Bactericidal Activity of Multilayered Hybrid Structures Comprising Titania Nanoparticles and CdSe Quantum Dots under Visible Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Titania NPs and CdSe QDs
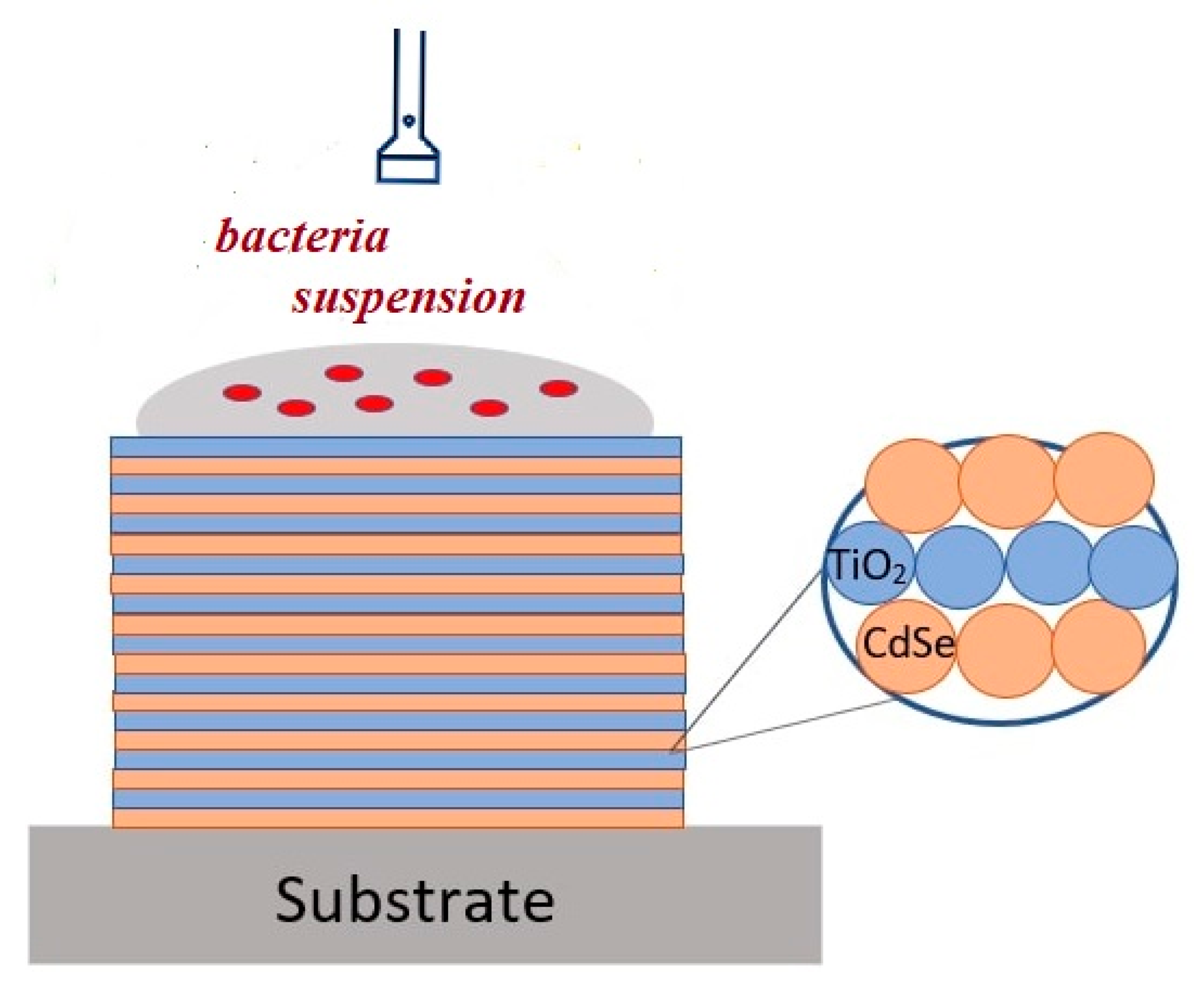
2.2. Formation of Titania NP/CdSe QD Multilayered Hybrid Structures
2.3. Study of ROS Generation by the Hybrid Structures
2.4. Experiments with Bacteria
3. Results and Discussions
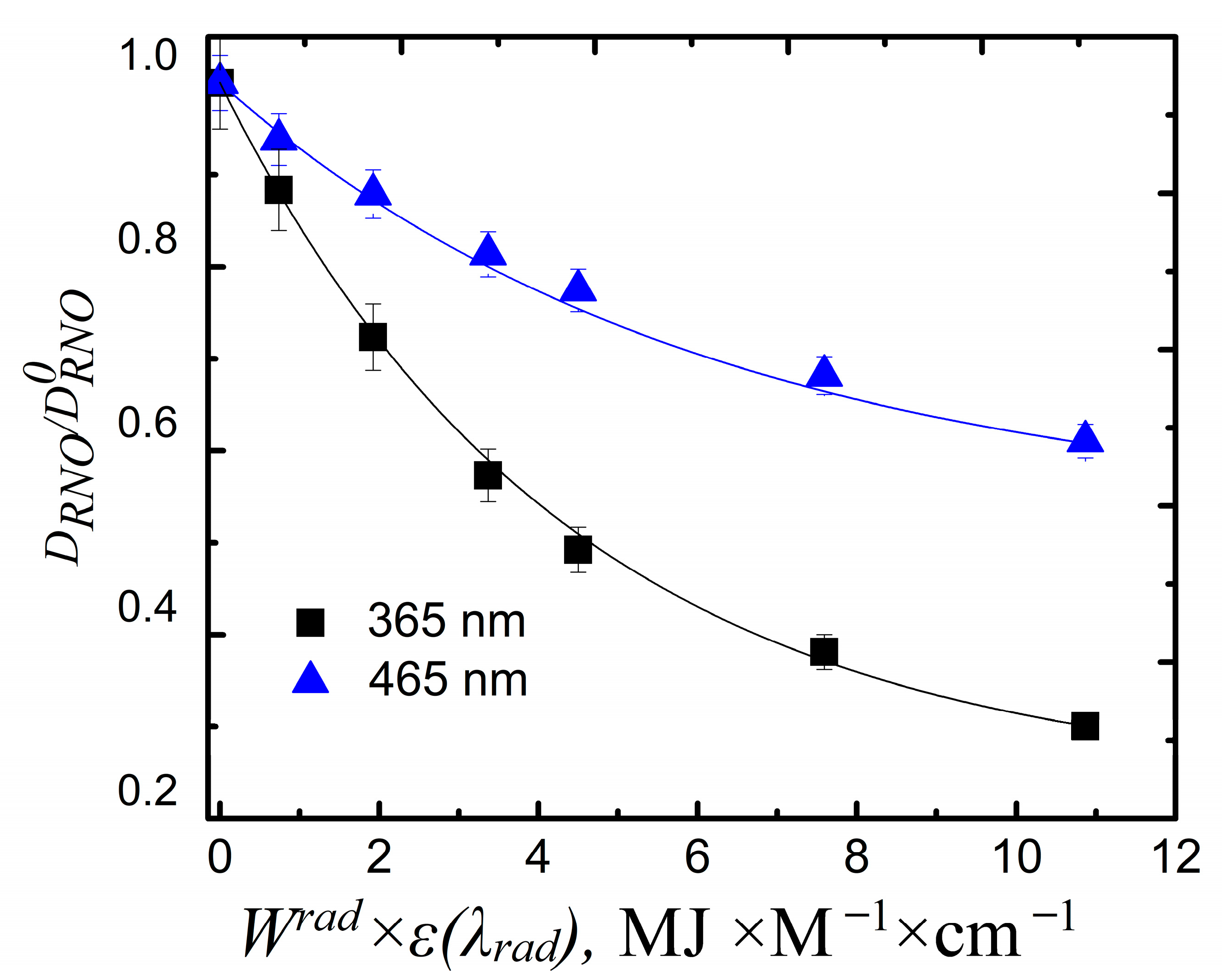
3.1. Photophysical Properties of Multilayered Titania NP/CdSe QD Hybrid Structures
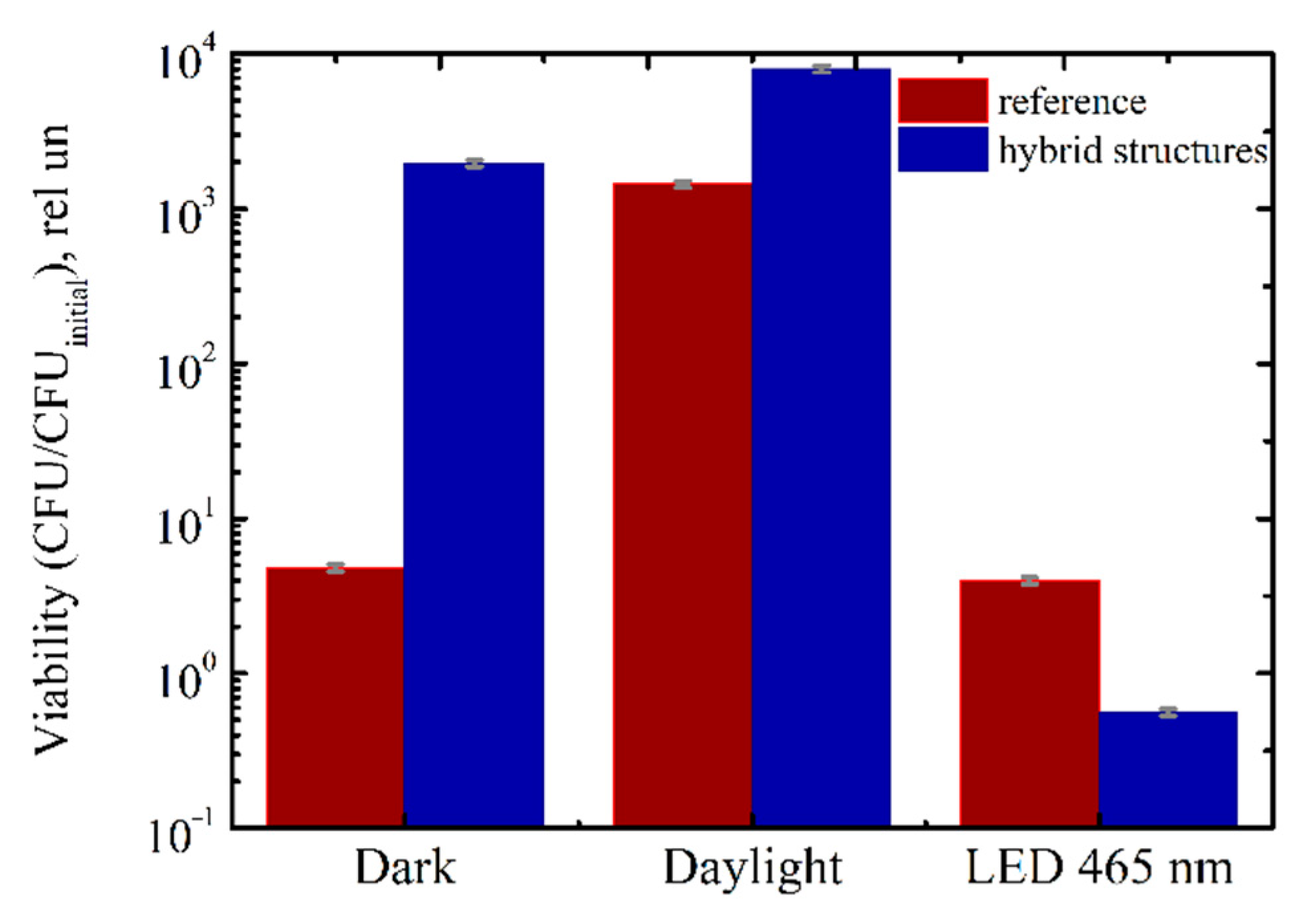
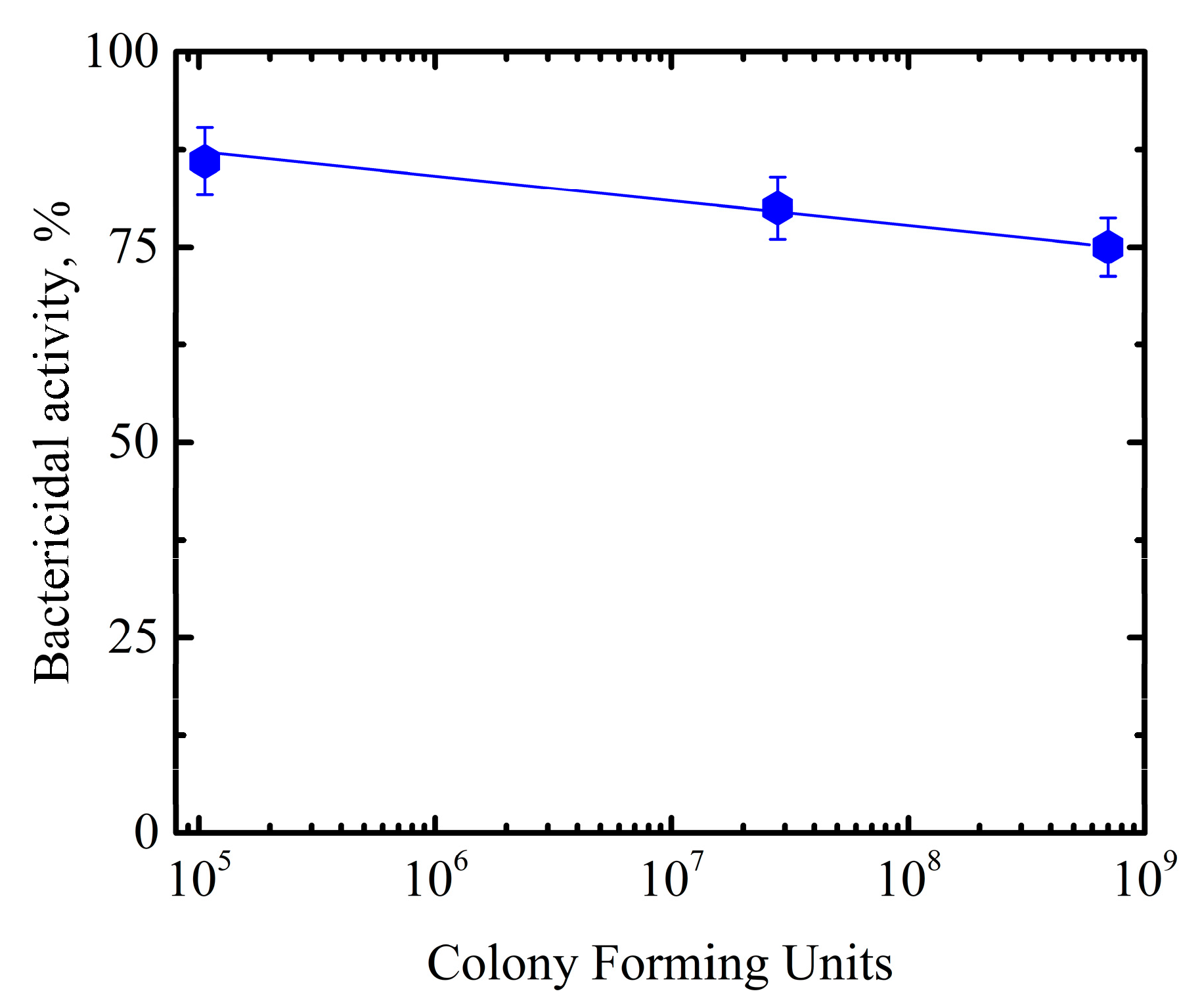
3.2. Interaction of Titania NP/CdSe QD Multilayered Hybrid Structures with Bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiadeh, M.N.; Moghadam, Z.B.; Adam, I.; Saber, V.; Bagheri, M.; Rostami, A. Human infectious diseases and risk of preeclampsia: An updated review of the literature. Infection 2017, 45, 589–600. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Blaskovich, M.A.; Cooper, M.A. Antibiotics in the clinical pipeline at the end of 2015. J. Antibiot. 2017, 70, 3–24. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1 (accessed on 1 September 2021).
- Montali, A. Antibacterial coating systems. Injury 2006, 37, S81–S86. [Google Scholar] [CrossRef]
- Benetti, G.; Cavaliere, E.; Brescia, R.; Salassi, S.; Ferrando, R.; Vantomme, A.; Van Bael, M.J. Tailored Ag–Cu–Mg multielemental nanoparticles for wide-spectrum antibacterial coating. Nanoscale 2019, 11, 1626–1635. [Google Scholar] [CrossRef]
- Nosrati, R.; Olad, A.; Shakoori, S. Preparation of an antibacterial, hydrophilic and photocatalytically active polyacrylic coating using TiO2 nanoparticles sensitized by graphene oxide. Mater. Sci. Eng. C 2017, 80, 642–651. [Google Scholar] [CrossRef]
- Allafchian, A.; Hosseini, S.S. Antibacterial magnetic nanoparticles for therapeutics: A review. IET Nanobiotechnol. 2019, 13, 786–799. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibacterial nanoparticles in endodontics: A review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef]
- Latvala, S.; Hedberg, J.; Di Bucchianico, S.; Möller, L.; Odnevall Wallinder, I.; Elihn, K.; Karlsson, H.L. Nickel release, ROS generation and toxicity of Ni and NiO micro-and nanoparticles. PLoS ONE 2016, 11, e0159684. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Cho, S.G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.J.; Dong, F.; Jiang, S.T.; Yin, G.Q.; Li, X.; Yang, H.B. Light-controlled generation of singlet oxygen within a discrete dual-stage metallacycle for cancer therapy. J. Am. Chem. Soc. 2019, 141, 8943–8950. [Google Scholar] [CrossRef]
- Azócar, M.I.; Alarcón, R.; Castillo, A.; Blamey, J.M.; Walter, M.; Paez, M. Capping of silver nanoparticles by anti-inflammatory ligands: Antibacterial activity and superoxide anion generation. J. Photochem. Photobiol. B Biol. 2019, 193, 100–108. [Google Scholar] [CrossRef]
- Li, N.; Liu, X. Synthesis of dendrimer-stabilized au nanoparticles and their application in the generation of hydroxyl radicals. ChemistrySelect 2019, 4, 9897–9900. [Google Scholar] [CrossRef]
- Ran, P.; Song, J.; Mo, F.; Wu, J.; Liu, P.; Fu, Y. Nitrogen-doped graphene quantum dots coated with gold nanoparticles for electrochemiluminescent glucose detection using enzymatically generated hydrogen peroxide as a quencher. Microchim. Acta 2019, 186, 276. [Google Scholar] [CrossRef]
- Shi, K.; Gao, Z.; Shi, T.Q.; Song, P.; Ren, L.J.; Huang, H.; Ji, X.J. Reactive oxygen species-mediated cellular stress response and lipid accumulation in oleaginous microorganisms: The state of the art and future perspectives. Front. Microbiol. 2017, 8, 793. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic. Biol. Med. 2016, 100, 81–85. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Paludetti, G.; Troiani, D. Targeting dysregulation of redox homeostasis in noise-induced hearing loss: Oxidative stress and ROS signaling. Free Radic. Biol. Med. 2019, 135, 46–59. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Visnapuu, M. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Micro Nano Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, P.; Antony, A.P.; Sharan, A.; Siddhardha, B.; Kasinathan, K.; Bahkali, N.A.; Syed, A. Antimicrobial photodynamic activity of toluidine blue encapsulated in mesoporous silica nanoparticles against Pseudomonas aeruginosa and Staphylococcus aureus. Biofouling 2019, 35, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Espina, M.; López-Machado, A.; Cajal, Y.; Rabanal, F.; Souto, E.B. State-of-the-art polymeric nanoparticles as promising therapeutic tools against human bacterial infections. J. Nanobiotechnol. 2020, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Sarwar, H.S.; Amin, U.; Naveed, S.; Ali, I.; Razzaq, S.; Shahnaz, G. Tuberculosis resistance and nanoparticles: Combating the dual role of reactive oxygen species in macrophages for tuberculosis management. Crit. Rev. Ther. Drug Carrier Syst. 2020, 37, 161–182. [Google Scholar] [CrossRef]
- Pekárek, S.; Mikeš, J.; Krýsa, J. Comparative study of TiO2 and ZnO photocatalysts for the enhancement of ozone generation by surface dielectric barrier discharge in air. Appl. Catal A Gen. 2015, 502, 122–128. [Google Scholar] [CrossRef]
- Benson, R.S. Use of radiation in biomaterials science. Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. Atoms 2002, 191, 752–757. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Zhou, B.; Li, J.; Chen, H.; Wang, J.; Cai, W. Highly stable CdS-modified short TiO2 nanotube array electrode for efficient visible-light hydrogen generation. Int. J. Hydrogen Energy 2011, 36, 167–174. [Google Scholar] [CrossRef]
- Jin, S.; Lian, T. Electron transfer dynamics from single CdSe/ZnS quantum dots to TiO2 nanoparticles. Nano Lett. 2009, 9, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Chuang, C.Y.; Lee, Y.Y.; Li, F.C.; Chang, Y.M.; Liu, I.P.; Lee, Y.L. Charge transfer in the heterointerfaces of CdS/CdSe cosensitized TiO2 photoelectrode. J. Phys. Chem. C 2012, 116, 1550–1555. [Google Scholar] [CrossRef]
- Wang, L.; Han, J.; Feng, J.; Wang, X.; Su, D.; Hou, X.; Dou, S.X. Simultaneously efficient light absorption and charge transport of CdS/TiO2 nanotube array toward improved photoelectrochemical performance. Int. J. Hydrogen Energy 2019, 44, 30899–30909. [Google Scholar] [CrossRef]
- Ma, X.; Xiang, Q.; Liao, Y.; Wen, T.; Zhang, H. Visible-light-driven CdSe quantum dots/graphene/TiO2 nanosheets composite with excellent photocatalytic activity for E. coli disinfection and organic pollutant degradation. Appl. Surf. Sci. 2018, 457, 846–855. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Zhang, T.; Sun, D.D.; Ng, W. Hierarchical TiO2/CdS “spindle-like” composite with high photodegradation and antibacterial capability under visible light irradiation. J. Hazard. Mater. 2012, 229, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Lu, Q.Z.; Liu, S.H.; Yang, L.X.; Wen, L.F.; Luo, S.L.; Cai, Q.Y. A ternary hybrid CdS/Pt–TiO2 nanotube structure for photoelectrocatalytic bactericidal effects on Escherichia coli. Biomaterials 2010, 31, 3317–3326. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.X.; Zhang, Z.L.; Zhang, M.X.; Xie, H.Y.; Tian, Z.Q.; Chen, P.; Pang, D.W. Core/shell quantum-dot-photosensitized nano-TiO2 films: Fabrication and application to the damage of cells and DNA. J. Phys. Chem B 2005, 109, 22663–22666. [Google Scholar] [CrossRef]
- Palmer, B.R.; Marinus, M.G. The dam and dcm strains of Escherichia coli—A review. Gene 1994, 143, 1–12. [Google Scholar] [CrossRef]
- Ragusa, J.; Gonzalez, D.; Li, S.; Noriega, S.; Skotak, M.; Larsen, G. Glucosamine/L-lactide copolymers as potential carriers for the development of a sustained rifampicin release system using Mycobacterium smegmatis as a tuberculosis model. Heliyon 2019, 5, e01539. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Even-Desrumeaux, K.; Chames, P.; Baty, D.; Artemyev, M.; Oleinikov, V.; Nabiev, I. Engineering of ultra-small diagnostic nanoprobes through oriented conjugation of single-domain antibodies and quantum dots. Protoc. Exch. 2012, 10, 1–23. [Google Scholar] [CrossRef]
- Niederberger, M.; Bartl, M.H.; Stucky, G.D. Benzyl alcohol and transition metal chlorides as a versatile reaction system for the nonaqueous and low-temperature synthesis of crystalline nano-objects with controlled dimensionality. J. Am. Chem. Soc. 2002, 124, 13642–13643. [Google Scholar] [CrossRef]
- Alaferdov, A.V.; Savu, R.; Rackauskas, S.; Rackauskas, T.; Canesqui, M.A.; Gromova, Y.A.; Moshkalev, S.A. New Hybrid Structures Based on CdSe/ZnS Quantum Dots and Multilayer Graphene for Photonics Applications; IEEE: Manhattan, NY, USA, 2015; pp. 1–4. [Google Scholar]
- Muff, J.; Bennedsen, L.R.; Søgaard, E.G. Detailed parameter study on the mechanisms in electrochemical oxidation of p-nitrosodimethylaniline in chloride electrolyte. In Proceedings of the 2nd European Conference on Environmental Applications of Advanced Oxidation Processes, Nicosia, Cyprus, 9–11 September 2009. [Google Scholar]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C: Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Moulis, J.M.; Thévenod, F. New perspectives in cadmium toxicity: An introduction. Biometals 2010, 23, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.T.; Law, W.C.; Hu, R.; Ye, L.; Liu, L.; Swihart, M.T.; Prasad, P.N. Nanotoxicity assessment of quantum dots: From cellular to primate studies. Chem. Soc. Rev. 2013, 42, 1236–1250. [Google Scholar] [CrossRef]
- Li, K.G.; Chen, J.T.; Bai, S.S.; Wen, X.; Song, S.Y.; Yu, Q.; Wang, Y.Q. Intracellular oxidative stress and cadmium ions release induce cytotoxicity of unmodified cadmium sulfide quantum dots. Toxicol. In Vitro 2009, 23, 1007–1013. [Google Scholar] [CrossRef]
- Robel, I.; Kuno, M.; Kamat, P.V. Size-dependent electron injection from excited CdSe quantum dots into TiO2 nanoparticles. J. Am. Chem. Soc. 2007, 129, 4136–4137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Song, N.; Lian, T. Controlling charge separation and recombination rates in CdSe/ZnS type I core−shell quantum dots by shell thicknesses. J. Am. Chem. Soc. 2010, 132, 15038–15045. [Google Scholar] [CrossRef] [PubMed]
- Kolesova, E.P.; Safin, F.M.; Maslov, V.G.; Dubavik, A.; Gun’ko, Y.K.; Orlova, A.O. Photophysics of Titania Nanoparticle/Quantum dot hybrid structures. Opt. Spectrosc. 2020, 128, 1256–1261. [Google Scholar] [CrossRef]
- Kolesova, E.P.; Safin, F.M.; Maslov, V.G.; Gun’ko, Y.K.; Orlova, A.O. The influence of photoinduced processes on a Quantum dot surface on the electron transfer efficiency in TiO2 Nanoparticle/Quantum dot structures. Opt. Spectrosc. 2019, 12, 548–1554. [Google Scholar] [CrossRef]
- Kolesova, E.; Maslov, V.; Safin, F.; Purcell-Milton, F.; Cleary, O.; Volkov, Y.; Gun’ko, Y.K.; Orlova, A. Photoinduced charge transfer in hybrid structures based on titanium dioxide NPs with multicomponent qd exciton luminescence decay. J. Phys. Chem. C 2019, 123, 14790–14796. [Google Scholar] [CrossRef]
- Saita, M.; Kobatashi, K.; Yoshino, F.; Hase, H.; Nonami, T.; Kimoto, K.; Masaichi, C.I. ESR investigation of ROS generated by H2O2 bleaching with TiO2 coated HAp. Dent. Mater. J. 2012, 31, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of reactive oxygen and nitrogen species by electron paramagnetic resonance (EPR) technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef] [PubMed]
- Kőrösi, L.; Bognár, B.; Bouderias, S.; Castelli, A.; Scarpellini, A.; Pasquale, L.; Prato, M. Highly-efficient photocatalytic generation of superoxide radicals by phase-pure rutile TiO2 nanoparticles for azo dye removal. Appl. Surf. Sci. 2019, 493, 719–728. [Google Scholar] [CrossRef]
- Kolesova, E.P.; Maslov, V.G.; Gun’ko, Y.K.; Orlova, A.O. A Method for estimating the functionality of TiO2/Quantum dot multilayer hybrid structures based on the generation of reactive oxygen species. Opt. Spectrosc. 2019, 127, 347–351. [Google Scholar] [CrossRef]
- Durisic, N.; Wiseman, P.W.; Grütter, P.; Heyes, C.D. A common mechanism underlies the dark fraction formation and fluorescence blinking of quantum dots. ACS Nano 2009, 3, 1167–1175. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical relevance of oxidative stress in male factor infertility: An update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef]
- Fu, L.M.; Fu-Liu, C.S. Is Mycobacterium tuberculosis a closer relative to Gram-positive or Gram–negative bacterial pathogens? Tuberculosis 2002, 82, 85–90. [Google Scholar] [CrossRef]
- Cummins, C.S.; Harris, H. The chemical composition of the cell wall in some gram-positive bacteria and its possible value as a taxonomic character. Microbiology 1956, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Touati, D.; Jacques, M.; Tardat, B.; Bouchard, L.; Despied, S. Lethal oxidative damage and mutagenesis are generated by iron in delta fur mutants of Escherichia coli: Protective role of superoxide dismutase. J. Bacteriol. Res. 1995, 177, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, E.; Ichihara, K.; Noda, Y.; Kusunose, M. Superoxide dismutase from Mycobacterium tuberculosis. J. Biochem. 1976, 80, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Gregory, E.M.; Fridovich, I. Oxygen toxicity and the superoxide dismutase. J. Bacteriol. Res. 1973, 114, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, R.L.; Eze, M.O. Escherichia coli superoxide dismutase expression does not change in response to iron challenge during lag phase: Is the ferric uptake regulator to blame? Adv. Enzyme Res. 2013, 1, 132. [Google Scholar] [CrossRef][Green Version]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef] [PubMed]


| Bacteria * | Type |
|---|---|
| Escherichia coli ATCC 25922 | Gram-negative |
| Pseudomonas aeruginosa ATCC 27853 | Gram-negative |
| Staphylococcus aureus FDA 209P | Gram-positive |
| Bacillus subtilis ATCC 6633 | Gram-positive |
| Mycobacterium smegmatis mc2 155 | Gram-positive |
| E. coli ATCC 25922, CFU/mL | P. aeruginosa ATCC 27853, CFU/mL | S. aureus FDA 209P, CFU/mL | B. subtilis ATCC 6633, CFU/mL | M. smegmatis mc2 155, CFU/mL | |
|---|---|---|---|---|---|
| Reference samples * | 1.2 × 107 | ~2.4 × 109 | ~6 × 106 | 3.5 × 107 | 5.8 × 106 |
| Titania NP/CdSe QD hybrid structures | 7 × 106 | ~2.4 × 109 | ~107 | 3.8 × 105 | ~1.5 × 106 |
| Decrease in the bacteria number by a factor | ~1.5 | 1 | 1 | ~90 | ~4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesova, E.; Bulgakova, A.; Maslov, V.; Veniaminov, A.; Dubavik, A.; Gun’ko, Y.; Efremenkova, O.; Oleinikov, V.; Orlova, A. Bactericidal Activity of Multilayered Hybrid Structures Comprising Titania Nanoparticles and CdSe Quantum Dots under Visible Light. Nanomaterials 2021, 11, 3331. https://doi.org/10.3390/nano11123331
Kolesova E, Bulgakova A, Maslov V, Veniaminov A, Dubavik A, Gun’ko Y, Efremenkova O, Oleinikov V, Orlova A. Bactericidal Activity of Multilayered Hybrid Structures Comprising Titania Nanoparticles and CdSe Quantum Dots under Visible Light. Nanomaterials. 2021; 11(12):3331. https://doi.org/10.3390/nano11123331
Chicago/Turabian StyleKolesova, Ekaterina, Anastasia Bulgakova, Vladimir Maslov, Andrei Veniaminov, Aliaksei Dubavik, Yurii Gun’ko, Olga Efremenkova, Vladimir Oleinikov, and Anna Orlova. 2021. "Bactericidal Activity of Multilayered Hybrid Structures Comprising Titania Nanoparticles and CdSe Quantum Dots under Visible Light" Nanomaterials 11, no. 12: 3331. https://doi.org/10.3390/nano11123331
APA StyleKolesova, E., Bulgakova, A., Maslov, V., Veniaminov, A., Dubavik, A., Gun’ko, Y., Efremenkova, O., Oleinikov, V., & Orlova, A. (2021). Bactericidal Activity of Multilayered Hybrid Structures Comprising Titania Nanoparticles and CdSe Quantum Dots under Visible Light. Nanomaterials, 11(12), 3331. https://doi.org/10.3390/nano11123331










