google Scholar] [CrossRef]
- Raja, K.; Sowmya, R.; Sudhagar, R.; Moorthy, P.S.; Govindaraju, K.; Subramanian, K.S. Biogenic ZnO and Cu Nanoparticles to Improve Seed Germination Quality in Blackgram (Vigna mungo). Mater. Lett. 2019, 235, 164–167. [Google Scholar] [CrossRef]
- Maroufpour, N.; Mousavi, M.; Abbasi, M.; Ghorbanpour, M. Biogenic Nanoparticles as Novel Sustainable Approach for Plant Protection. In Biogenic Nano-Particles and Their Use in Agro-Ecosystems; Ghorbanpour, M., Bhargava, P., Varma, A., Choudhary, D.K., Eds.; Springer: Singapore, 2020; pp. 161–172. ISBN 9789811529856. [Google Scholar]
- Fraceto, L.F.; Maruyama, C.R.; Guilger, M.; Mishra, S.; Keswani, C.; Singh, H.B.; Lima, R.D. Trichoderma Harzianum-Based Novel Formulations: Potential Applications for Management of Next-Gen Agricultural Challenges. J. Chem. Technol. Biotechnol. 2018, 93, 2056–2063. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, Y.; Dong, H.; Niu, J.; Tang, J.; Yang, J.; Tang, G.; Zhou, Z.; Tang, R.; Shi, X.; et al. A Bioresponsive System Based on Mesoporous Organosilica Nanoparticles for Smart Delivery of Fungicide in Response to Pathogen Presence. ACS Sustain. Chem. Eng. 2020, 8, 5716–5723. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Hilmi, N.H.Z.; Daim, L.D.J. Phytotoxicity of Chitosan-Based Agronanofungicides in the Vegetative Growth of Oil Palm Seedling. PLoS ONE 2020, 15, e0231315. [Google Scholar] [CrossRef]
- Kavetsou, E.; Koutsoukos, S.; Daferera, D.; Polissiou, M.G.; Karagiannis, D.; Perdikis, D.C.; Detsi, A. Encapsulation of Mentha pulegium Essential Oil in Yeast Cell Microcarriers: An Approach to Environmentally Friendly Pesticides. J. Agric. Food Chem. 2019, 67, 4746–4753. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of Botanical Insecticides for Sustainable Agriculture: Future Perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef]
- Pereira, A.E.S.; Silva, P.M.; Oliveira, J.L.; Oliveira, H.C.; Fraceto, L.F. Chitosan Nanoparticles as Carrier Systems for the Plant Growth Hormone Gibberellic Acid. Colloids Surf. B Biointerfaces 2017, 150, 141–152. [Google Scholar] [CrossRef]
- Yi, Z.; Hussain, H.I.; Feng, C.; Sun, D.; She, F.; Rookes, J.E.; Cahill, D.M.; Kong, L. Functionalized Mesoporous Silica Nanoparticles with Redox-Responsive Short-Chain Gatekeepers for Agrochemical Delivery. ACS Appl. Mater. Interfaces 2015, 7, 9937–9946. [Google Scholar] [CrossRef]
- Guo, H.; White, J.C.; Wang, Z.; Xing, B. Nano-Enabled Fertilizers to Control the Release and Use Efficiency of Nutrients. Curr. Opin. Environ. Sci. Health 2018, 6, 77–83. [Google Scholar] [CrossRef]
- Du, B.D.; Ngoc, D.T.B.; Thang, N.D.; Tuan, L.N.A.; Thach, B.D.; Hien, N.Q. Synthesis and in Vitro Antifungal Efficiency of Alginate-Stabilized Cu2O-Cu Nanoparticles against Neoscytalidium dimidiatum Causing Brown Spot Disease on Dragon Fruit Plants (Hylocereus undatus). Vietnam J. Chem. 2019, 57, 318–323. [Google Scholar] [CrossRef]
- Ji, M.; Sun, X.; Guo, X.; Zhu, W.; Wu, J.; Chen, L.; Wang, J.; Chen, M.; Cheng, C.; Zhang, Q. Green Synthesis, Characterization and in Vitro Release of Cinnamaldehyde/Sodium Alginate/Chitosan Nanoparticles. Food Hydrocoll. 2019, 90, 515–522. [Google Scholar] [CrossRef]
- Qi, T.; Lü, S.; Zhang, S.-F.; Bai, X.; Chen, J.; Huang, M.; Liu, M. Zein Coated Porous Carboxymethyl Starch Fertilizer for Iron Promoting and Phosphate Sustainable Release. J. Clean. Prod. 2020, 258, 120778. [Google Scholar] [CrossRef]
- Coelho, C.C.S.; Michelin, M.; Cerqueira, M.A.; Gonçalves, C.; Tonon, R.V.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.C.; Teixeira, J.A. Cellulose Nanocrystals from Grape Pomace: Production, Properties and Cytotoxicity Assessment. Carbohydr. Polym. 2018, 192, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, M.; Luan, Q.; Tang, H.; Huang, F.; Xiang, X.; Yang, C. Cellulose Anionic Hydrogels Based on Cellulose Nanofibers as Natural Stimulants for Seed Germination and Seedling Growth. J. Agric. Food Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; Oliveira, J.L.D.; da Silva, C.M.G.; Pascoli, M.; Pasquoto, T.; Lima, R.; Abhilash, P.C.; Fernandes Fraceto, L. Polymeric and Solid Lipid Nanoparticles for Sustained Release of Carbendazim and Tebuconazole in Agricultural Applications. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Santos, V.; Badan Ribeiro, A.P.; Andrade Santana, M.H. Solid Lipid Nanoparticles as Carriers for Lipophilic Compounds for Applications in Foods. Food Res. Int. 2019, 122, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan Nanoparticle Based Delivery Systems for Sustainable Agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Dasgupta, A.; Sarkar, J.; Panda, K.; Acharya, K. Chitosan Nanoparticles: A Positive Modulator of Innate Immune Responses in Plants. Sci. Rep. 2015, 5, 15195. [Google Scholar] [CrossRef]
- Jiménez-Arias, D.; Morales-Sierra, S.; Borges, A.A.; Díaz Díaz, D. Biostimulant Nanoencapsulation: The New Keystone To Fight Hunger. J. Agric. Food Chem. 2020, 68, 7083–7085. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. Available online: https://www.hindawi.com/journals/jnm/2019/3702518/ (accessed on 5 May 2020).
- Fischer, J.; Beckers, S.J.; Yiamsawas, D.; Thines, E.; Landfester, K.; Wurm, F.R. Targeted Drug Delivery in Plants: Enzyme-Responsive Lignin Nanocarriers for the Curative Treatment of the Worldwide Grapevine Trunk Disease Esca. Adv. Sci. 2019, 6, 1802315. [Google Scholar] [CrossRef]
- Panyuta, O.; Belava, V.; Fomaidi, S.; Kalinichenko, O.; Volkogon, M.; Taran, N. The Effect of Pre-Sowing Seed Treatment with Metal Nanoparticles on the Formation of the Defensive Reaction of Wheat Seedlings Infected with the Eyespot Causal Agent. Nanoscale Res. Lett. 2016, 11, 92. [Google Scholar] [CrossRef]
- Schmitt, C.C.; Moreira, R.; Neves, R.C.; Richter, D.; Funke, A.; Raffelt, K.; Grunwaldt, J.-D.; Dahmen, N. From Agriculture Residue to Upgraded Product: The Thermochemical Conversion of Sugarcane Bagasse for Fuel and Chemical Products. Fuel Process. Technol. 2020, 197, 106199. [Google Scholar] [CrossRef]
- Matyjaszczyk, E. Comparison between Seed and Foliar Treatment as a Tool in Integrated Pest Management. J. Agric. Food Chem. 2017, 65, 6081–6086. [Google Scholar] [CrossRef] [PubMed]
- Farias, B.V.; Pirzada, T.; Mathew, R.; Sit, T.L.; Opperman, C.; Khan, S.A. Electrospun Polymer Nanofibers as Seed Coatings for Crop Protection. ACS Sustain. Chem. Eng. 2019, 7, 19848–19856. [Google Scholar] [CrossRef]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of Nanomaterials in the Environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.V.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Ha, C.V. Copper Nanoparticle Application Enhances Plant Growth and Grain Yield in Maize under Drought Stress Conditions. Plant Biol. 2020. [Google Scholar] [CrossRef]
- Barbieri, M.; Sappa, G.; Nigro, A. Soil Pollution: Anthropogenic versus Geogenic Contributions over Large Areas of the Lazio Region. J. Geochem. Explor. 2018, 195, 78–86. [Google Scholar] [CrossRef]
- Qayyum, M.F.; Rehman, M.Z.U.; Ali, S.; Rizwan, M.; Naeem, A.; Maqsood, M.A.; Khalid, H.; Rinklebe, J.; Ok, Y.S. Residual Effects of Monoammonium Phosphate, Gypsum and Elemental Sulfur on Cadmium Phytoavailability and Translocation from Soil to Wheat in an Effluent Irrigated Field. Chemosphere 2017, 174, 515–523. [Google Scholar] [CrossRef]
- Plaksenkova, I.; Kokina, I.; Petrova, A.; Jermaļonoka, M.; Gerbreders, V.; Krasovska, M. The Impact of Zinc Oxide Nanoparticles on Cytotoxicity, Genotoxicity, and MiRNA Expression in Barley (Hordeum vulgare L.) Seedlings. Available online: https://www.hindawi.com/journals/tswj/2020/6649746/ (accessed on 5 January 2021).
- Raliya, R.; Tarafdar, J.C.; Biswas, P. Enhancing the Mobilization of Native Phosphorus in the Mung Bean Rhizosphere Using ZnO Nanoparticles Synthesized by Soil Fungi. J. Agric. Food Chem. 2016, 64, 3111–3118. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, F.; Yue, L.; Li, T.; Jiang, Z.; Xu, Z.; Wang, Z.; Xing, B. Uptake, Transport, and Transformation of CeO2 Nanoparticles by Strawberry and Their Impact on the Rhizosphere Bacterial Community. ACS Sustain. Chem. Eng. 2020, 8, 4792–4800. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, M.; Zhang, W.; Gardea-Torresdey, J.L.; White, J.C.; Ji, R.; Zhao, L. Silver Nanoparticles Alter Soil Microbial Community Compositions and Metabolite Profiles in Unplanted and Cucumber-Planted Soils. Environ. Sci. Technol. 2020, 54, 3334–3342. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Dang, F.; Hintelmann, H.; Yin, B.; Zhou, D. Alteration of Crop Yield and Quality of Three Vegetables upon Exposure to Silver Nanoparticles in Sludge-Amended Soil. ACS Sustain. Chem. Eng. 2020, 8, 2472–2480. [Google Scholar] [CrossRef]
- Zhai, Y.; Hunting, E.R.; Liu, G.; Baas, E.; Peijnenburg, W.J.G.M.; Vijver, M.G. Compositional Alterations in Soil Bacterial Communities Exposed to TiO2 Nanoparticles Are Not Reflected in Functional Impacts. Environ. Res. 2019, 178, 108713. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.; Nadanathangam, V. Shifts in Metabolic Patterns of Soil Bacterial Communities on Exposure to Metal Engineered Nanomaterials. Ecotoxicol. Environ. Saf. 2020, 189, 110012. [Google Scholar] [CrossRef] [PubMed]
- Santaella, C.; Plancot, B. Interactions of Nanoenabled Agrochemicals with Soil Microbiome. In Nanopesticides: From Research and Development to Mechanisms of Action and Sustainable Use in Agriculture; Fraceto, L.F., S.S. de Castro, V.L., Grillo, R., Ávila, D., Caixeta Oliveira, H., Lima, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 137–163. ISBN 978-3-030-44873-8. [Google Scholar]
- Hamidat, M.; Barakat, M.; Ortet, P.; Chanéac, C.; Rose, J.; Bottero, J.-Y.; Heulin, T.; Achouak, W.; Santaella, C. Design Defines the Effects of Nanoceria at a Low Dose on Soil Microbiota and the Potentiation of Impacts by the Canola Plant. Available online: https://pubs.acs.org/doi/pdf/10.1021/acs.est.6b01056 (accessed on 31 July 2020).
- Simonin, M.; Colman, B.P.; Tang, W.; Judy, J.D.; Anderson, S.M.; Bergemann, C.M.; Rocca, J.D.; Unrine, J.M.; Cassar, N.; Bernhardt, E.S. Plant and Microbial Responses to Repeated Cu (OH)2 Nanopesticide Exposures under Different Fertilization Levels in an Agro-Ecosystem. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A Critical Evaluation of Nanopesticides and Nanofertilizers against Their Conventional Analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
| Nanoparticle System | Characteristics | Main Effects | Applications | Reference |
|---|---|---|---|---|
| Biogenic silver nanoparticles produced using kaffir lime leaf extracts | Spherical nanoparticles with particle size of 6–26 nm | Concentrations: 10 and 20 mg/mL Species: Rice seeds (Oriza sativa L. cv. KDML 105). Effects: Water uptake ↑, Aquaporin gene expression ↑, Enzyme activity ↑, Seed and seedlings vigor ↑ Plant morphology ↑, and biomass ↑ |  | [14] |
| Iron oxide nanoparticles | Particle size <50 nm, with surface area of 180 m/g2 | Concentrations: 10, 50, 100, and 500 mg/L. Species: Sorghum (Sorghum bicolor (L.) Moench) KDML 105. Effects: Seed and seedlings vigor ↑, Biochemical activity ↑, Biomass ↑, and water content in leaves ↑ |  | [65] |
| Biogenic iron nanoparticles produced using onion extracts | Particle size of 19–30 nm, with low-crystalline or amorphous Fe2O3 | Concentrations: 20, 40, 80, and 160 mg/L. Species: Watermelon (Citrullus lanatus (Thunb.) Matsum and Nakay varieties). Effects: Seed and seedlings vigor ↑, Plant morphology ↑, Phytotoxic effects ↓. Enzyme activity ↑, and Plant growth regulator (jasmonate) ↑ |  | [31] |
| Zinc oxide and iron oxide nanoparticles | Zinc oxide nanoparticles with sizes of 20–30 cm; iron oxide nanoparticles (Fe3O4) with sizes of 50–100 nm | Concentrations: Zinc nanoparticles at 25, 50, 75, and 100 mg/L; iron nanoparticles at 5, 10, 15, and 20 mg/L. Species: Wheat (Triticum aestivum L.). Effects: Plant morphology ↑, Biomass ↑, Biochemical activity ↑, Cadmium uptake ↓, and Biofortification ↑ |  | [66] |
| Silicon nanoparticles | Spherical nanoparticles with size around 90 nm | Concentrations: The nanoparticles were evaluated at concentrations of 300, 600, 900, and 1200 mg/L. Species: Wheat (Triticum aestivum L.). Effects: Biomass ↑, Biochemical activity ↑, ROS levels ↑, and Cadmium uptake ↓ |  | [30] |
| Biogenic zinc nanoparticles produced using brown seaweed (Turbinaria ornata) extracts | Spherical and hexagonal nanoparticles with average size of 15–52 nm | Concentrations: Nanoparticles at concentrations of 5, 10, 25, 50, 100, and 200 mg/L. Species: Rice (Oryza sativa L.). Effects: Seed and seedlings vigor ↑, Antioxidant enzymes ↑, and Biofortification ↑ |  | [64] |
| Nanoparticles of zinc, titanium, and silver | Zinc oxide nanoparticles (35–40 nm), titanium oxide nanoparticles (100 nm), and silver nanoparticles (85 nm), with spherical, cylindrical, and needle-like morphologies, respectively | Concentrations: Nanoparticles at concentrations of 750, 1000, and 1250 mg/kg. Species: Chilli (Capsicum annuum L.). Effects: Seed and seedlings vigor ↑, Plant morphology ↑, Antimicrobial activity ↑ |  | [67] |
| Chitosan/tripolyphosphate nanoparticles | Nanoparticles with size of 259.4 ± 4.7 nm, PDI (polydispersity index) of 0.28 ± 0.0016, and zeta potential of 40.0 ± 2.9 mV | Concentrations: Nanoparticles at concentrations of 1–100 µg/mL. Species: Wheat (Triticum aestivum L.). Effects: Plant morphology ↑, Biochemical activity ↑, and Plant growth regulator (auxin) ↑ |  | [62] |
| Biogenic gold nanoparticles synthesized using galangal rhizome extracts | Spherical nanoparticles with size of 10–30 nm | Concentrations: Nanoparticles at concentrations of 5, 10, and 15 ppm. Species: Maize (Zea mays L.). Effects: Seed and seedlings vigor (aged seeds) ↑, and Biochemical activity ↑ |  | [34] |
| Manganese(III) oxide nanoparticles | Spherical nanoparticles with size of 50 nm, and zeta potential of 29.2 ± 1.8 mV | Concentrations: 0.1, 0.5, and 1 mg/mL. Species: Jalapeño (Capsicum annuum L.). Effects: Salinity resistance ↑, Antioxidant enzymes ↑ |  | [29] |
| Iron(II) sulfide aqua nanoparticles | Spherical nanoparticles with size around 6–20 nm | Concentration: 30 µg/mL. Species: Rice (Oryza sativa L.). Effects: Seed and seedlings vigor ↑ Plant morphology ↑, Antimicrobial activity ↑ |  | [68] |
| Copper nanoparticles | Nanoparticles with sizes of 25, 40, and 80 nm, and zeta potentials of 15–25 mV | Concentrations: 1, 10, 100, and 1000 mg/L. Species: Common bean (Phaseolus vulgaris L.). Effects: Seed and seedlings vigor ↑↓ (High concentrations inhibited seed germination, independent of nanoparticle size), and Biomass ↑ |  | [53] |
| Chitosan nanoparticles of and carbon nanotubes | Chitosan nanoparticles with size of 95 ± 2 nm and zeta potential of +123.5 mV; carbon nanotubes with size of 40 ± 0.4 nm, and zeta potential of −8.5 mV | Concentrations: 10% of both nanomaterials were used, with a concentration of 20 µg/L−. Species: Common bean (Phaseolus vulgaris L.). Effects: Plant morphology ↓, ROS levels ↑, and Biochemical activity ↓ |  | [69] |
| Zinc nanoparticles | Zinc nanoparticles with size of 20 nm and spherical shape, and sizes of 40 and 60 nm with elongated shapes | Concentrations: 1, 10, 100, 1000, and 5000 mg/L. Species: Common bean (Phaseolus vulgaris L.). Effects: Biomass ↑ |  | [33] |
| Chitosan nanoparticles | Chitosan nanoparticles with sizes of 20–170 nm | Concentrations: 0.5, 1, 1.5, 10, 15, and 20 mg/mL. Species: Rice (Oryza sativa L.). Effects: Plant morphology ↓, and Biomass ↑ |  | [70] |
| Zinc nanoparticles | Mean size of 21.3 nm | Concentrations: 20, 40, and 60 mg/L. Species: Lupin (Lupinis termis L.). Effects: Salinity resistance ↑, Biochemical activity ↑, ROS levels ↓ |  | [63] |
| Biogenic silver and gold nanoparticles produced using onion extract | Silver nanoparticles with size of 11.6 ± 2.40 nm and zeta potential of −2.20 ± 0.29 mV, with spherical and ellipsoidal morphology; gold nanoparticles with size of 93.68 ± 2.06 nm, and zeta potential of −8.51 ± 1.26 mV, with anisotropic morphology | Concentrations: Silver nanoparticles at 31.3 µg/mL and gold nanoparticles at 31.3 µg/mL. Species: Onion (Allium cepa L.) Effects: Seed and seedlings vigor ↑ Plant morphology ↑, Biochemical activity ↑ |  | [19] |
| Platinum nanoparticles stabilized with poly(vinylpyrrolidone) | Nanoparticles with size of 3.2 ± 0.8 nm and spherical morphology | Concentrations: Concentrated solution at 1.0 mM. Species: Pea (Pisum sativum L.). Effects: Seed and seedlings vigor ↑ Plant morphology ↑, and microorganisms colonization (arbuscular mycorrhizal fungi and rhizobia) ↓ |  | [71] |
| Biogenic silver nanoparticles produced using Phyllanthus emblica | Nanoparticles with size of 10–35 nm, irregular shape, and zeta potential of −23.8 mV | Concentrations: 0, 5, 10, 25, and 50 mg/L. Species: Wheat seeds (Triticum aestivum L.). Effects: ROS levels ↓, Seed and seedlings vigor (non-biogenic silver nanoparticles) ↓, Seed and seedlings vigor (biogenic nanoparticles) ↑ |  | [72] |
| Chitosan guar nanoparticles | Nanoparticles with size of 122 nm, PDI of 0.358, and zeta potential of −30 mV | Concentrations: 0.05, 0.1, and 0.2%. Species: Rice (Oryza sativa L.). Effects: Seed and seedlings vigor ↑↓ (0.05% and 0.1%, the nanoparticles promoted seed germination, while the use of 0.2% reduced seed germination), Plant morphology ↑, Biomass ↑, Biochemical activity ↑ and Antimicrobial activity ↑ | [73] | |
| Iron nanoparticles | Nanoparticles with size of ~80 nm, and zeta potential of −44 mV | Concentrations: 25, 50, 100, 200, 300, 400, 500, and 1000 µg/mL. Species: Wheat (Triticum aestivum L.), types WL711 (low-iron genotype) and IITR26 (high-iron genotype). Effects: Seed and seedlings vigor ↑↓ (dose dependent), Plant morphology ↑↓ (High concentrations caused inhibition of plant growth), and Harvest ↑ |  | [74] |
| Zero-valent iron nanoparticles (priming of aged seeds) | Nanoparticles with size of 33.8 ± 3.59 nm, and zeta potential of −39 mV | Concentrations: 10, 20, 40, 80, and 160 mg/L. Species: Rice (Oryza sativa L.). Effects: Seed and seedlings vigor ↑↓, Water uptake ↑↓, Plant morphology ↑, Biochemical activity ↑, Enzymatic activity ↑, ROS levels ↓. Dose dependent effects |  | [75] |
| Biogenic silver nanoparticles and turmeric nanoemulsions | Turmeric nanoemulsion with particle size of 171.3 ± 0.52 nm, PDI of 0.25, and zeta potential of -1.23 ± 0.16 mV; biogenic silver nanoparticles with size of 141.3 ± 0.78 nm, PDI of 0.18 ± 0.03, and zeta potential of −1.23 ± 0.16 mV | Concentrations: 10-fold diluted turmeric nanoemulsion and biogenic silver nanoparticles at 31.3 µg/mL. Species: Watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai). Effects: Seed and seedlings vigor ↑, Enzymatic activity ↑, Plant morphology ↑, Biochemical activity ↑, and Harvest ↑ |  | [12] |
| Multi-walled carbon nanotubes | Nanotubes with diameter of 13–14 nm | Concentrations: 70, 80, and 90 µg/mL. Species: Wheat (Triticum aestivum L.). Effects: Seed and seedlings vigor ↑, Plant morphology ↑, and Harvest ↑ |  | [76] |
| Nano-pyrite (FeS2) | Nanoparticles with sizes in the range 25–100 nm, with spherical morphology | Concentrations: 50 µg/mL. Species: Rice (Oryza sativa L.). Effects: Enzymatic activity ↑, Seed and seedlings vigor ↑, and Fertilizer ↓ |  | [77] |
| Mesoporous silica nanoparticles containing cinnamon essential oil | Nanoparticles with size of ~100 nm and pores of around 2–2.8 nm | Concentration: 2 mg/mL. Species: Pea seeds (Pisum sativum L.). Effects: Seed and seedlings vigor ↑, and antimicrobial activity ↓ |  | [78] |
| Copper and iron nanoparticles | Copper nanoparticles with sizes of around 15–30 nm; iron nanoparticles with sizes of around 20–30 nm | Concentrations: 20, 25, 30, 35, and 40 ppm Species: Wheat (Triticum aestivum L.) seeds of varieties galaxy-13, Pakistan-13, and NARC-11. Effects: Enzymatic activity ↑, Biochemical activity ↑ Anti-oxidant activity ↑, Abiotic stress resistance ↑, and Harvest ↑↓ (dose dependent) |  | [79] |
| Cobalt and molybdenum oxide nanoparticles | Both types of nanoparticle had sizes of 60–80 nm and spherical morphology | Concentrations: 1 L of stock commercial solution for 40 kg of seeds. Species: Soybean seeds (Glycine max (L). Merr.). Effects: Seed and seedlings vigor ↑, Plant morphology ↑, Biomass ↑, and Enzymatic activity ↑ |  | [11] |
| Biochar nanoparticles produced from rice and wood sawdust | Nanoparticles with sizes of ~22–56 nm and spherical morphology | Concentrations: 0.5, 5, and 50 mg/L. Species: Rice (Oryza sativa L.) and tomato (Lycopersicon esculentum Mill.). Effects: Seed and seedlings vigor ↑, Plant morphology ↑, and Biomass ↑ |  | [80] |
| Chitosan nanoparticles loaded with thiamine | Nanoparticles with size of 560 nm, polydispersity <1, and zeta potential of 37.7 mV | Concentration: 0.1%. Species: Chickpea (Cicer arietinum L.) Effects: Seed and seedlings vigor ↑, Plant morphology ↑, and Plant growth regulator (auxins) ↑ |  | [81] |
| Lignin nanoparticles loaded with gibberellic acid | Nanoparticles with sizes around 200–250 nm, polydispersity of 0.17–0.38, and spherical morphology | Concentrations: 0.5, 1, and 1.5 mg/mL. Species: Arugula (Eruca visicaria (L.) Cav. subsp. sativa), tomato (Solanum lycopersicum L. cv. Ciliegino), and chickpea (Cicer arietinum L.). Effects: Seed and seedlings vigor ↑ (The effects varied according to the time of sowing after the treatment.) |  | [54] |
| Chitosan nanoparticles loaded with gibberellic acid | Alginate/chitosan nanoparticles with 450 ± 10 nm, PDI of 0.3 and zeta potential of −29 mV. Chitosan/tripolyphosphate nanoparticles with 195 ± 1 nm, PDI of 0.3, and zeta potential of 27 mV. Spherical morphology | Concentrations: 0.05, 0.005, and 0.0005 mg/mL. Species: tomato (Solanum lycopersicum var. cerasiforme). Effects: Seed and seedlings vigor ↑, Plant morphology ↑, and Harvest ↑ |  | [82] |
| Biogenic silver nanoparticles produced using the fungus Macrophomina phaseolina | Nanoparticles with size of 5–30 nm | Concentrations: 10, 20, and 50 µg/mL. Species: Soybean (Glycine max (L.) Merr.). Effects: Antimicrobial activity ↑ |  | [83] |
| Chitosan nanoparticles | Nanoparticles with size of 400 nm and spherical morphology | Concentration: 250 mg/kg. Species: Pearl millet (Pennisetum glaucum (L.) R.Br.). Effects: Seed and seedlings vigor ↑, Antimicrobial activity ↑, Enzymatic activity ↑, Biochemical activity ↑, and Plant growth regulators ↑ |  | [61] |
| Biogenic zinc oxide nanoparticles produced using Eclipta alba extracts | Nanoparticles with size of 32 nm | Concentrations: 50, 100, 150, 200, 250, and 500 ppm. Species: Pearl millet (Pennisetum glaucum (L.) R.Br.). Effects: Seed and seedlings vigor ↑, Plant morphology ↑, and Antimicrobial resistance ↑ |  | [84] |
| Chitosan nanoparticles containing copper | Nanoparticles with size of 374.3 ± 8.2 nm, and zeta potential of 22.6 mV | Concentrations: 0.01, 0.04, 0.08, 0.12, and 0.16% w/v. Species: Maize seeds (Zea mays L.). Effects: Seed and seedlings vigor ↑↓, Enzymatic activity ↑↓ (Dose dependent) |  | [85] |
| Chitosan nanoparticles containing zinc | Nanoparticles with size of 387.7 ± 4 nm, spherical morphology, polydispersity of 0.22, and zeta potential of +34 mV | Concentrations: 0.01, 0.04, 0.08, 0.12, and 0.16% w/v. Species: Maize seeds (Zea mays L.). Effects: Seed and seedlings vigor ↑, Enzymatic activity ↑, Anti-oxidant enzymes ↑, Biotic resistance ↑, and Harvest ↑ |  | [86] |
| Molybdenum nanoparticles combined with the bacteria Mesorhizobium ciceri ST282 and Bacillus subtilis Ch13 | Nanoparticles with size of 35–50 nm | Concentrations: 10 mg/L of nanoparticles, 108 CFU/mL− of M. cicero ST282, and 107 CFU/mL of B. subtilis Ch13. Species: Chickpea (Cicer arietinum L.). Effects: Seed and seedlings vigor ↑, Plant morphology ↑ Anti-oxidant enzymes ↑, and Harvest ↑ | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology Potential in Seed Priming for Sustainable Agriculture. Nanomaterials 2021, 11, 267. https://doi.org/10.3390/nano11020267
do Espirito Santo Pereira A, Caixeta Oliveira H, Fernandes Fraceto L, Santaella C. Nanotechnology Potential in Seed Priming for Sustainable Agriculture. Nanomaterials. 2021; 11(2):267. https://doi.org/10.3390/nano11020267
Chicago/Turabian Styledo Espirito Santo Pereira, Anderson, Halley Caixeta Oliveira, Leonardo Fernandes Fraceto, and Catherine Santaella. 2021. "Nanotechnology Potential in Seed Priming for Sustainable Agriculture" Nanomaterials 11, no. 2: 267. https://doi.org/10.3390/nano11020267
APA Styledo Espirito Santo Pereira, A., Caixeta Oliveira, H., Fernandes Fraceto, L., & Santaella, C. (2021). Nanotechnology Potential in Seed Priming for Sustainable Agriculture. Nanomaterials, 11(2), 267. https://doi.org/10.3390/nano11020267