Constructing Co3O4/g-C3N4 Ultra-Thin Nanosheets with Z-Scheme Charge Transfer Pathway for Efficient Photocatalytic Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of g-C3N4
2.3. Synthesis of the Ultra-Thin Co3O4/g-C3N4 Catalysts
2.4. Characterization
2.5. Photocatalytic Activity
3. Results and Discussion
3.1. XRD Analysis
3.2. EA and ICP
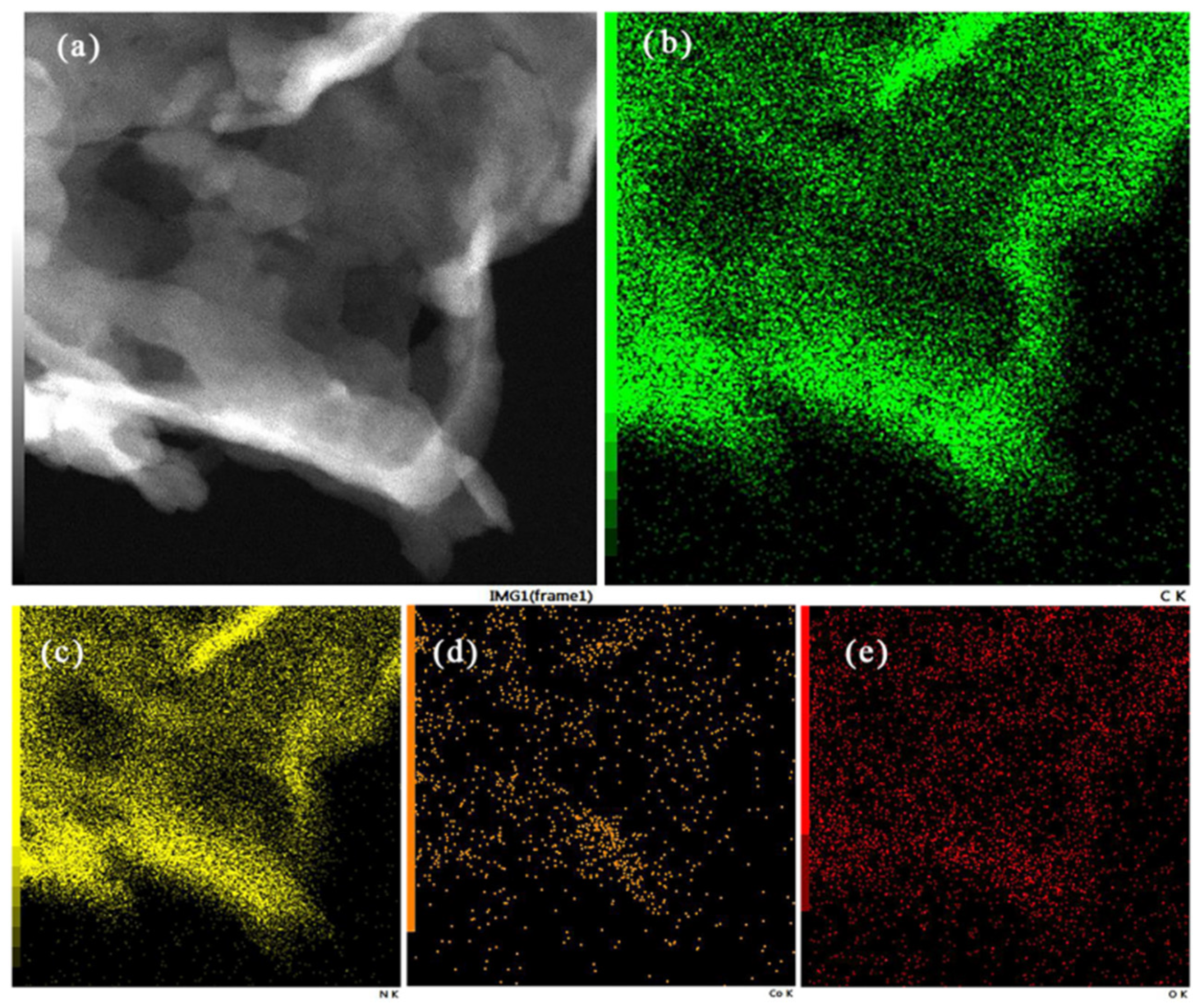
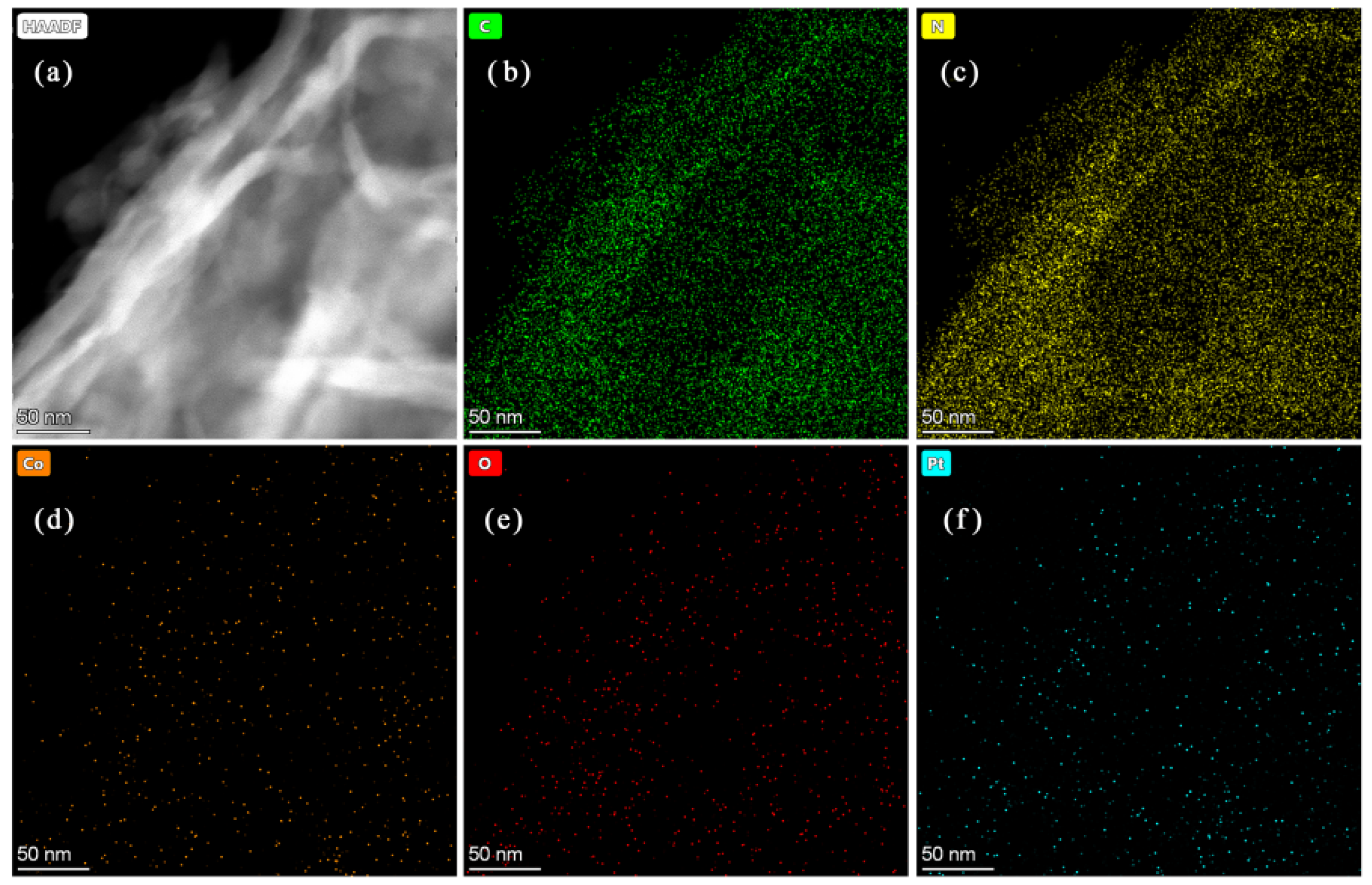
3.3. Morphology Analysis
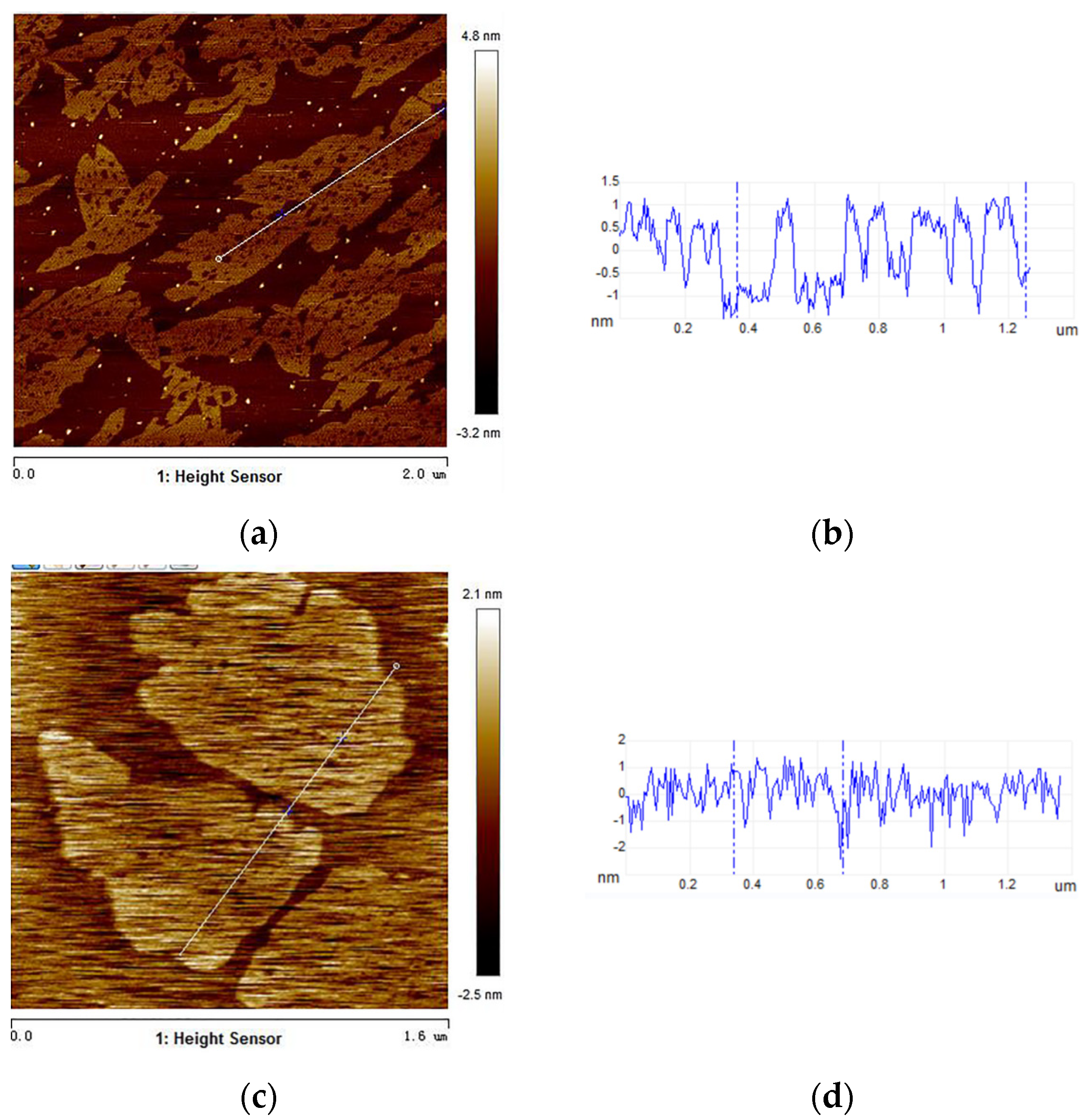
3.4. AFM Analysis
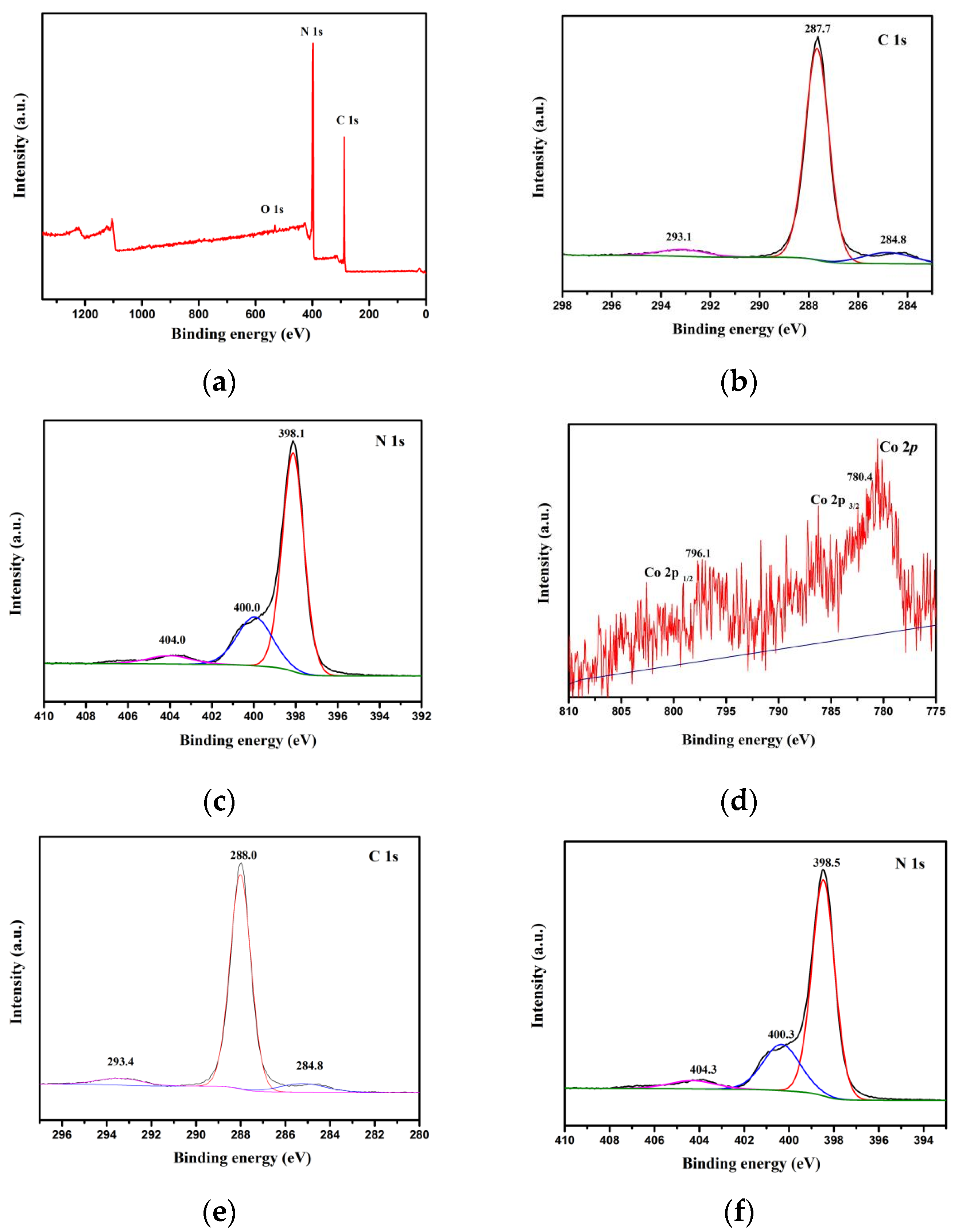
3.5. XPS Analysis
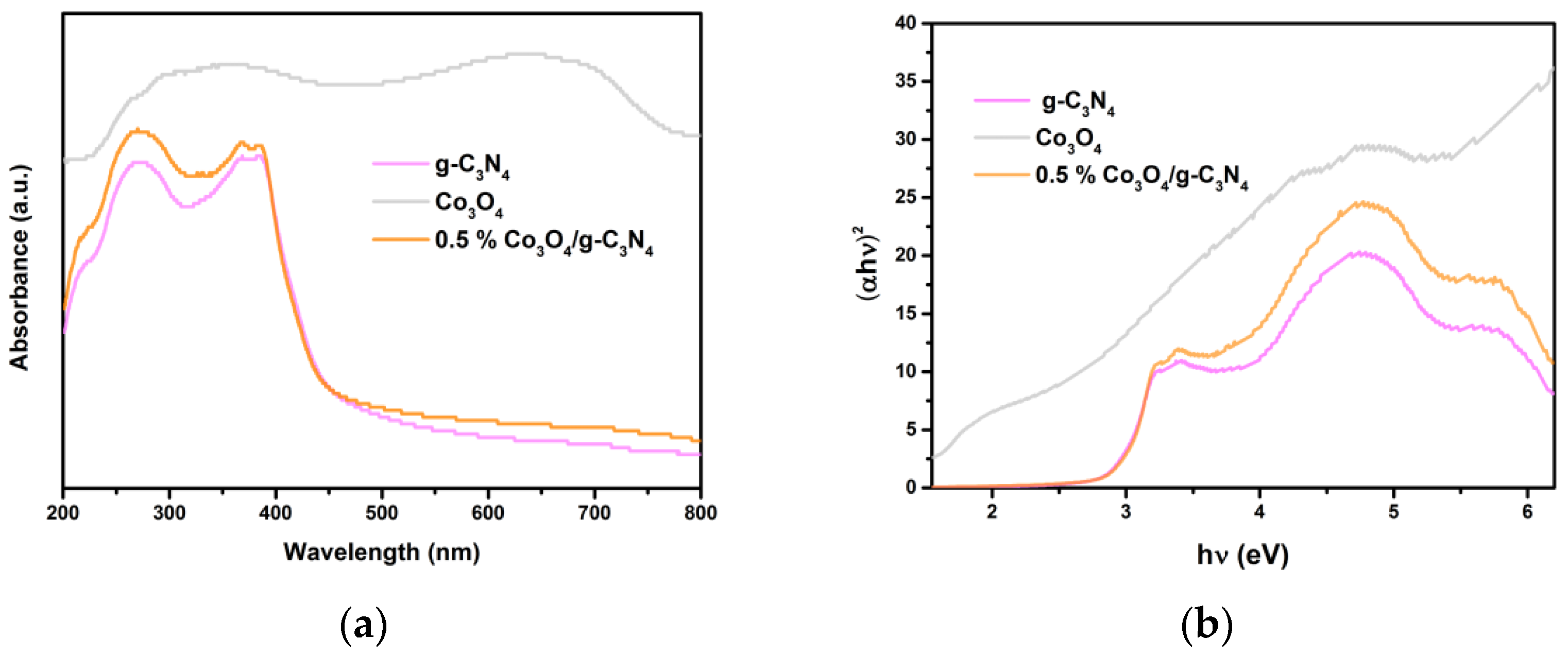
3.6. UV–Vis DRS Analysis
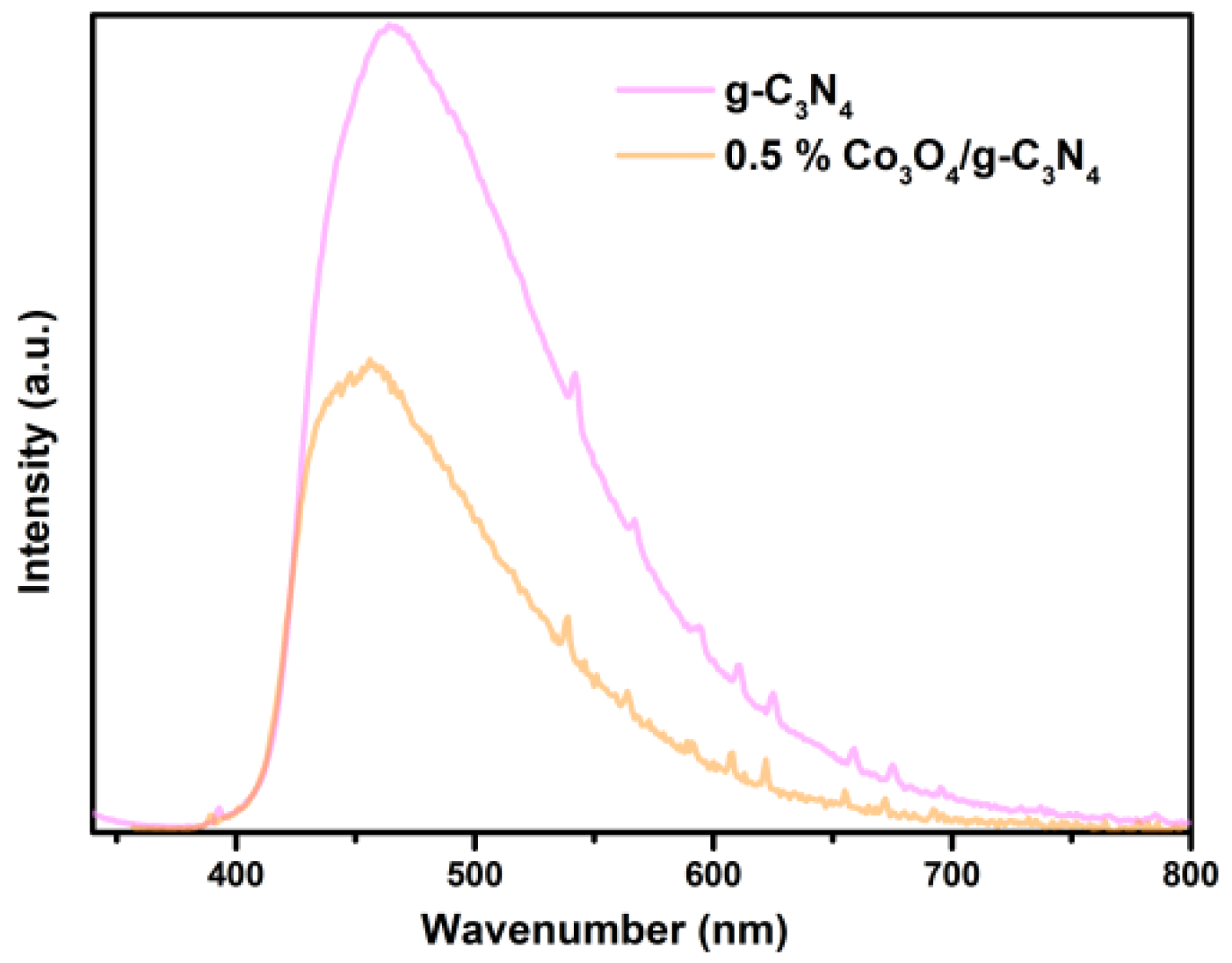
3.7. PL
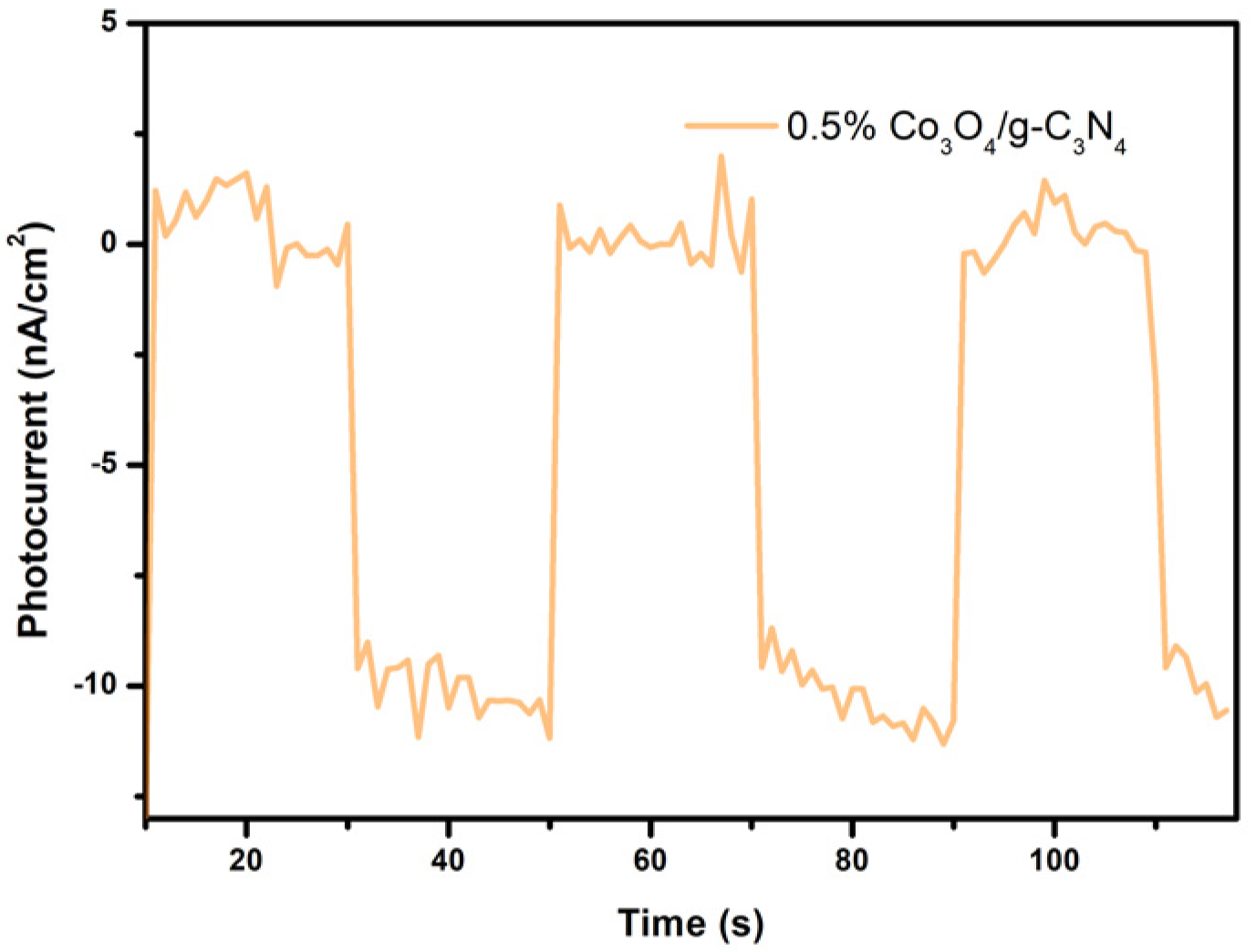
3.8. Photoelectrochemical Analysis
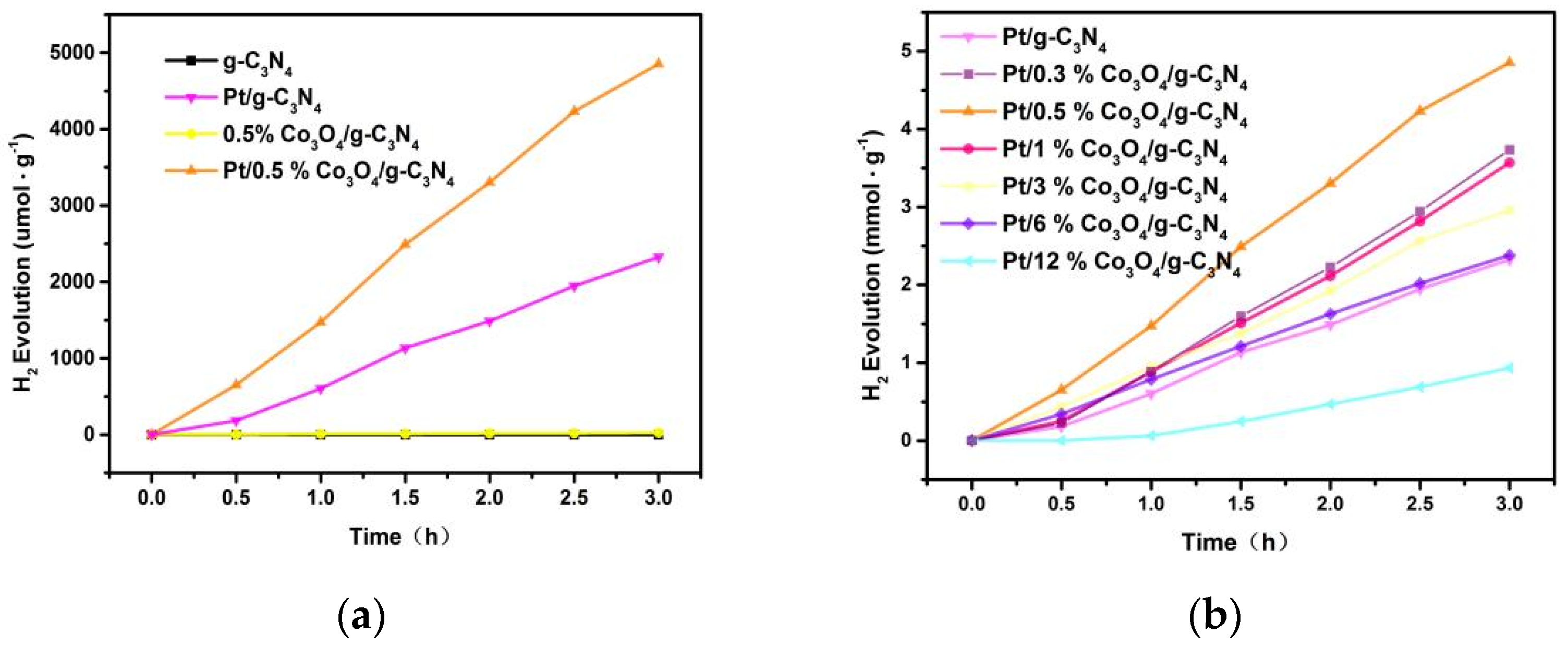
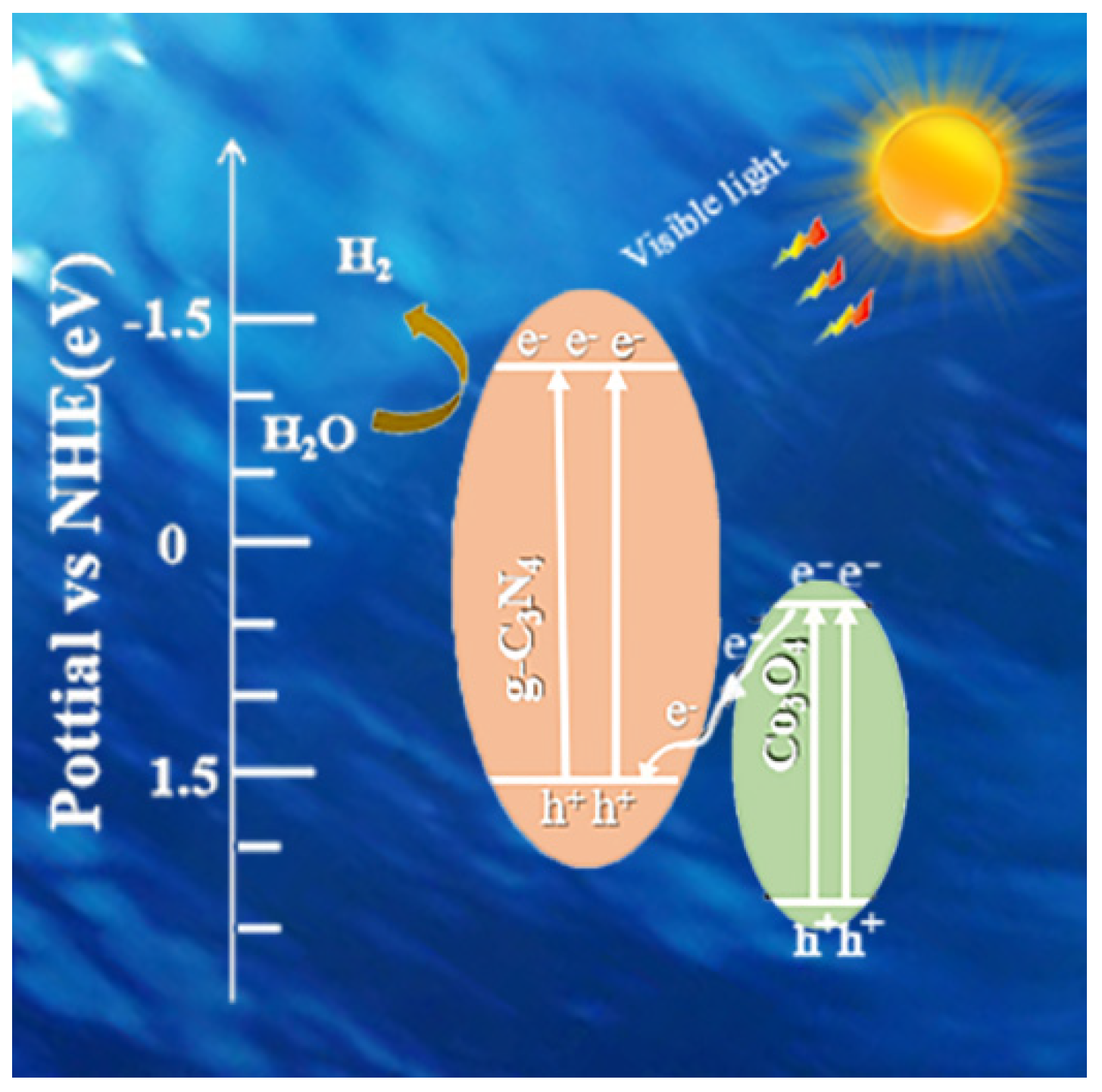
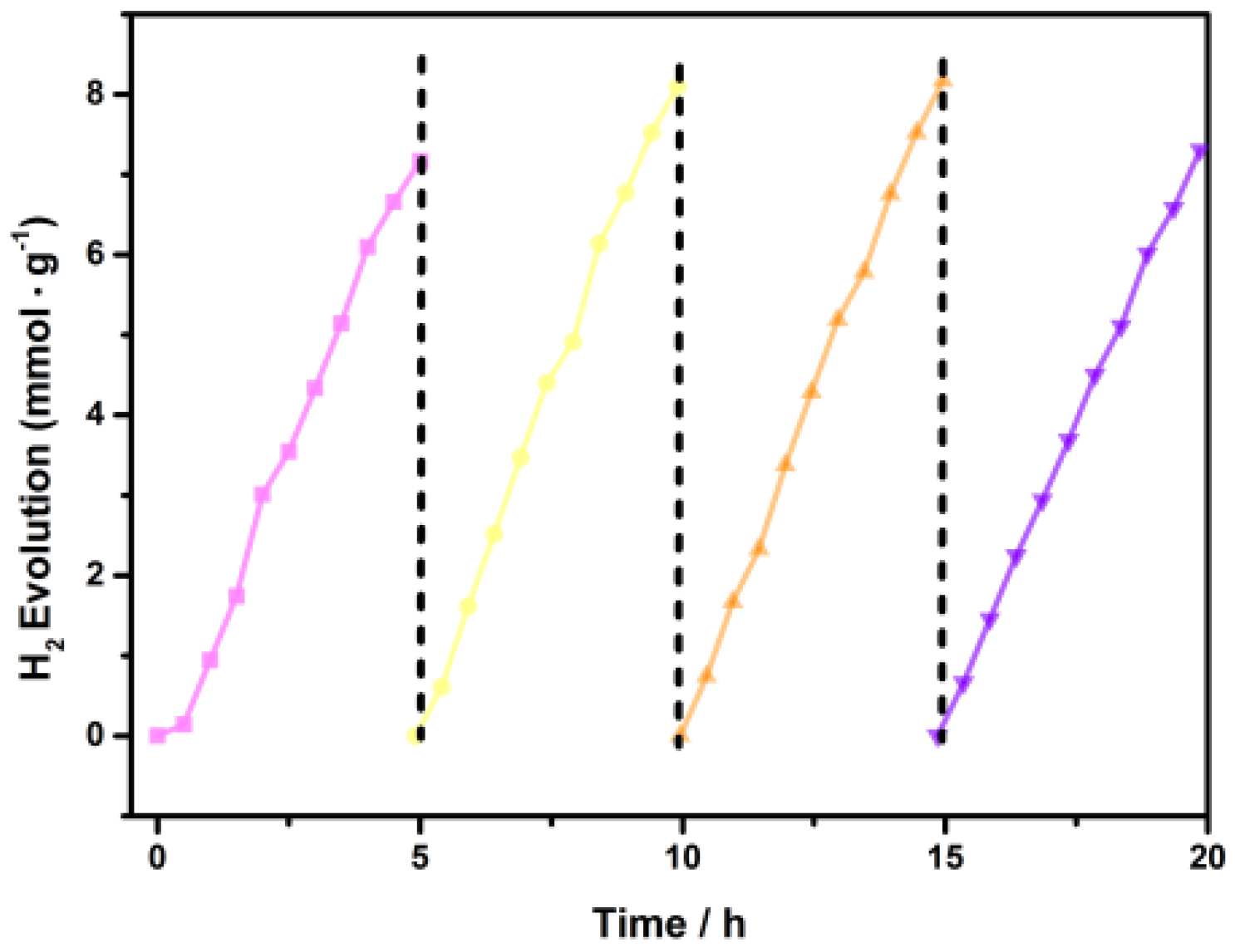
3.9. Photocatalytic Activity and Cycling Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Z.F.; Song, J.; Wang, X.; Pan, L.; Li, K.; Zhang, X.W.; Wang, L.; Zou, J.J. Switching charge transfer of C3N4/W18O49 from type-II to Z-scheme by interfacial band bending for highly efficient photocatalytic hydrogen evolution. Nano Energy 2017, 40, 308–316. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Jia, Q.; Tokudome, H.; Zhong, M.; Wang, C.Z.; Pan, Z.H.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef]
- Tan, M.; Yu, C.; Li, J.; Li, Y.; Tao, C.D.; Liu, C.B.; Meng, H.M.; Su, Y.J.; Qiao, L.J.; Bai, Y. Engineering of g-C3N4-based photocatalysts to enhance hydrogen evolution. Adv. Colloid Interface Sci. 2021, 295, 102488. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xi, X.; Gong, C.; Zhang, X.L.; Fan, G.L. Open porous BiVO4 nanomaterials: Electronspinning fabrication and enhanced visible light photocatalytic activity. Mater. Res. Bull. 2016, 74, 258–264. [Google Scholar] [CrossRef]
- Su, T.; Hood, Z.D.; Naguib, M.; Bai, L.; Luo, S.; Rouleau, C.M.; Ivanov, I.N.; Ji, H.B.; Qin, Z.Z.; Wu, Z.L. 2D/2D heterojunction of Ti3C2/g-C3N4 nanosheets for enhanced photocatalytic hydrogen evolution. Nanoscale 2019, 11, 8138–8149. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Piao, L.; Chen, X. Emerging photocatalysts for hydrogen evolution. Trends Chem. 2020, 2, 57–70. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Meng, F.Y.; Ma, C.C.; Yan, Y.S.; Meng, M.J. Nitrogen-doped hydrogenated TiO2 modified with CdS nanorods with enhanced optical absorption, charge separation and photocatalytic hydrogen evolution. Chem. Eng. J. 2020, 384, 123275. [Google Scholar] [CrossRef]
- Chen, J.; Shen, Z.; Lv, S.; Shen, K.; Wu, R.F.; Jiang, X.F.; Fan, T.; Chen, J.Y.; Li, Y.W. Fabricating sandwich-shelled ZnCdS/ZnO/ZnCdS dodecahedral cages with “one stone” as Z-scheme photocatalysts for highly efficient hydrogen production. J. Mater. Chem. A 2018, 6, 19631–19642. [Google Scholar] [CrossRef]
- Zhou, H.; Qu, Y.; Zeid, T.; Duan, X.F. Towards highly efficient photocatalysts using semiconductor nanoarchitectures. Energy Environ. Sci. 2012, 5, 6732–6743. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate photocatalysts for light-driven water splitting: Mechanisms, challenges, and design strategies. Chem. Rev. 2019, 120, 919–985. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L.Q. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, S.H.; Li, J.; Zheng, X.C.; Guan, X.X. Constructing of 3D graphene aerogel-g-C3N4 metal-free heterojunctions with superior purification efficiency for organic dyes. J. Mol. Liq. 2020, 310, 113242. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, Y.; Yuan, X.; Zou, D.S.; Fang, J.; Jiang, L.B.; Zhang, J.; Yang, H.L.; Xiao, Z.H. MXene Ti3C2 derived Z–scheme photocatalyst of graphene layers anchored TiO2/g-C3N4 for visible light photocatalytic degradation of refractory organic pollutants. Chem. Eng. J. 2020, 394, 124921. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.K.; Yu, J.G. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ. 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, S.L.; Yan, W. Tin diselinide a stable co-catalyst coupled with branched TiO2 fiber and g-C3N4 quantum dots for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 270, 118900. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Wu, Q.; Du, P.W.; Zhu, J.; Dai, S.Y.; Yang, S.F. Incorporating graphitic carbon nitride (g-C3N4) quantum dots into bulk-heterojunction polymer solar cells leads to efficiency enhancement. Adv. Funct. Mater. 2016, 26, 1719–1728. [Google Scholar] [CrossRef]
- Mo, Z.; Zhu, X.; Jiang, Z.; Song, Y.H.; Liu, D.B.; Li, H.P.; Yang, X.F.; She, Y.B.; Lei, Y.C.; Yuan, S.Q.; et al. Porous nitrogen-rich g-C3N4 nanotubes for efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. 2019, 256, 117854. [Google Scholar] [CrossRef]
- Liu, G.; Liao, M.; Zhang, Z.; Wang, H.Y.; Chen, D.H.; Feng, Y.J. Enhanced photodegradation performance of Rhodamine B with g-C3N4 modified by carbon nanotubes. Sep. Purif. Technol. 2020, 244, 116618. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Kandula, S.; Shanker, V. Fe-doped and-mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J. Mater. Chem. A 2014, 2, 6772–6780. [Google Scholar] [CrossRef]
- Lu, X.; Xu, K.; Chen, P.; Jia, K.C.; Liu, S.; Wu, C.Z. Facile one step method realizing scalable production of g-C3N4 nanosheets and study of their photocatalytic H2 evolution activity. J. Mater. Chem. A 2014, 2, 18924–18928. [Google Scholar] [CrossRef]
- Meng, N.; Ren, J.; Liu, Y.; Huang, Y.; Petit, T.; Zhang, B. Engineering oxygen-containing and amino groups into two-dimensional atomically-thin porous polymeric carbon nitrogen for enhanced photocatalytic hydrogen production. Energy Environ. Sci. 2018, 11, 566–571. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Wang, T.; Zhang, H.; Meng, X.G.; Hao, D.; Chang, K.; Li, P.; Kako, T.; Ye, J.H. Nature-inspired environmental “phosphorylation” boosts photocatalytic H2 production over carbon nitride nanosheets under visible-light irradiation. Angew. Chem. 2015, 127, 13765–13769. [Google Scholar] [CrossRef]
- Wu, M.; He, X.; Jing, B.; Wang, T.; Wang, C.Y.; Qin, Y.L.; Ao, Z.M.; Wang, S.B.; An, T.C. Novel carbon and defects co-modified g-C3N4 for highly efficient photocatalytic degradation of bisphenol A under visible light. J. Hazard. Mater. 2020, 384, 121323. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Chen, G.; Yu, Y.; Hao, S.; Zhou, Y.S.; Zheng, Y. Facile approach to synthesize g-PAN/g-C3N4 composites with enhanced photocatalytic H2 evolution activity. ACS Appl. Mater. Interfaces 2014, 6, 7171–7179. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xiao, M.; Yu, S.; Wu, H.H.; Wang, Y.; Sun, J.X.; Chen, G.; Li, C.M. Insight into the Activity and Stability of RhxP Nano-Species Supported on g-C3N4 for Photocatalytic H2 Production. ACS Catal. 2019, 10, 458–462. [Google Scholar] [CrossRef]
- Yan, Q.; Xie, X.; Liu, Y.; Wang, S.B.; Zhang, M.H.; Chen, Y.Y.; Si, Y.S. Constructing a new Z-scheme multi-heterojunction photocataslyts Ag-AgI/BiOI-Bi2O3 with enhanced photocatalytic activity. J. Hazard. Mater. 2019, 371, 304–315. [Google Scholar] [CrossRef]
- Qu, J.; Wang, X.; Zhang, Y.; Yuan, G.L. Multifunctional Ag nanoparticles in heterostructured Ag2MoO4/Ag/AgBr cubes with boosted photocatalytic performances. Sol. Energy 2018, 170, 124–131. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Gui, P.; Zheng, L.N.; Liang, J.S.; Xue, G. Hydrothermal synthesis of sandwich interspersed LaCO3OH/Co3O4/graphene oxide composite and the enhanced catalytic performance for methane combustion. Catal. Today 2019, 327, 134–142. [Google Scholar] [CrossRef]
- Jin, C.; Wang, M.; Li, Z.; Kang, J.; Zhao, Y.; Han, J.; Wu, Z.M. Two dimensional Co3O4/g-C3N4 Z-scheme heterojunction: Mechanism insight into enhanced peroxymonosulfate-mediated visible light photocatalytic performance. Chem. Eng. J. 2020, 398, 125569. [Google Scholar] [CrossRef]
- Gao, H.; Yang, H.; Xu, J.; Zhang, S.W.; Li, J.X. Strongly coupled g-C3N4 nanosheets-Co3O4 quantum dots as 2D/0D heterostructure composite for peroxymonosulfate activation. Small 2018, 14, 1801353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Grzelczak, M.; Hou, Y.; Maeda, K.; Domen, K.; Fu, X.Z.; Antonietti, M.; Wang, X.C. Photocatalytic oxidation of water by polymeric carbon nitride nanohybrids made of sustainable elements. Chem. Sci. 2012, 3, 443–446. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.A.; Wang, X. Surface engineering of graphitic carbon nitride polymers with cocatalysts for photocatalytic overall water splitting. Chem. Sci. 2017, 8, 5261–5274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Liu, Y.; Li, Y.; Cao, Y.H.; Huang, J.N.; Wang, H.J.; Yu, H.; Liang, H.; Peng, F. Dual functional CuO1–x clusters for enhanced photocatalytic activity and stability of a Pt cocatalyst in an overall water-splitting reaction. ACS Sustain. Chem. Eng. 2018, 6, 17340–17351. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Peng, S.; Cao, Y.; Zhou, F.; Xu, Z.D.; Li, Y.X. CoP decorated with Co3O4 as a cocatalyst for enhanced photocatalytic hydrogen evolution via dye sensitization. Appl. Surf. Sci. 2019, 487, 315–321. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Wang, H.; Wang, H.; Zhang, J.J.; Pan, B.C.; Xie, Y. Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, T.; Li, C.; Huang, Z.Q.; Luo, Z.B.; Gong, J.L. Adjusting the reduction potential of electrons by quantum confinement for selective photoreduction of CO2 to methanol. Angew. Chem. Int. Ed. 2019, 58, 3804–3808. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Qiu, P.; Chen, H.; Jiang, F. Cobalt modified mesoporous graphitic carbon nitride with enhanced visible-light photocatalytic activity. RSC Adv. 2014, 4, 39969–39977. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, Z.; Fu, X.; Wang, X.C. Construction of conjugated carbon nitride nanoarchitectures in solution at low temperatures for photoredox catalysis. Angew. Chem. Int. Ed. 2012, 51, 11814–11818. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Si, X.; Yu, J.; Bai, H.Y.; Zhang, X.H. Doping nano-Co3O4 surface with bigger nanosized Ag and its photocatalytic properties for visible light photodegradation of organic dyes. Appl. Surf. Sci. 2015, 330, 191–199. [Google Scholar] [CrossRef]
- Chen, G.L.; Guyon, C.; Zhang, Z.X.; Ognier, S.; Beem, J.; Tatoulian, M. The different structure characteristics of nanosized Co3O4 film crystallized by the annealing and plasma techniques. Mater. Lett. 2013, 107, 111–114. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhu, C.; Zuo, G.; Guo, Y.; Xiao, W.; Dai, Y.X.; Kong, J.J.; Xu, X.M.; Zhou, Y.X.; Xie, A.M.; et al. 0D/2D Co3O4/TiO2 Z-Scheme heterojunction for boosted photocatalytic degradation and mechanism investigation. Appl. Catal. B Environ. 2020, 278, 119298. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Zhang, J.; Wang, R.; Liu, H.L. Rational construction of a direct Z-scheme g-C3N4/CdS photocatalyst with enhanced visible light photocatalytic activity and degradation of erythromycin and tetracycline. Appl. Surf. Sci. 2019, 478, 1056–1064. [Google Scholar] [CrossRef]
- Chen, M.; Wu, J.; Lu, C.; Luo, X.; Huang, Y.Q.; Jin, B.; Gao, H.X.; Zhang, X.W.; Argyle, M.; Liang, Z.W. Photoreduction of CO2 in the presence of CH4 over g-C3N4 modified with TiO2 nanoparticles at room temperature. Green Energy Environ. 2020, 6, 938–951. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Zhang, P.; Zhang, J.J.; Li, A.; Gong, J.L. Enhanced surface reaction kinetics and charge separation of p–n heterojunction Co3O4/BiVO4 photoanodes. J. Am. Chem. Soc. 2015, 137, 8356–8359. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, Y.; Tian, J.; Song, X.J.; Zhang, X.J.; Wang, X.Z.; Cui, H.Z. Silver oxide decorated graphitic carbon nitride for the realization of photocatalytic degradation over the full solar spectrum: From UV to NIR region. Sol. Energy Mater. Sol. Cells 2017, 168, 100–111. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Liu, W.; Duan, W.; Wang, S.; Jia, L.; Zhang, G.; Zhu, B.; Huang, W.; Zhang, S. Constructing Co3O4/g-C3N4 Ultra-Thin Nanosheets with Z-Scheme Charge Transfer Pathway for Efficient Photocatalytic Water Splitting. Nanomaterials 2021, 11, 3341. https://doi.org/10.3390/nano11123341
Guo Y, Liu W, Duan W, Wang S, Jia L, Zhang G, Zhu B, Huang W, Zhang S. Constructing Co3O4/g-C3N4 Ultra-Thin Nanosheets with Z-Scheme Charge Transfer Pathway for Efficient Photocatalytic Water Splitting. Nanomaterials. 2021; 11(12):3341. https://doi.org/10.3390/nano11123341
Chicago/Turabian StyleGuo, Yuan, Wanqing Liu, Wei Duan, Siyu Wang, Liqun Jia, Guoqing Zhang, Baolin Zhu, Weiping Huang, and Shoumin Zhang. 2021. "Constructing Co3O4/g-C3N4 Ultra-Thin Nanosheets with Z-Scheme Charge Transfer Pathway for Efficient Photocatalytic Water Splitting" Nanomaterials 11, no. 12: 3341. https://doi.org/10.3390/nano11123341
APA StyleGuo, Y., Liu, W., Duan, W., Wang, S., Jia, L., Zhang, G., Zhu, B., Huang, W., & Zhang, S. (2021). Constructing Co3O4/g-C3N4 Ultra-Thin Nanosheets with Z-Scheme Charge Transfer Pathway for Efficient Photocatalytic Water Splitting. Nanomaterials, 11(12), 3341. https://doi.org/10.3390/nano11123341






