Effect of Substrates on Femtosecond Laser Pulse-Induced Reductive Sintering of Cobalt Oxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparataion of the Co3O4 Nanoparticle Ink
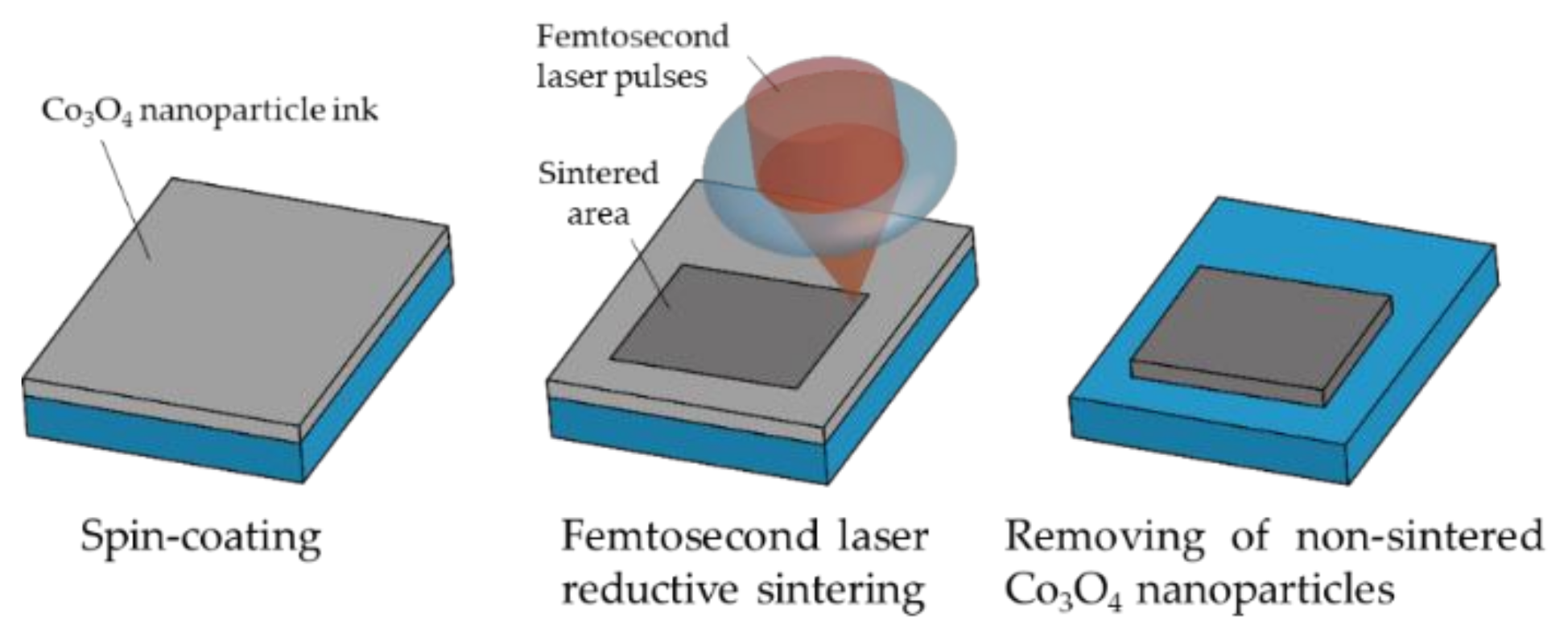
2.2. Femtosecond Laser Direct Writing Process
-> Co/CoO + 2C2H4O2 + H2O(g).
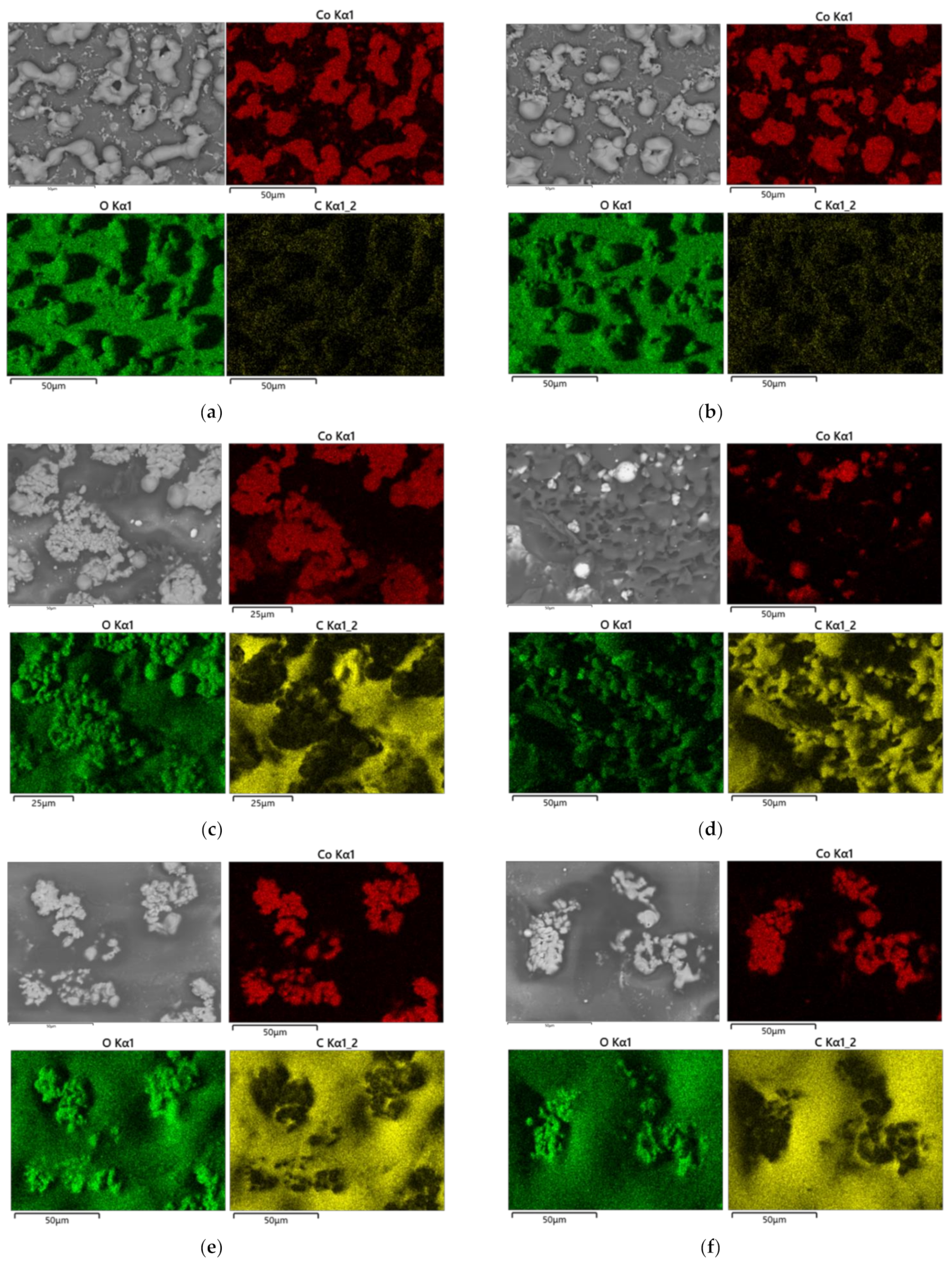
2.3. Evaluation of the Patterns and Substrates
3. Results and Discussion
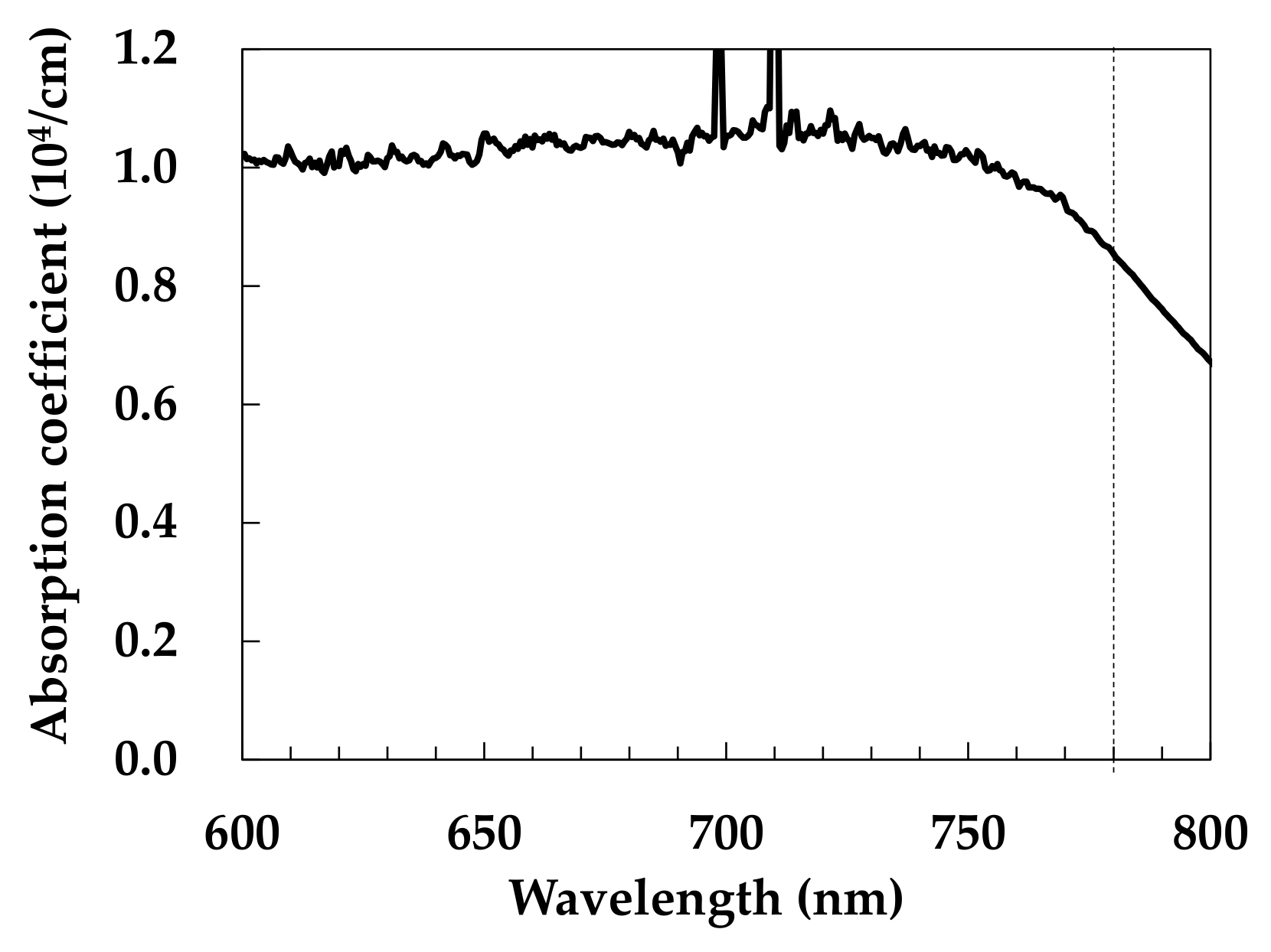
3.1. Absorption Properties of the Co3O4 Nanoparticle Ink
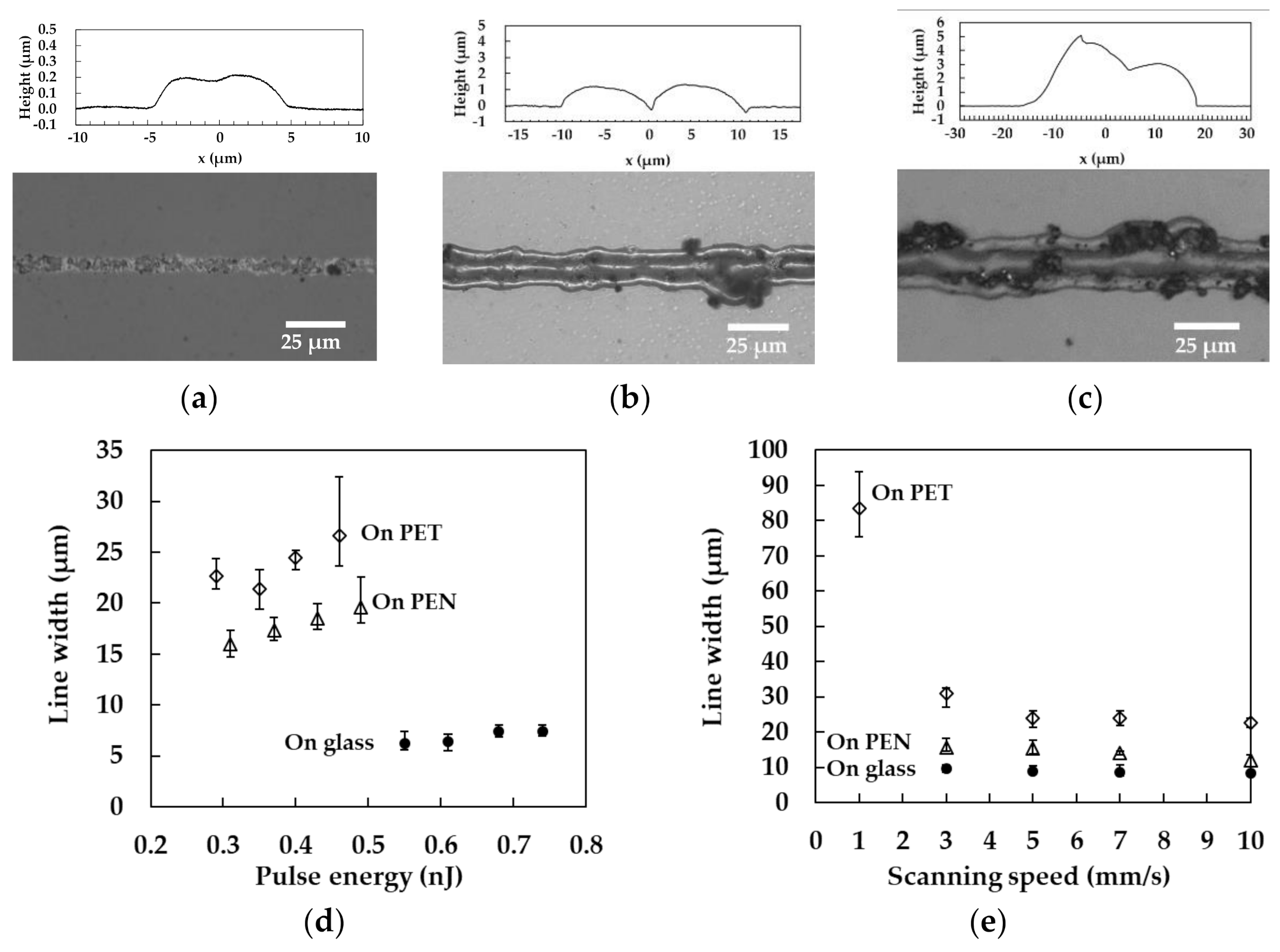
3.2. Line Width
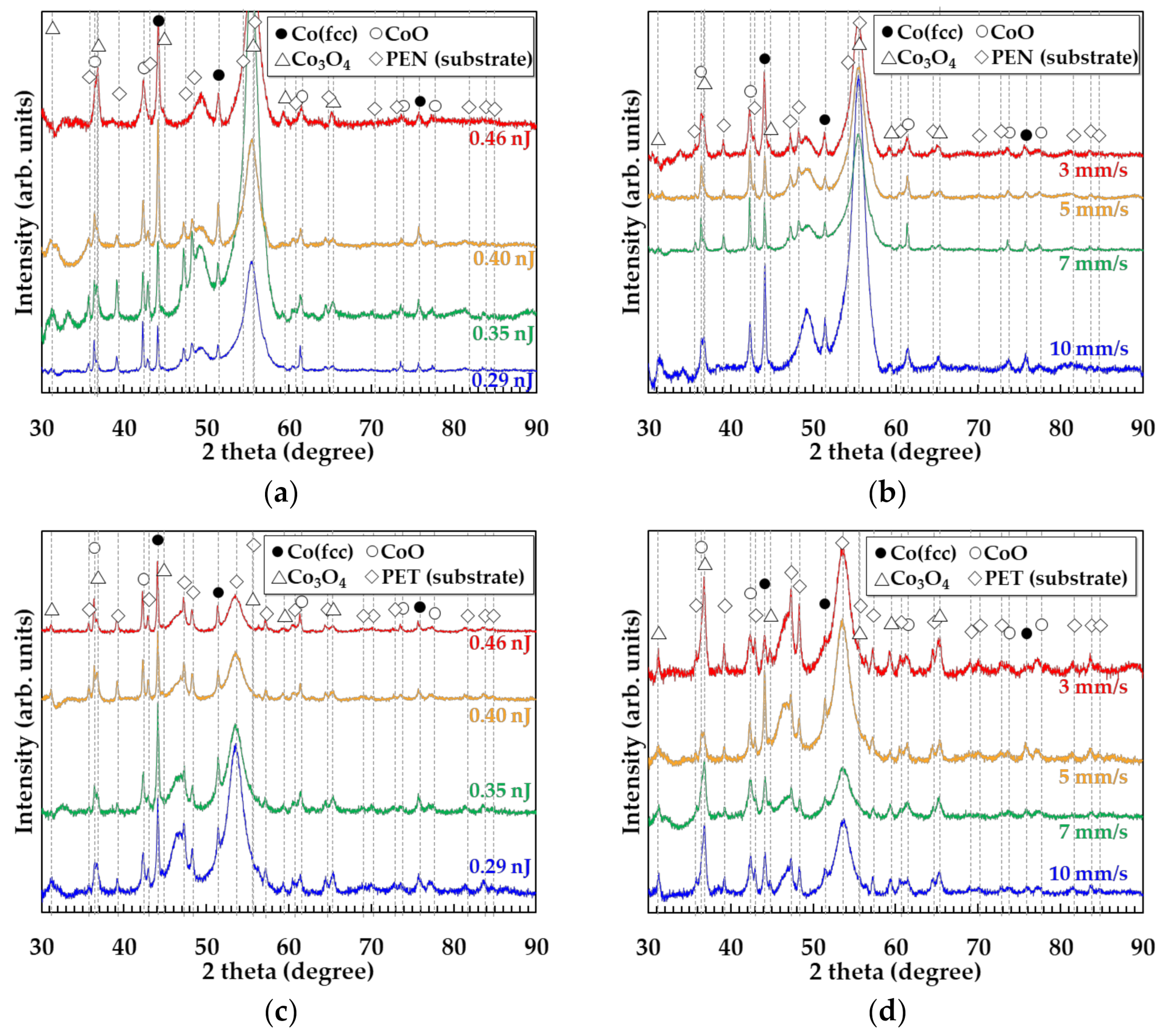
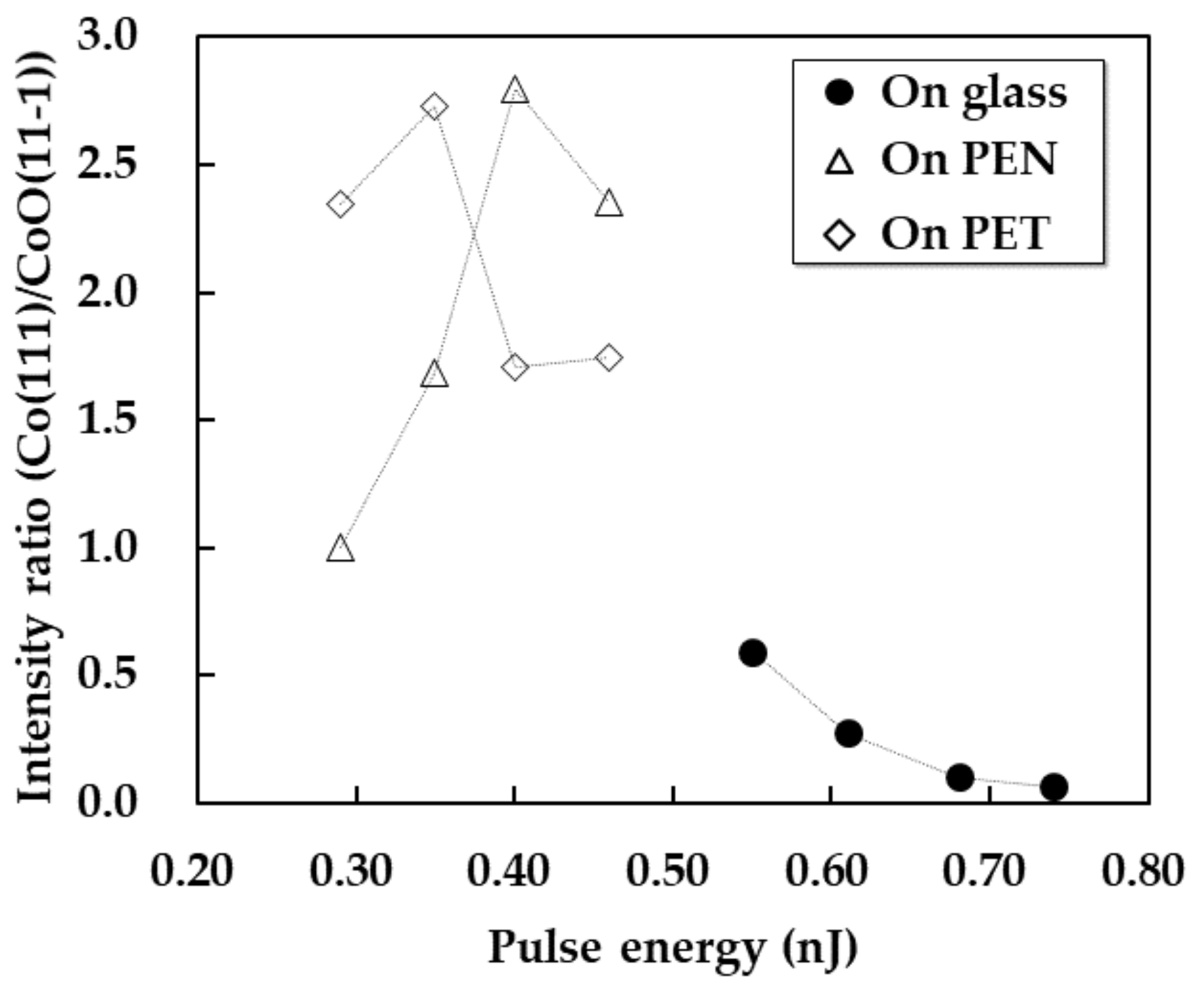
3.3. Crystal Structures of the Patterns on Various Substrates
(remained/re-oxidized) (in air).
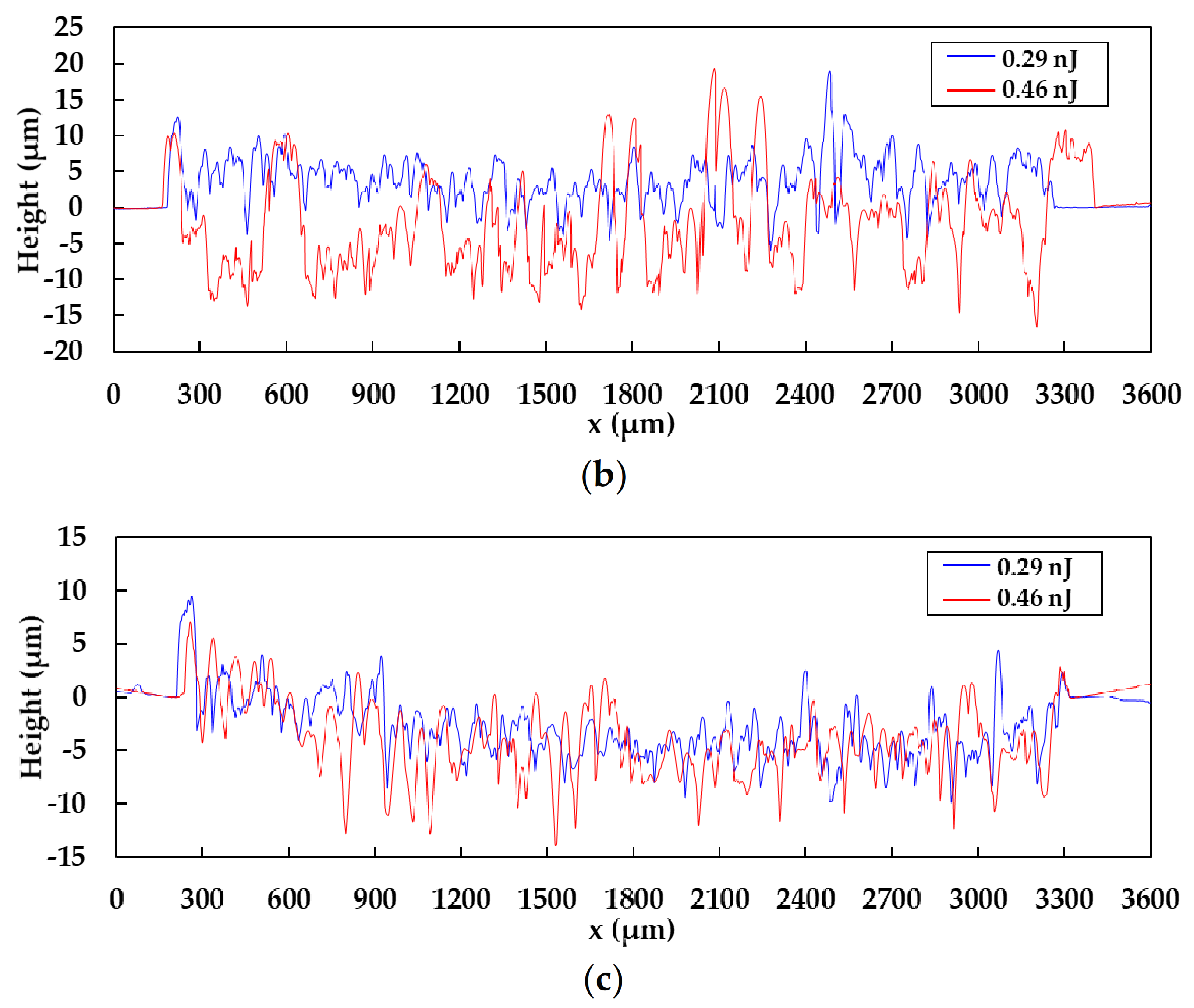
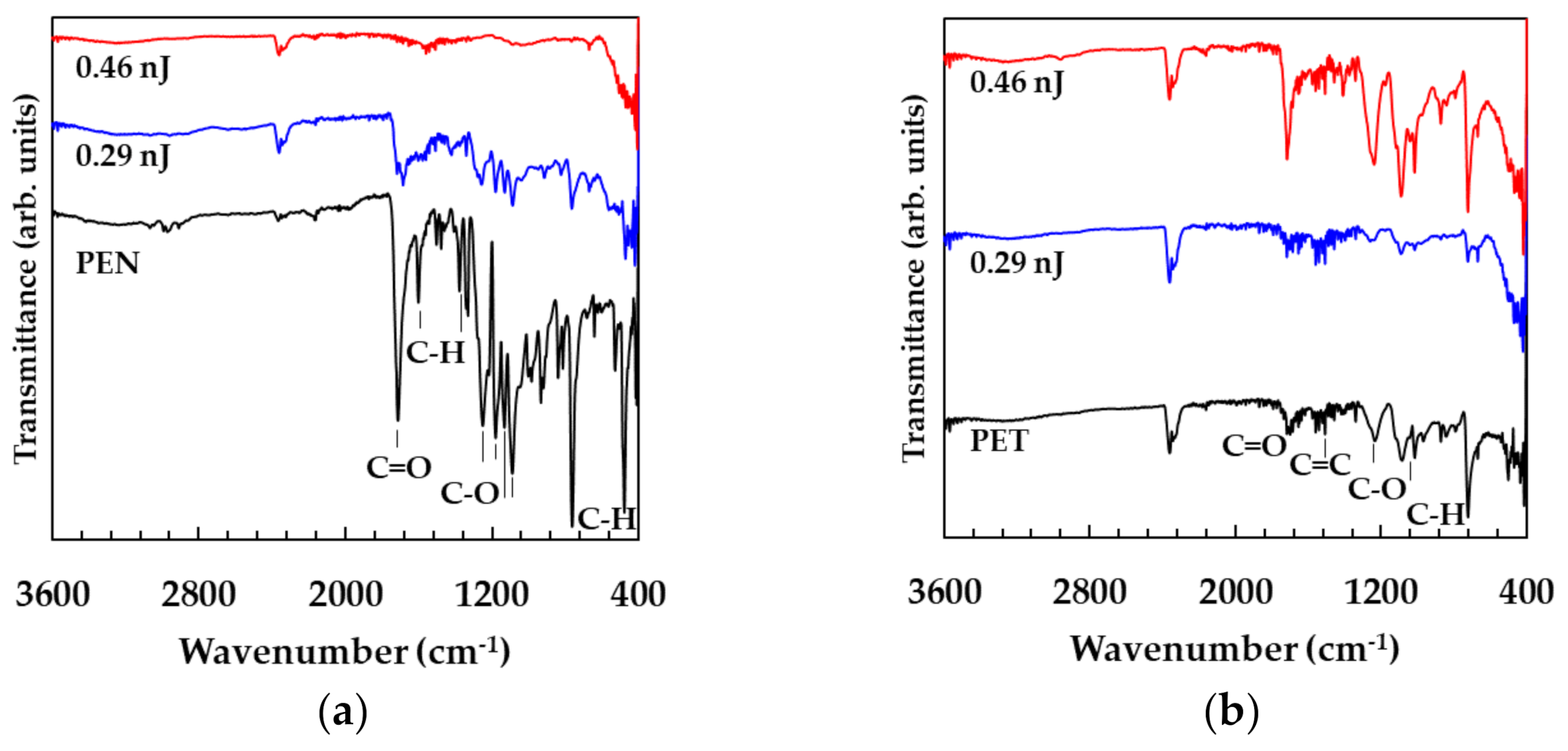
3.4. Damage of the Substrates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tumkin, I.I.; Khairullina, E.M.; Panov, M.S.; Yoshidomi, K.; Mizoshiri, M. Copper and nickel microsensors produced by selective laser reductive sintering for non-enzymatic glucose detection. Materials 2021, 14, 2493. [Google Scholar] [CrossRef] [PubMed]
- Zenou, M.; Ermak, O.; Saar, A.; Kotler, Z. Laser sintering of copper nanoparticles. J. Phys. D Appl. Phys. 2014, 47, 025501. [Google Scholar] [CrossRef]
- Soltani, A.; Khorramdel Vahed, B.; Mardoukhi, A.; Mantysalo, M. Laser sintering of copper nanoparticles on top of silicon substrates. Nanotechnology 2016, 27, 035203. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, H.S.; Ryu, J.; Thomas Hahn, H.; Jang, S.; Joung, J.W. Inkjet printed electronics using copper nanoparticle ink. J. Mater. Sci. Mater. Electron. 2010, 21, 1213–1220. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.; Han, S.; Kim, J.; Ko, S.; Yang, M. One-step fabrication of copper electrode by laser-induced direct local reduction and agglomeration of copper oxide nanoparticle. J. Phys. Chem. C 2011, 115, 23664–23670. [Google Scholar] [CrossRef]
- Lee, H.; Yang, M. Effect of solvent and PVP on electrode conductivity in laser-induced reduction process. Appl. Phys. A 2015, 119, 317–323. [Google Scholar] [CrossRef]
- Lee, D.; Paeng, D.; Park, H.K.; Grigoropoulos, C.P. Vacuum-free, maskless patterning of Ni electrodes by laser reductive sintering of NiO nanoparticle ink and its application to transparent conductors. ACS Nano 2014, 8, 9807–9814. [Google Scholar] [CrossRef] [PubMed]
- Paeng, D.; Lee, D.; Yeo, J.; Yoo, J.-H.; Allen, F.I.; Kim, E.; So, H.; Park, H.K.; Minor, A.M.; Grigoropoulos, C.P. Laser-induced reductive sintering of nickel oxide nanoparticles under ambient conditions. J. Phys. Chem. C 2015, 119, 6363–6372. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Arakane, S.; Sakurai, J.; Hata, S. Direct writing of Cu-based micro-temperature detectors using femtosecond laser reduction of CuO nanoparticles. Appl. Phys. Express 2016, 9, 036701. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Kondo, Y. Direct writing of Cu-based fine micropatterns using femtosecond laser pulse-induced sintering of Cu2O nanospheres. Jpn. J. Appl. Phys. 2019, 58, SDDF05. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Yoshidomi, K. Cu patterning using femtosecond laser reductive sintering of CuO nanoparticles under inert gas injection. Materials 2021, 14, 3285. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Hata, S. Selective fabrication of p-type and n-type thermoelectric micropatterns by the reduction of CuO/NiO mixed nanoparticles using femtosecond laser pulses. Appl. Phys. A 2017, 124, 64. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Nishitani, K.; Hata, S. Effect of heat accumulation on femtosecond laser reductive sintering of mixed CuO/NiO nanoparticles. Micromachines 2018, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; Gu, C.D.; Wang, X.L.; Tu, J.P. Correlation between microstructure and electrochemical behavior of the mesoporous Co3O4 sheet and its ionothermal synthesized hydrotalcite-like α-Co(OH)2 precursor. J. Phys. Chem. C 2013, 118, 911–923. [Google Scholar] [CrossRef]
- Yu, J.; Ni, Y.; Zhai, M. Highly selective non-enzyme glucose detection based on Co-CoO-Co3O4 nanocomposites prepared via a solution-combustion and subsequent heat-treating route. J. Alloys Compd. 2017, 723, 904–911. [Google Scholar] [CrossRef]
- Xu, J.M.; Cheng, J.P. The advances of Co3O4 as gas sensing materials: A review. J. Alloys Compd. 2016, 686, 753–768. [Google Scholar] [CrossRef]
- Smikhovskaia, A.V.; Panov, M.S.; Tumkin, I.I.; Khairullina, E.M.; Ermakov, S.S.; Balova, I.A.; Ryazantsev, M.N.; Kochemirovsky, V.A. In Situ laser-induced codeposition of copper and different metals for fabrication of microcomposite sensor-active materials. Anal. Chim. Acta 2018, 1044, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Smikhovskaia, A.V.; Andrianov, V.S.; Khairullina, E.M.; Lebedev, D.V.; Ryazantsev, M.N.; Panov, M.S.; Tumkin, I.I. In Situ laser-induced synthesis of copper-silver microcomposite for enzyme-free d-glucose and l-alanine sensing. Appl. Surf. Sci. 2019, 488, 531–536. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Ito, Y.; Arakane, S.; Sakurai, J.; Hata, S. Direct fabrication of Cu/Cu2O composite micro-temperature sensor using femtosecond laser reduction patterning. Jpn. J. Appl. Phys. 2016, 55, 06GP05. [Google Scholar] [CrossRef]
- Back, S.; Kang, B. Low-cost optical fabrication of flexible copper electrode via laser-induced reductive sintering and adhesive transfer. Opt. Lasers Eng. 2018, 101, 78–84. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Mikami, M.; Ozaki, K. Thermal-photovoltaic hybrid solar generator using thin-film thermoelectric modules. Jpn. J. Appl. Phys. 2012, 51, 06FL07. [Google Scholar] [CrossRef]
- Zacharatos, F.; Karvounis, P.; Theodorakos, I.; Hatziapostolou, A.; Zergioti, I. Single step laser transfer and laser curing of ag nanowires: A digital process for the fabrication of flexible and transparent microelectrodes. Materials 2018, 11, 1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.films.toray/en/technical/lumirror/lum_002.html (accessed on 10 October 2021).
- Pal, J.; Chauhan, P. Study of physical properties of cobalt oxide (Co3O4) nanocrystals. Mater. Charact. 2010, 61, 575–579. [Google Scholar] [CrossRef]
- Makhlouf, S.A.; Bakr, Z.H.; Aly, K.I.; Moustafa, M.S. Structural, electrical and optical properties of Co3O4 nanoparticles. Superlattices Microstruct. 2013, 64, 107–117. [Google Scholar] [CrossRef]
- Chen, M.; Hallstedt, B.; Ludwig, J.G. Thermodynamic assessment of the Co-O system. J. Phase Equilibria 2003, 24, 212–227. [Google Scholar] [CrossRef]
- Ha, N.P.; Ohishi, T.; Mizoshiri, M. Direct writing of Cu patterns on polydimethylsiloxane substrates using femtosecond laser pulse-induced reduction of glyoxylic acid copper complex. Micromachines 2021, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Mizoshiri, M.; Aoyama, K.; Uetsuki, A.; Ohishi, T. Direct writing of copper micropatterns using near-infrared femtosecond laser-pulse-induced reduction of glyoxylic acid copper complex. Micromachines 2019, 10, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbull, L.; Liggat, J.J.; MacDonald, W.A. Thermal degradation chemistry of poly(ethylene naphthalate)—A study by thermal volatilisation analysis. Polym. Degrad. Stab. 2013, 98, 2244–2258. [Google Scholar] [CrossRef] [Green Version]
- Khairullina, E.M.; Panov, M.S.; Andriianov, V.S.; Ratautas, K.; Tumkin, I.I.; Račiukaitis, G. High rate fabrication of copper and copper–gold electrodes by laser-induced selective electroless plating for enzyme-free glucose sensing. RSC Adv. 2021, 11, 19521–19530. [Google Scholar] [CrossRef]



| Thickness (mm) | Thermal Conductivity (W/K·m) | Heat Capacity (J/K·kg) | Density (kg/m3) | Glass Transition Temperature (°C) | Melting Point (°C) | Ref. | |
|---|---|---|---|---|---|---|---|
| Glass | 1 | 1.38 | 703 | 2203 | ~1700 | >1700 | [21] |
| PEN (Teonex, Teijin) | ~0.25 | ~0.2 | 0.87 | ~1330 | 121 | 269 | [22] |
| PET (Lumirror, Toray) | ~0.25 | ~0.14 | 0.32 | ~1400 | 155 | 263 | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizoshiri, M.; Yoshidomi, K.; Darkhanbaatar, N.; Khairullina, E.M.; Tumkin, I.I. Effect of Substrates on Femtosecond Laser Pulse-Induced Reductive Sintering of Cobalt Oxide Nanoparticles. Nanomaterials 2021, 11, 3356. https://doi.org/10.3390/nano11123356
Mizoshiri M, Yoshidomi K, Darkhanbaatar N, Khairullina EM, Tumkin II. Effect of Substrates on Femtosecond Laser Pulse-Induced Reductive Sintering of Cobalt Oxide Nanoparticles. Nanomaterials. 2021; 11(12):3356. https://doi.org/10.3390/nano11123356
Chicago/Turabian StyleMizoshiri, Mizue, Kyohei Yoshidomi, Namsrai Darkhanbaatar, Evgenia M. Khairullina, and Ilya I. Tumkin. 2021. "Effect of Substrates on Femtosecond Laser Pulse-Induced Reductive Sintering of Cobalt Oxide Nanoparticles" Nanomaterials 11, no. 12: 3356. https://doi.org/10.3390/nano11123356
APA StyleMizoshiri, M., Yoshidomi, K., Darkhanbaatar, N., Khairullina, E. M., & Tumkin, I. I. (2021). Effect of Substrates on Femtosecond Laser Pulse-Induced Reductive Sintering of Cobalt Oxide Nanoparticles. Nanomaterials, 11(12), 3356. https://doi.org/10.3390/nano11123356







