Temperature-Dependent Photoluminescence of Manganese Halide with Tetrahedron Structure in Anti-Perovskites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. The Preparation of [MnBr4]BrCs3 Films
2.3. Characterization of [MnBr4]BrCs3
2.4. Temperature Dependence Experiment
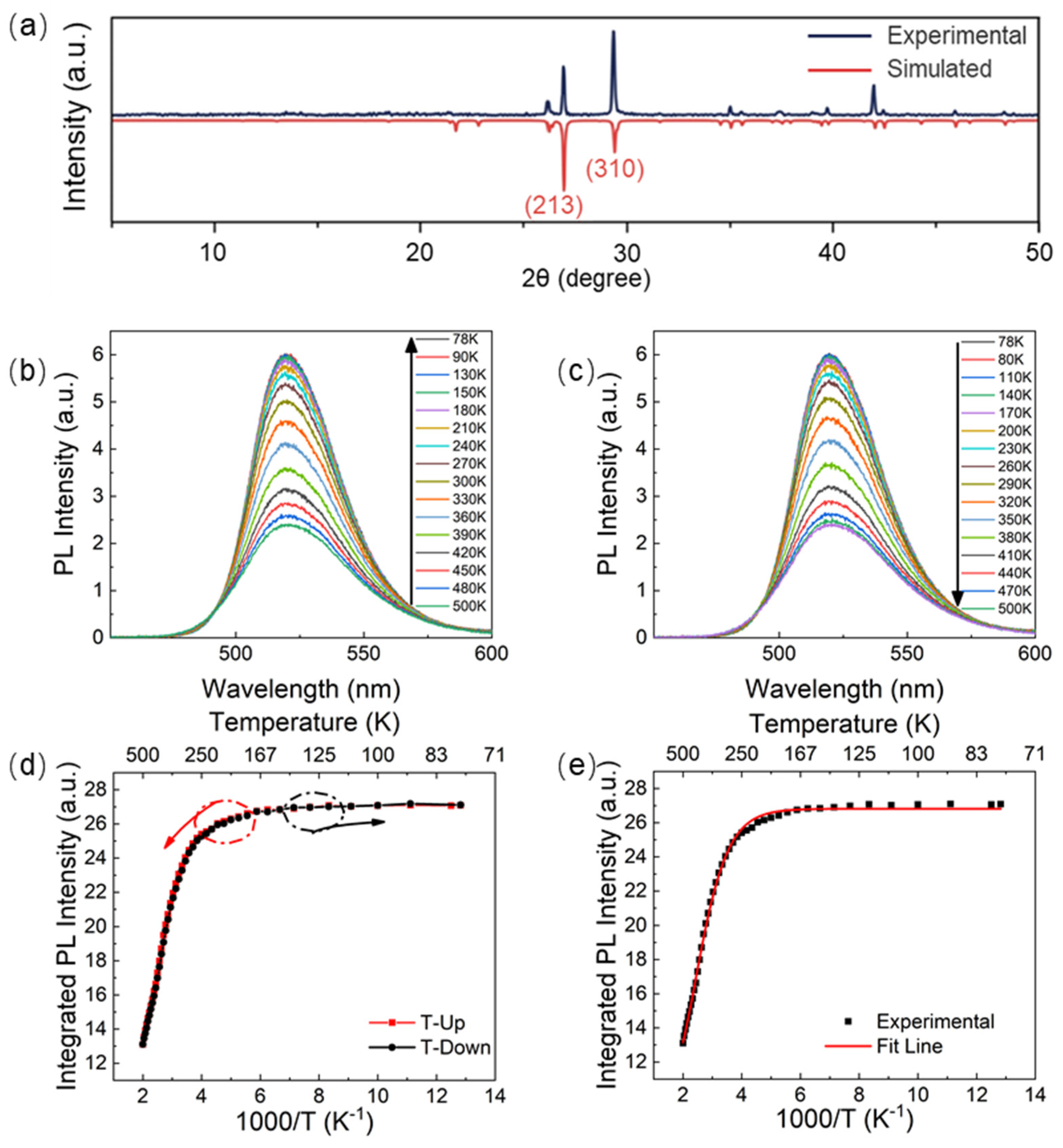
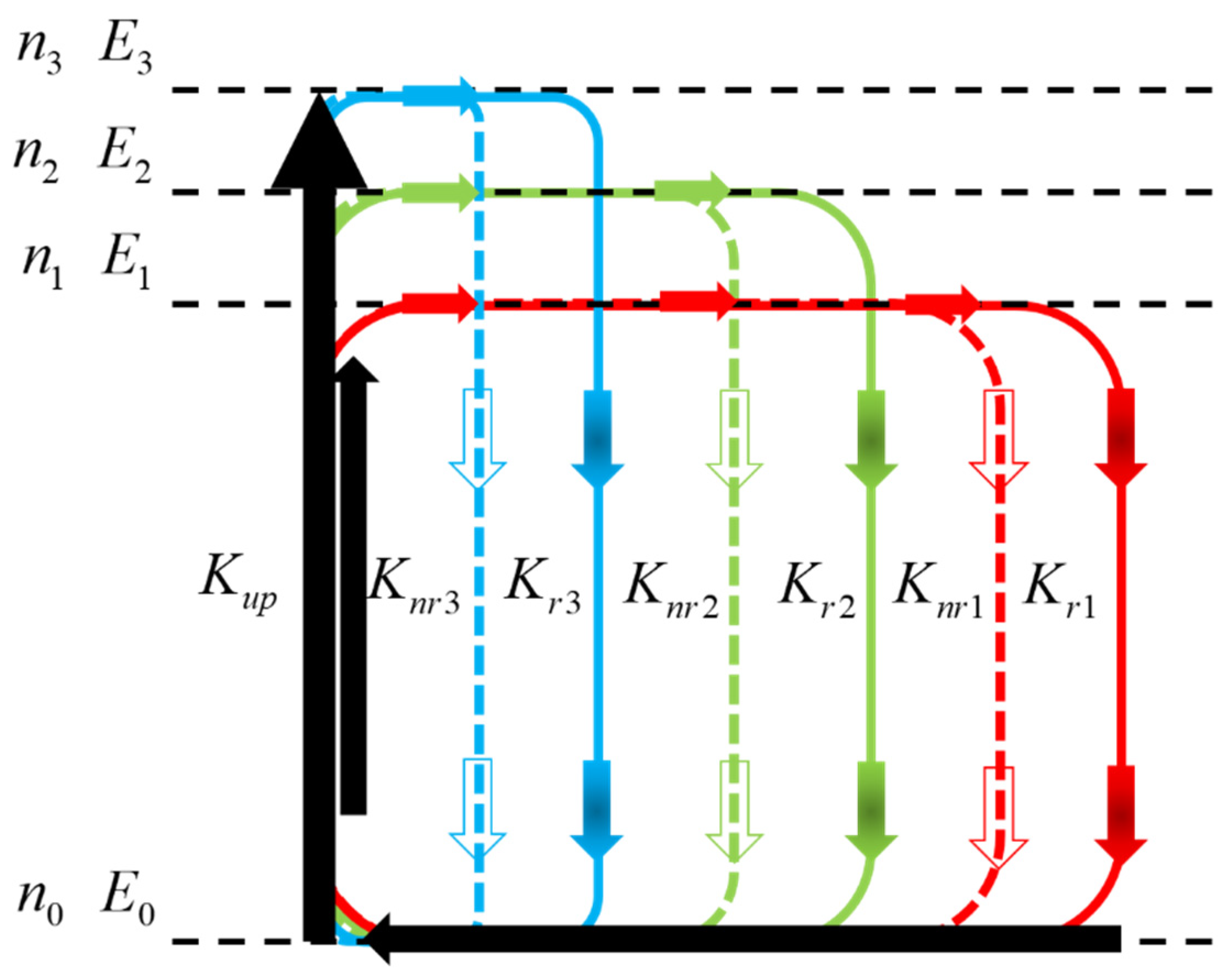
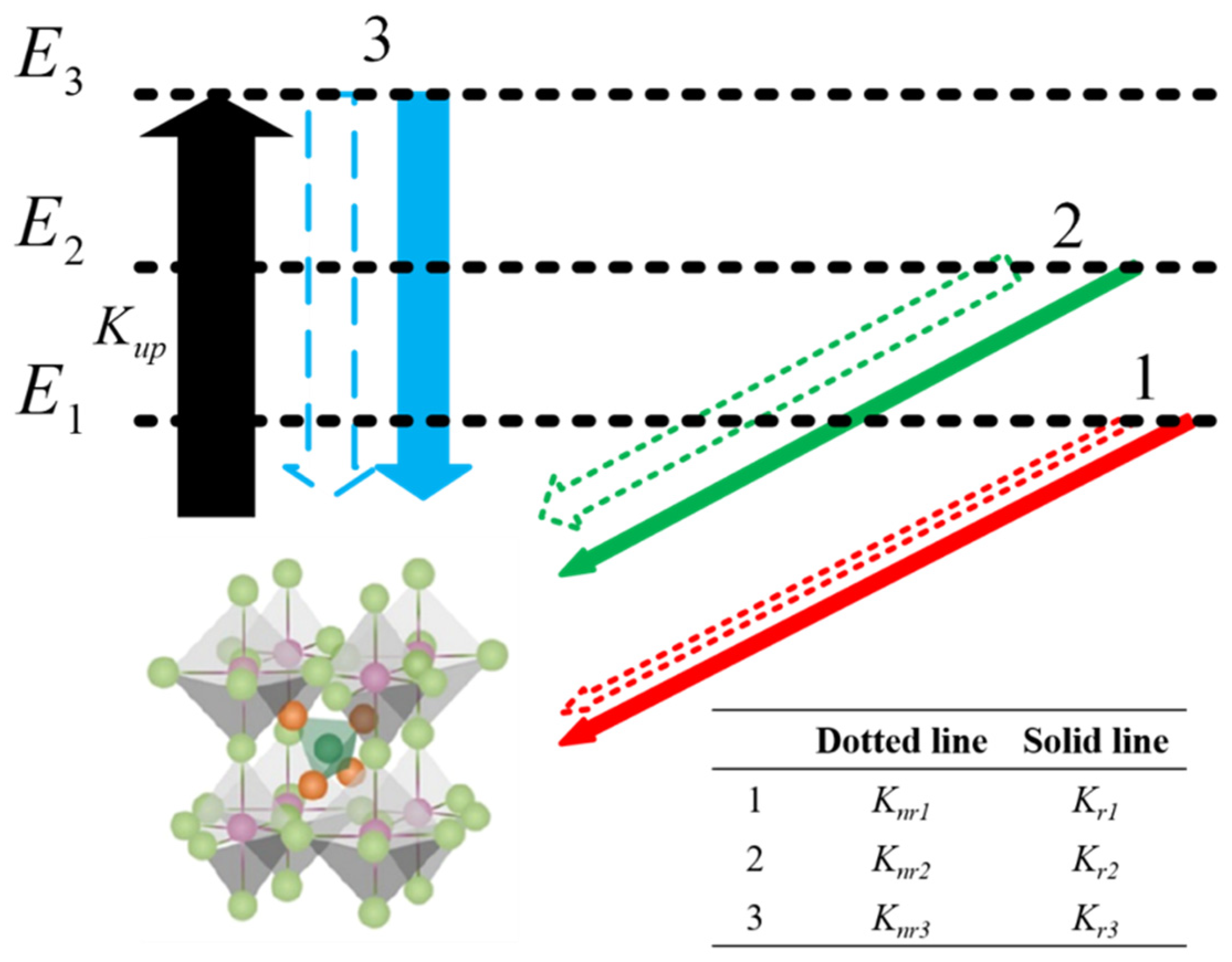
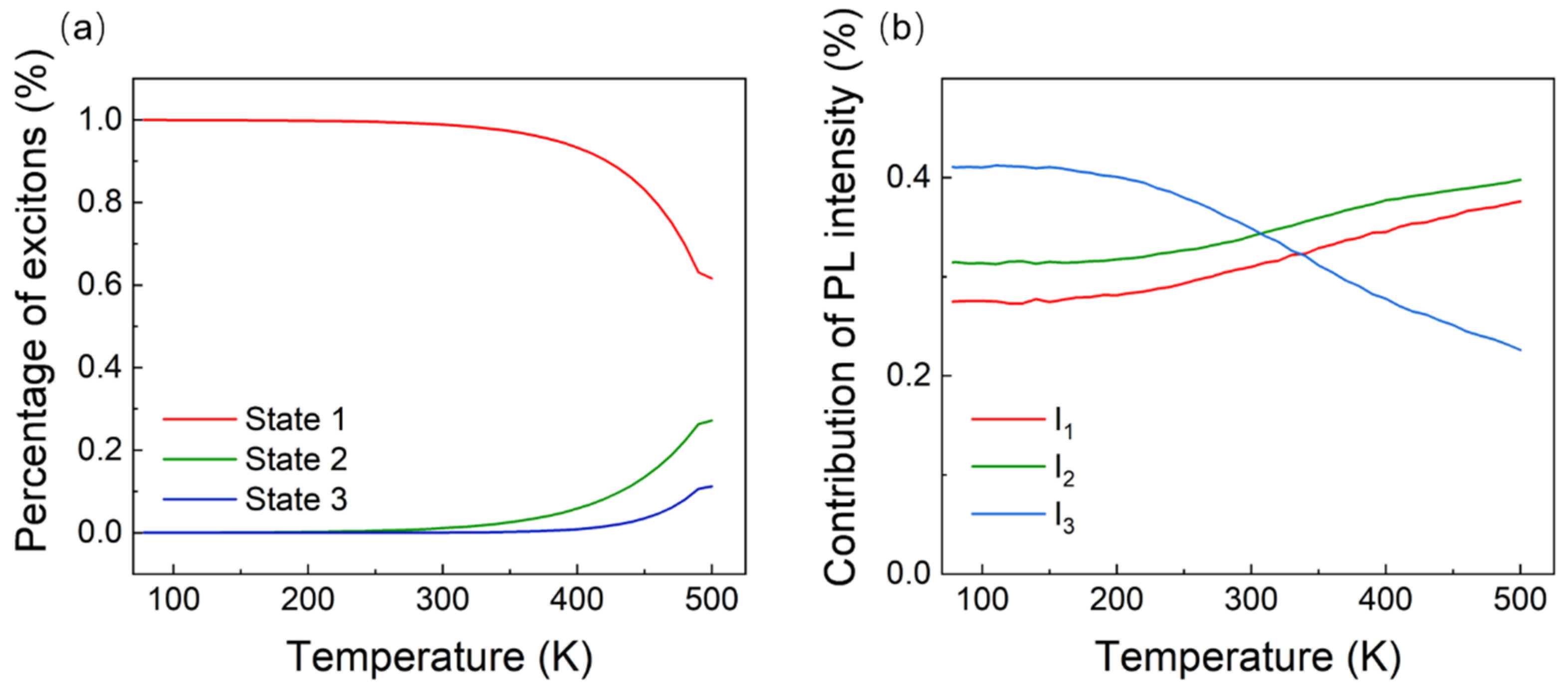
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High charge carrier mobilities and lifetimes in organolead trihalide perovskites. Adv. Mater. 2014, 26, 1584–1589. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Li, X.; Wu, Y. Space-confined growth of CsPbBr3 film achieving photodetectors with high performance in all figures of merit. Adv. Funct. Mater. 2018, 28, 1804394. [Google Scholar] [CrossRef]
- Zhi-Kuang, T.; Reza Saberi, M.; May Ling, L.; Reza Saberi, M.; May Ling, L. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]
- Hoye, R.L.Z.; Chua, M.R.; Musselman, K.P.; Li, G.; Lai, M.; Tan, Z.; Greenham, N.C.; MacManus-Driscoll, J.L.; Friend, R.H.; Credgington, D. Enhanced performance in fluorene-free organometal halide perovskite light-emitting diodes using tunable, low electron affinity oxide electron injectors. Adv. Mater. 2015, 27, 1414–1419. [Google Scholar] [CrossRef] [Green Version]
- Sichert, J.A.; Tong, Y.; Mutz, N.; Vollmer, M.; Fischer, S.; Milowska, K.Z.; Cortadella, R.G.; Nickel, B.; Cardenas-Daw, C.; Stolarczyk, J.K.; et al. Quantum size effect in organometal halide perovskite nanoplatelets. Nano Lett. 2015, 15, 6521–6527. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Yuan, M.; Yang, X. Fast postmoisture treatment of luminescent perovskite films for efficient light-emitting diodes. Small 2018, 14, 1703410. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; De Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 percent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Zhao, B. High-efficiency perovskite–polymer bulk heterostructure light-emitting diodes. Nat. Photon. 2018, 12, 783–789. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, N.; Tian, H.; Guo, J.; Wei, Y.; Chen, H.; Miao, Y.; Zou, W.; Pan, K.; He, Y.; et al. Perovskite light-emitting diodes based on spontaneously formed submicrometre-scale structures. Nature 2018, 562, 249–253. [Google Scholar] [CrossRef]
- Hu, G.; Xu, B.; Wang, A.; Guo, Y.; Wu, J.; Muhammad, F.; Meng, W.; Wang, C.; Sui, S.; Liu, Y.; et al. Stable and Bright Pyridine Manganese Halides for Efficient White Light-Emitting Diodes. Adv. Funct. Mater. 2021, 31, 2011191. [Google Scholar] [CrossRef]
- Yang, J.; Siempelkamp, B.D.; Liu, D.; Kelly, T. Investigation of CH3NH3PbI3 degradation rates and mechanisms in controlled humidity environments using in situ techniques. ACS Nano 2015, 9, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, Z.; Shao, J.; Yao, D.; Gao, H.; Liu, Y.; Yu, W.; Zhang, H.; Yang, B. CsPbxMn1–xCl3 perovskite quantum dots with high Mn substitution ratio. ACS Nano 2017, 11, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Cho, H.; Wolf, C.; Kim, J.S.; Yun, H.J.; Bae, J.S.; Kim, H.; Heo, J.; Ahn, S.; Lee, T. High-efficiency solution-processed inorganic metal halide perovskite light-emitting diodes. Adv. Mater. 2017, 29, 1700579.1–1700579.8. [Google Scholar] [CrossRef]
- Saidaminov, M.; Haque, A.; Almutlaq, J.; Sarmah, S.; Miao, X.-H.; Begum, R.; Zhumekenov, A.A.; Dursun, I.; Cho, N.; Murali, B.; et al. Inorganic lead halide perovskite single crystals: Phase-selective low-temperature growth, carrier transport properties, and self-powered photodetection. Adv. Opt. Mater. 2017, 5, 160070. [Google Scholar] [CrossRef]
- Chen, L.-J.; Lee, C.-R.; Chuang, Y.-J.; Wu, Z.-H.; Chen, C. Synthesis and Optical Properties of Lead-Free Cesium Tin Halide Perovskite Quantum Rods with High-Performance Solar Cell Application. J. Phys. Chem. Lett. 2016, 7, 5028–5035. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Guo, Y.; Zhou, Z.; Niu, X.; Wang, Y.; Muhammad, F.; Li, H.; Zhang, T.; Wang, J.; Nie, S.; et al. Aqueous acid-based synthesis of lead-free tin halide perovskites with near-unity photoluminescence quantum efficiency. Chem. Sci. 2019, 10, 4573–4579. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.-H.; Liao, W.-Q.; Li, P.-F.; Wang, Z.-X.; Mao, C.-Y.; Zhang, Y. Dielectric and photoluminescence properties of a layered perovskite-type organic–inorganic hybrid phase transition compound: NH3(CH2)5NH3MnCl4. J. Mater. Chem. C 2016, 4, 1881–1885. [Google Scholar] [CrossRef]
- Yan, S.; Tang, K.; Lin, Y.; Ren, Y.; Tian, W. Light-Emitting Diodes with Manganese Halide Tetrahedron Embedded in Anti-Perovskites. ACS Energy Lett. 2021, 6, 1901–1911. [Google Scholar] [CrossRef]
- Lee, S.M.; Moon, C.J.; Lim, H.; Lee, Y.; Choi, M.Y.; Bang, J. Temperature-Dependent Photoluminescence of Cesium Lead Halide Perovskite Quantum Dots: Splitting of the Photoluminescence Peaks of CsPbBr3 and CsPb(Br/I)3 Quantum Dots at Low Temperature. J. Phys. Chem. C 2017, 121, 26054–26062. [Google Scholar] [CrossRef]
- Savchyn, P.V.; Vistovskyy, V.V.; Pushak, A.S.; Voloshinovskii, A.S.; Popov, A.I. Synchrotron radiation studies on luminescence of Eu2+-doped LaCl3 microcrystals embedded in a NaCl matrix. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 275, 78–82. [Google Scholar] [CrossRef]
- Wu, K.; Bera, A.; Ma, C.; Du, Y.; Yang, Y.; Li, L.; Wu, T. Temperature-dependent excitonic photoluminescence of hybrid organometal halide perovskite films. Phys. Chem. Chem. Phys. 2014, 16, 22476–22481. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, J.-F.; Huang, Z.; Wei, J.; Wang, X.; Li, W.; Chen, H.; Kuang, D.; Su, C. A Highly red emissive lead-free indium-based perovskite single crystal for sensitive water detection. Angew. Chem. Int. 2019, 58, 5277–5281. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Neu, J.; Zhou, Y.; Chaaban, M.; Lee, S.; Worku, M.; Chen, B.; Clark, R.J.; Cheng, W.; et al. Green Emitting Single-Crystalline Bulk Assembly of Metal Halide Clusters with Near-Unity Photoluminescence Quantum Efficiency. ACS Energy Lett. 2019, 4, 1579–1583. [Google Scholar] [CrossRef]
- Baldo, M.A.; Obrien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.D.; Thompson, M.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Zhong, G.; Ding, X.; Zhou, J.; Jiang, N.; Huang, W.; Hou, X. Temperature-dependent photoluminescence of organic light-emitting materials: Types and characteristics of excitons involved in the emitting process. Chem. Phys. Lett. 2006, 420, 347–353. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, Y.; Liu, S.; Li, H.; Chen, J. Narrow-Band Green-Emitting Sr2MgAl22O36: Mn2+ Phosphors with Superior Thermal Stability and Wide Color Gamut for Backlighting Display Applications. Adv. Opt. Mater. 2019, 7, 1801419.1–1801419.9. [Google Scholar] [CrossRef]
- Reshchikov, M.A. Temperature dependence of defect-related photoluminescence in III-V and II-VI semiconductors. J. Appl. Phys. 2014, 115, 012010. [Google Scholar] [CrossRef] [Green Version]
- Benabdesselam, M.; Petitfils, A.; Wrobel, F.; Butler, J.E.; Mady, F. Thermal quenching investigation in CVD diamond by simultaneous detection of thermally stimulated luminescence and conductivity. J. Appl. Phys. 2008, 103, 114908. [Google Scholar] [CrossRef]
- Hu, T.; Smith, M.D.; Dohner, E.R.; Sher, M.-J.; Wu, X.; Trinh, M.T.; Fisher, A.; Corbett, J.; Zhu, X.-Y.; Karunadasa, H.I.; et al. Lindenberg. Mechanism for Broadband White-Light Emission from Two-Dimensional (110) Hybrid Perovskites. J. Phys. Chem. Lett. 2016, 7, 2258–2263. [Google Scholar] [CrossRef]
- Wasylishen, R.E.; Knop, O.; Macdonald, J.B. Cation rotation in methylammonium lead halides. Solid State Commun. 1985, 56, 581–582. [Google Scholar] [CrossRef]


| Material | Exciton Labels | |||
|---|---|---|---|---|
| [MnBr4]BrCs3 | 1 | 1 | 1 | 0 |
| 2 | 1 | 38 | 143.92 | |
| 3 | 64 | 66 | 241.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Du, S.; Huang, P.; Wu, L.; Yan, S.; Wang, W.; Zhong, G. Temperature-Dependent Photoluminescence of Manganese Halide with Tetrahedron Structure in Anti-Perovskites. Nanomaterials 2021, 11, 3310. https://doi.org/10.3390/nano11123310
Xia Y, Du S, Huang P, Wu L, Yan S, Wang W, Zhong G. Temperature-Dependent Photoluminescence of Manganese Halide with Tetrahedron Structure in Anti-Perovskites. Nanomaterials. 2021; 11(12):3310. https://doi.org/10.3390/nano11123310
Chicago/Turabian StyleXia, Yijie, Shuaishuai Du, Pengju Huang, Luchao Wu, Siyu Yan, Weizhi Wang, and Gaoyu Zhong. 2021. "Temperature-Dependent Photoluminescence of Manganese Halide with Tetrahedron Structure in Anti-Perovskites" Nanomaterials 11, no. 12: 3310. https://doi.org/10.3390/nano11123310
APA StyleXia, Y., Du, S., Huang, P., Wu, L., Yan, S., Wang, W., & Zhong, G. (2021). Temperature-Dependent Photoluminescence of Manganese Halide with Tetrahedron Structure in Anti-Perovskites. Nanomaterials, 11(12), 3310. https://doi.org/10.3390/nano11123310






