Colloidal Stability of Silica-Modified Magnetite Nanoparticles: Comparison of Various Dispersion Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe3O4 MNPs
2.2. Synthesis of Fe3O4/APTES MNPs
2.3. Dispersion by Sonication
2.4. Dispersion by Stirring
2.5. Humic Preparation Characterization
Characterization of Samples
3. Results
3.1. Characterization of MNP Microstructure
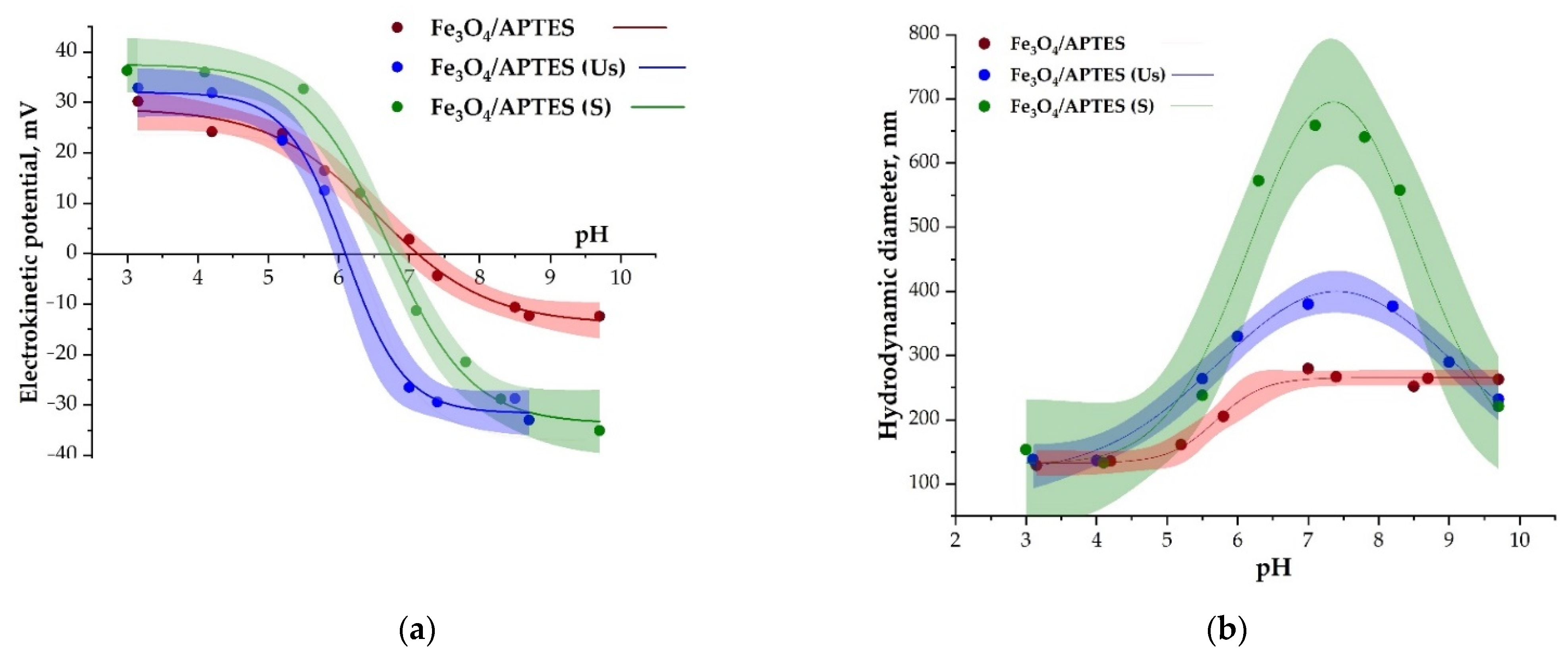
3.2. Characterization of the Zeta Potential and Hydrodynamic Size of MNPs
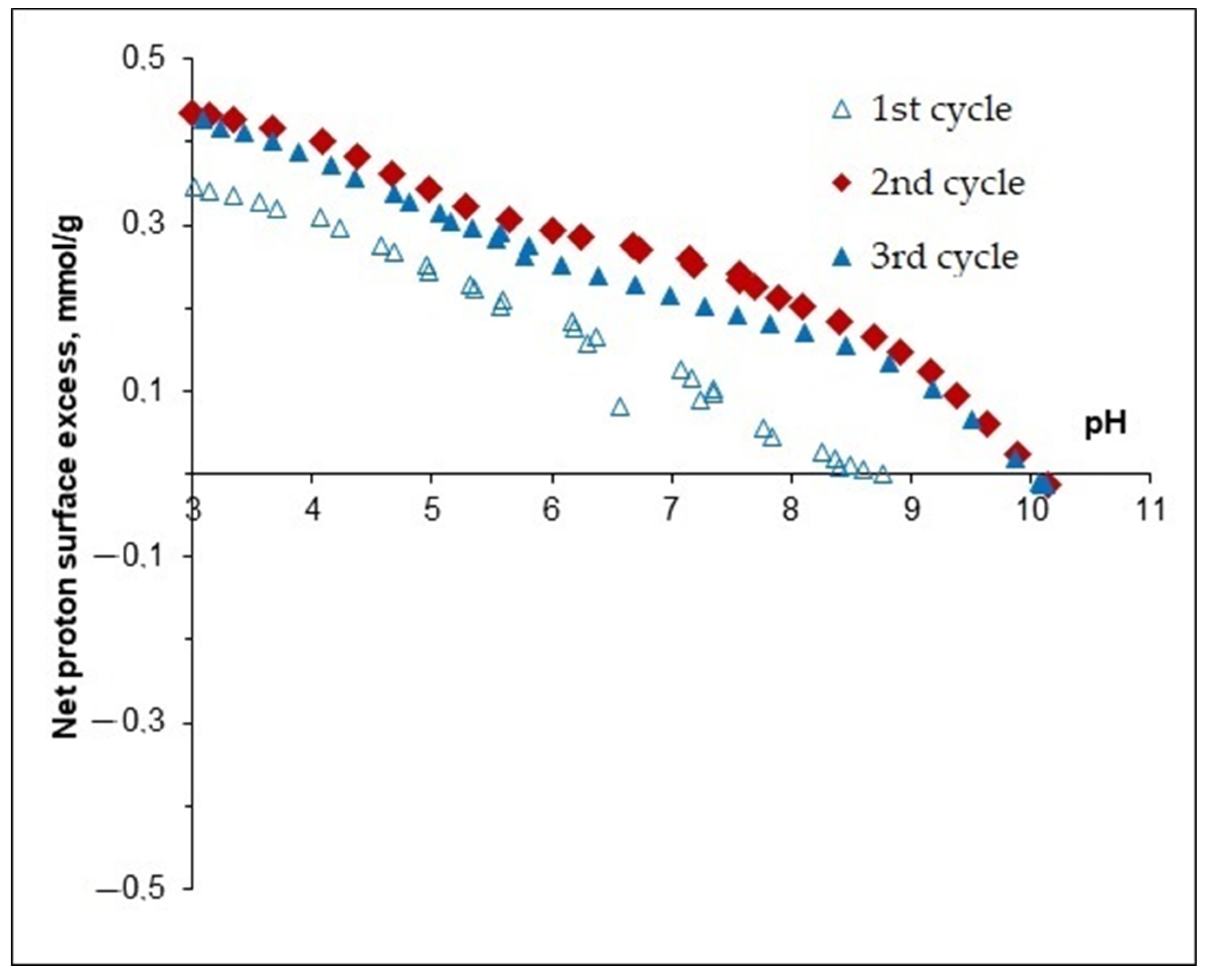
3.2.1. Surface Charging of MNPs
3.2.2. Effects of Dialysis and Dispersion
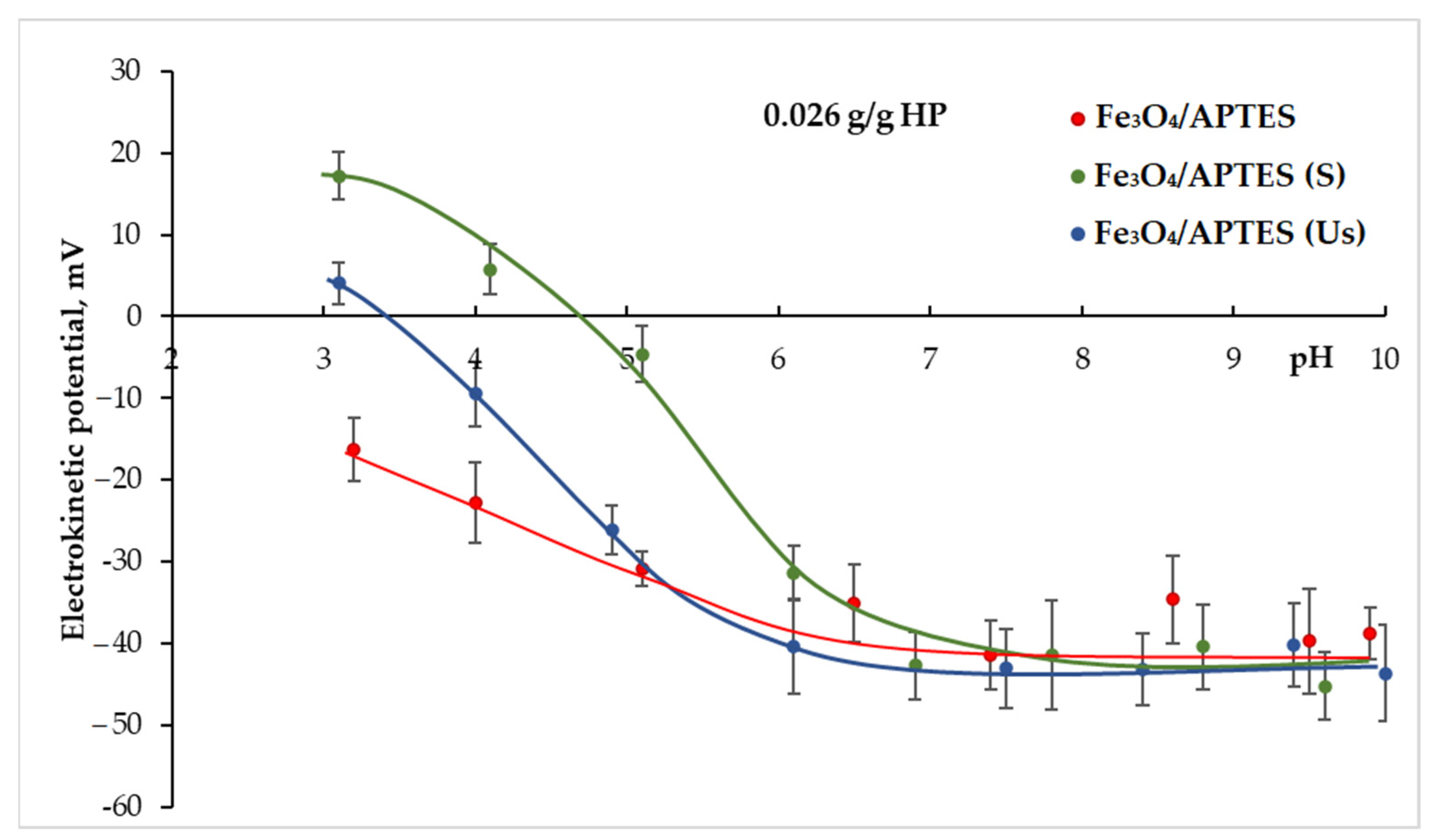
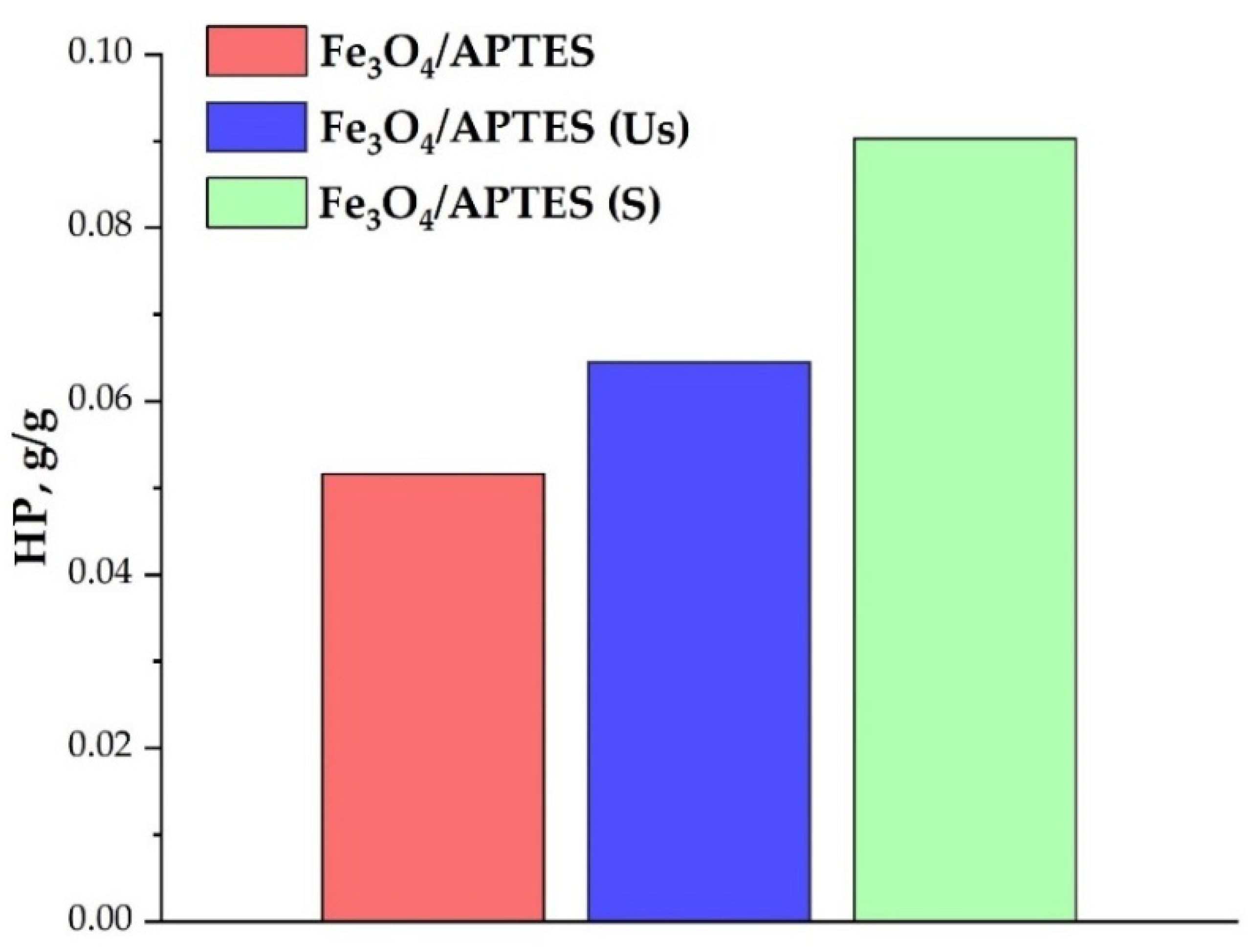
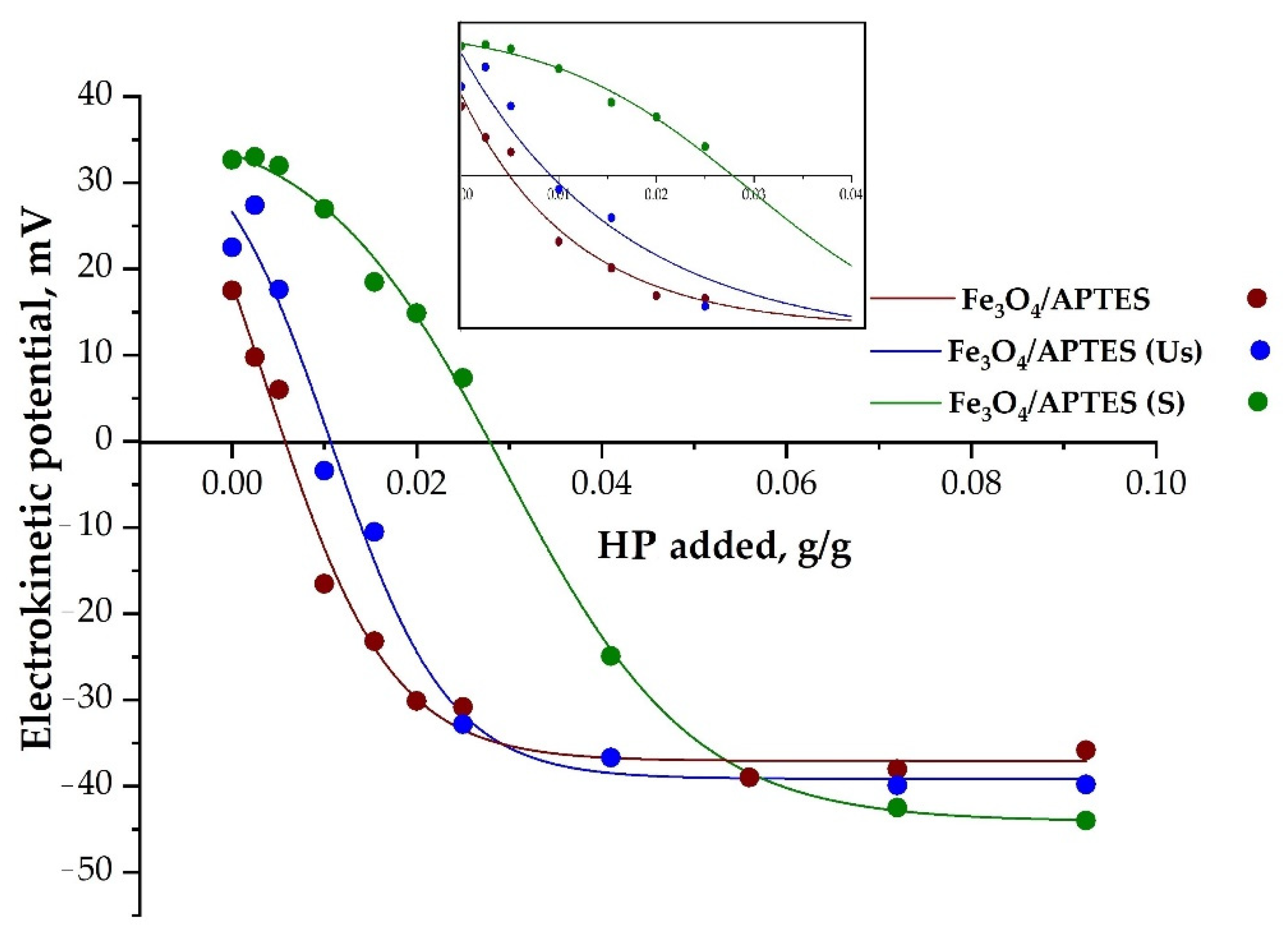
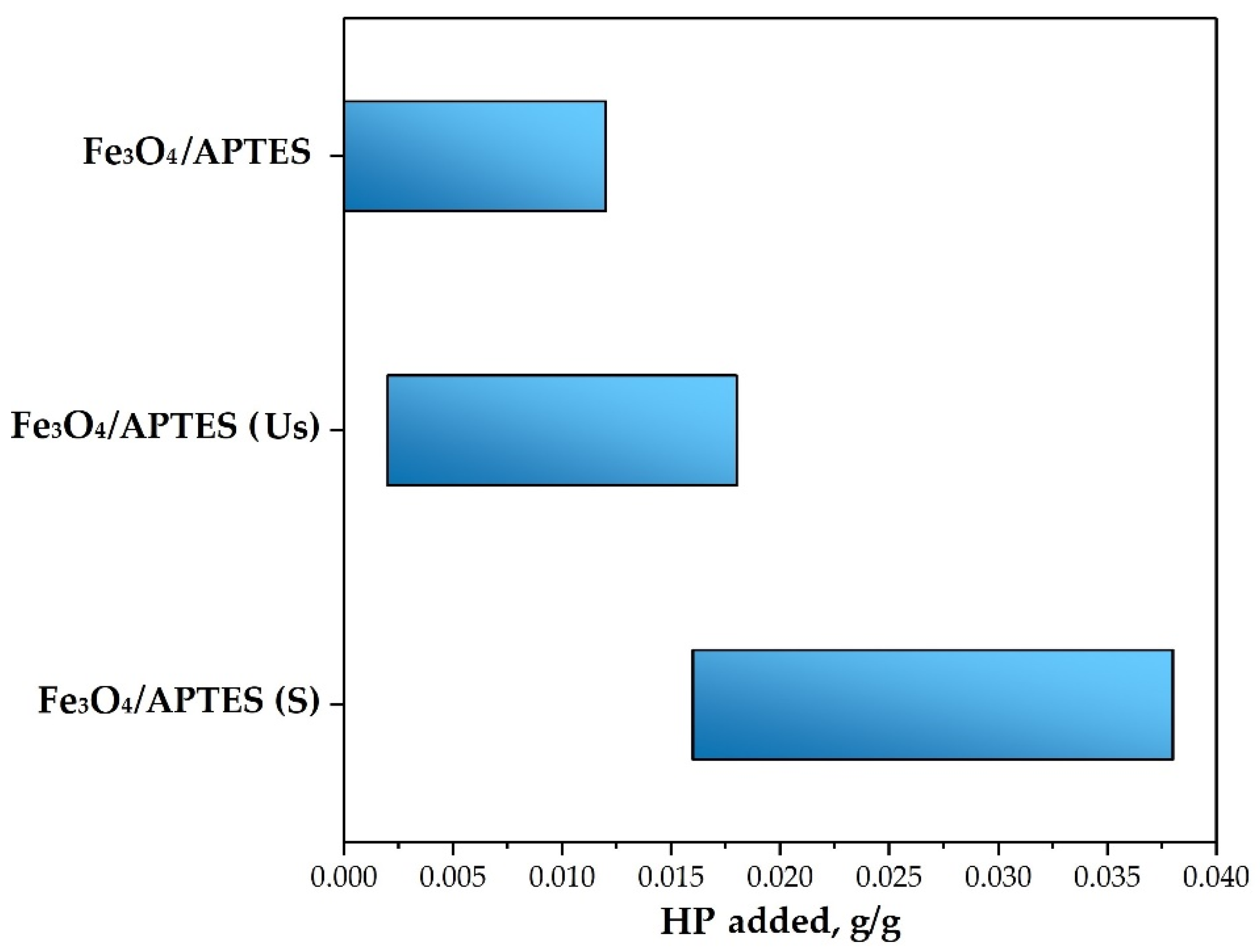
3.2.3. Loading Capacity towards Humic Preparation
3.2.4. Colloidal Stability of HP-Coated MNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nuzhina, J.; Shtil, A.A.; Prilepskii, A. Preclinical evaluation and clinical translation of magnetite-based nanomedicines. J. Drug Deliv. Sci. Tec. 2019, 5, 212–227. [Google Scholar] [CrossRef]
- Su, C. Environmental implications and applications of engineered nanoscale magnetite and its hybrid nanocomposites: A review of recent literature. J. Hazard. Mater. 2017, 322, 48–84. [Google Scholar] [CrossRef]
- Forte, P.; Patern, M.; Rustighi, E. A Magnetorheological fluid damper for rotor applications. Int. J. Rotating Mach. 2004, 10, 175–182. [Google Scholar] [CrossRef]
- Kumar, S.; Sehgal, R.; Wani, M.F.; Sharma, M.D. Stabilization and tribological properties of magnetorheological (MR) fluids: A review. J. Magn. Magn. Mater. 2021, 538, 168295. [Google Scholar] [CrossRef]
- Ruparelia, N.; Soni, U.; Desai, R.P.; Ray, A. Silica anchored colloidal suspension of magnetite nanorods. J. Solid State Chem. 2020, 290, 121574. [Google Scholar] [CrossRef]
- Díaz-Tena, E.; Lopez de Lacalle, L.N.; Campa, F.J.; Bocanegra, D.L.C. Use of magnetorheological fluids for vibration reduction on the milling of thin floor parts. Procedia Eng. 2013, 63, 835–842. [Google Scholar] [CrossRef]
- Batterbee, D.C.; Sims, N.D.; Stanway, R.; Wolejsza, Z. Magnetorheological landing gear: 1. A design methodology. Smart Mater. Struct. 2007, 16, 2429–2440. [Google Scholar] [CrossRef][Green Version]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.; Jafelicci, M. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef]
- Phenrat, T.; Kim, H.-J.; Fagerlund, F.; Illangasekare, T.; Tilton, R.D.; Lowry, G.V. Particle size distribution, concentration, and magnetic attraction affect transport of polymer-modified Fe0 nanoparticles in sand columns. Environ. Sci. Technol. 2009, 43, 5079–5085. [Google Scholar] [CrossRef]
- Johnson, R.L.; Nurmi, J.T.; Johnson, G.S.O.; Fan, D.; O’Brien Johnson, R.L.; Shi, Z.; Salter-Blanc, A.J.; Tratnyek, P.G.; Lowry, G.V. Field-scale transport and transformation of carboxymethylcellulose-stabilized nano zero-valent iron. Environ. Sci. Technol. 2013, 47, 1573–1580. [Google Scholar] [CrossRef]
- Pradhan, S.; Hedberg, J.; Blomberg, E. Effect of sonication on particle dispersion, administered dose and metal release of non-functionalized, non-inert metal nanoparticles. J. Nanopart. Res. 2016, 18, 285. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; DeLoid, G.; Pyrgiotakis, G.; Demokritou, P. Interactions of engineered nanomaterials in physiological media and implications for in vitro dosimetry. Nanotoxicology 2013, 7, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; DeLoid, G.M.; Demokritou, P. A critical review of in vitro dosimetry for engineered nanomaterials. Nanomedicine 2015, 10, 3015–3032. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Jensen, K.A.; Baun, A.; Rasmussen, K.; Rauscher, H.; Tantra, R.; Cupi, D.; Gilliland, D.; Pianella, F.; Riego Sintes, J.M. Techniques and protocols for dispersing nanoparticle powders in aqueous media—Is there a rationale for harmonization? J. Toxicol. Environ. Health B 2015, 18, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Midander, K.; Cronholm, P.; Karlsson, H.L.; Elihn, K.; Möller, L.; Leygraf, C.; Wallinder, I.O. Surface characteristics, copper release, and toxicity of nano-and micrometer-sized copper and copper (II) oxide particles: A cross-disciplinary study. Small 2009, 5, 389–399. [Google Scholar] [CrossRef]
- Alstrup, J.K.; Kembouche, Y.; Christiansen, E.; Jacobsen, N.; Wallin, H.; Guiot, C.; Spalla, O.; Witschger, O. Final protocol for producing suitable manufactured nanomaterial exposure media. The generic NANOGENOTOX dispersion protocol. Nanogenotox 2011, 1–33. Available online: https://www.anses.fr/en/system/files/nanogenotox_deliverable_5.pdf (accessed on 2 December 2021).
- Bonner, J.C.; Silva, R.M.; Taylor, A.J.; Brown, J.M.; Hilderbrand, S.C.; Castranova, V.; Porter, D.; Elder, A.; Oberdorster, G.; Harkema, J.R. Interlaboratory evaluation of rodent pulmonary responses to engineered nanomaterials: The NIEHS Nano GO Consortium. Environ. Health Perspect. 2013, 121, 676–682. [Google Scholar] [CrossRef]
- Cohen, J.M.; Teeguarden, J.G.; Demokritou, P. An integrated approach for the in vitro dosimetry of engineered nanomaterials. Part. Fibre Toxicol. 2014, 1, 11–20. [Google Scholar] [CrossRef]
- OECD. Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials. Series on the Safety of Manufactured Nanomaterials; No. 36; Organisation for Economic Co-operation and Development: Paris, France, 2012. [Google Scholar]
- Taurozzi, J.S.; Hackley, V.A.; Wiesner, M.R. A standardised approach for the dispersion of titanium dioxide nanoparticles in biological media. Nanotoxicology 2013, 7, 389–401. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Hedberg, Y.; Tornberg, M.; De Battice, L.; Svedhem, S.; Odnevall Wallinder, I. Cell membrane damage and protein interaction induced by copper containing nanoparticles—importance of the metal release process. Toxicology 2013, 313, 59–69. [Google Scholar] [CrossRef]
- Tiraferri, A.; Chen, K.L.; Sethi, R.; Elimelech, M. Reduced aggregation and sedimentation of zero-valent iron nanoparticles in the presence of guar gum. J. Colloid Interface Sci. 2008, 324, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Kralj, S.; Drofenik, M.; Makovec, D. Controlled surface functionalization of silica-coated magnetic nanoparticles with terminal amino and carboxyl groups. J. Nanoparticle Res. 2010, 13, 2829–2841. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S. Core–shell magnetic nanocomposite of Fe3O4@SiO2@NH2 as an efficient and highly recyclable adsorbent of methyl red dye from aqueous environments. Environ. Technol. Innov. 2019, 14, 100–113. [Google Scholar] [CrossRef]
- Feng, B.; Hong, R.Y.; Wang, L.S.; Guo, L.; Li, H.; Ding, J.; Zheng, Y.; Wei, D. Synthesis of Fe3O4/APTES/PEG di-acid functionalized magnetic nanoparticles for MR imaging. Colloids Surf. A Physicochem. Eng. Asp. 2008, 328, 52–59. [Google Scholar] [CrossRef]
- Durdureanu-Angheluta, A.; Dascalu, A.; Fifere, A.; Coroaba, A.; Pricop, L.; Chiriac, H.; Simionescu, B.C. Progress in the synthesis and characterization of magnetite nanoparticles with amino groups on the surface. J. Magn. Magn. Mater. 2012, 324, 1679–1689. [Google Scholar] [CrossRef]
- Dhavale, R.P.; Waifalkar, P.P.; Sharma, A.; Dhavale, R.P.; Sahoo, S.C.; Kollu, P.; Patil, P.B. Monolayer grafting of aminosilane on magnetic nanoparticles: An efficient approach for targeted drug delivery system. J. Colloid Interface Sci. 2018, 529, 415–425. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Jiang, G. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, Q.; Xu, W.; Yang, M.; Cai, Y.; Dionysiou, D.D.; O’Shea, K.E. Cr(VI) adsorption and reduction by humic acid coated on magnetite. Environ. Sci. Technol. 2014, 48, 8078–8085. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, D.; Zhang, S.; Zhang, X.; Meng, Z.; Cai, Y. Humic acid coated Fe3O4 magnetic nanoparticles as highly efficient Fenton-like catalyst for complete mineralization of sulfathiazole. J. Hazard. Mater. 2011, 190, 559–565. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Wu, Z.; Zhang, L.; Zeng, G.; Zhou, C. Adsorption of methylene blue onto humic acid-coated Fe3O4 nanoparticles. Colloids Surf. A 2013, 435, 85–90. [Google Scholar] [CrossRef]
- Schepetkin, I.; Khlebnikov, A.; Kwon, B.S. Medical drugs from humus matter: Focus on mumie. Drug Dev. Res. 2002, 57, 140–159. [Google Scholar] [CrossRef]
- Van Rensburg, C.E.J. The Antiinflammatory Properties of Humic Substances: A Mini Review. Phytother. Res. 2015, 29, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Pomogailo, A.D.; Kydralieva, K.A.; Zaripova, A.A.; Muratov, V.S.; Dzhardimalieva, G.I.; Pomogailo, S.I.; Golubeva, N.D.; Jorobekova, S.J. Magnetoactive humic-based nanocomposites. Macromol. Symp. 2011, 304, 18–23. [Google Scholar] [CrossRef]
- Ozmen, M.; Can, K.; Arslan, G.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Adsorption of Cu(II) from aqueous solution by using modified Fe3O4 magnetic nanoparticles. Desalination 2010, 254, 162–169. [Google Scholar] [CrossRef]
- Szekeres, M.; Tombácz, E. Surface charge characterization of metal oxides by potentiometric acid-base titration, revisited theory and experiment. Colloids Surf. A Physicochem. Eng. 2012, 414, 302–313. [Google Scholar] [CrossRef]
- Gorski, C.A.; Scherer, M.M. Determination of nanoparticulate magnetite stoichiometry by Mössbauer spectroscopy, acid dissolution, and powder X-ray diffraction: A critical review. Am. Mineral. 2010, 95, 1017–1026. [Google Scholar] [CrossRef]
- Bondarenko, L.; Illés, E.; Tombácz, E.; Dzhardimalieva, G.; Golubeva, N.; Tushavina, O.; Adachi, Y.; Kydralieva, K. Fabrication, microstructure and colloidal stability of humic acids loaded Fe3O4/APTES nanosorbents for environmental applications. Nanomaterials 2021, 11, 1418. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef]
- Romano, F.L.; Ambrosano, G.M.B.; Magnani, M.B.B.D.A.; Nouer, F. Analysis of the coefficient of variation in shear and tensile bond strength tests. J. Appl. Oral Sci. 2005, 13, 243–246. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The role of variable surface charge and surface complexation in the adsorption of humic acid on magnetite. Colloids Surf. A 2003, 230, 99–109. [Google Scholar] [CrossRef]
- Tombácz, E.; Illés, E.; Majzik, A.; Hajdú, A.; Rideg, N.A.; Szekeres, M. Ageing in the inorganic nanoworld: Example of magnetite nanoparticles in aqueous medium. Croat. Chem. Acta 2007, 80, 503–515. [Google Scholar]
- Fornaguera, C.; Solans, C. Analytical methods to characterize and purify polymeric nanoparticles. Int. J. Polym. Sci. 2018, 2018, 6387826. [Google Scholar] [CrossRef]
- Vauthier, C.; Cabane, B.; Labarre, D. How to concentrate nanoparticles and avoid aggregation? Eur. J. Pharm. Biopharm. 2008, 69, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macêdo, M.A.; Nakamura, M.; Toma, H.E. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Gdula, K.; Gładysz-Płaska, A.; Cristóvão, B.; Ferenc, W.; Skwarek, E. Amine-functionalized magnetite-silica nanoparticles as effective adsorbent for removal of uranium(VI) ions. J. Mol. Liq. 2019, 290, 111–125. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Linden, M. Towards establishing structure-activity relationships for mesoporous silica in drug delivery applications. J. Control Rel. 2008, 128, 157–164. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Shao, Y. Amino-functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interface Sci. 2010, 349, 293–299. [Google Scholar] [CrossRef]
- Sundman, A.; Vitzhum, A.-L.; Adaktylos-Surber, K.; Figueroa, A.I.; van der Laan, G.; Daus, B.; Byrne, J.M. Effect of Fe-metabolizing bacteria and humic substances on magnetite nanoparticle reactivity towards arsenic and chromium. J. Hazard. Mater. 2019, 384, 121450. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Zhou, Y. Adsorption-desorption characteristics and mechanisms of Pb(II) on natural vanadium, titanium-bearing magnetite-humic acid magnetic adsorbent. Powder Technol. 2019, 344, 947–958. [Google Scholar] [CrossRef]
- Golub, A.A.; Zubenko, A.I.; Zhmud, B.V. γ-APTES modified silica gels: The structure of the surface layer. J. Colloid Interface Sci. 1996, 179, 482–487. [Google Scholar] [CrossRef]
- Hajdú, A.; Szekeres, M.; Tóth, I.Y.; Bauer, R.A.; Mihály, J.; Zupkó, I.; Tombácz, E. Enhanced stability of polyacrylate-coated magnetite nanoparticles in biorelevant media. Colloids Surf. B 2012, 94, 242–249. [Google Scholar] [CrossRef] [PubMed]

| Sample | Fe3O4 | Fe3O4/APTES |
|---|---|---|
| a, A | 8.3813 | 8.3789 |
| Structure | Fe2.93O4 | Fe2.88O4 |
| % Fe3O4 | 78.8 | 63.7 |
| DXRD, nm | 17.1 ± 2.3 | 20.5 ± 3.3 |
| CV, % | 13.5 | 16.1 |
| Sample | Fe3O4/APTES | Fe3O4/APTES (Us) | Fe3O4/APTES (S) |
|---|---|---|---|
| IEP (ζ = 0) | 7.1 | 6.3 | 6.6 |
| Max ζ-potential, mV | 30.2 ± 7.2 | 32.9 ± 6.1 | 36.3 ± 7.5 |
| Min ζ-potential, mV | −12.4 ± 5.5 | −33.1 ± 5.2 | −35.1 ± 5.1 |
| HP Amount, g/g | Fe3O4/APTES | Fe3O4/APTES (Us) | Fe3O4/APTES (S) |
|---|---|---|---|
| for full neutralization of charge | 0.004 | 0.01 | 0.025 |
| to achieve −20 mV of zeta potential | 0.014 | 0.018 | 0.038 |
| to reach plateau | 0.04 | 0.028 | 0.072 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzhardimalieva, G.; Bondarenko, L.; Illés, E.; Tombácz, E.; Tropskaya, N.; Magomedov, I.; Orekhov, A.; Kydralieva, K. Colloidal Stability of Silica-Modified Magnetite Nanoparticles: Comparison of Various Dispersion Techniques. Nanomaterials 2021, 11, 3295. https://doi.org/10.3390/nano11123295
Dzhardimalieva G, Bondarenko L, Illés E, Tombácz E, Tropskaya N, Magomedov I, Orekhov A, Kydralieva K. Colloidal Stability of Silica-Modified Magnetite Nanoparticles: Comparison of Various Dispersion Techniques. Nanomaterials. 2021; 11(12):3295. https://doi.org/10.3390/nano11123295
Chicago/Turabian StyleDzhardimalieva, Gulzhian, Lyubov Bondarenko, Erzsébet Illés, Etelka Tombácz, Nataliya Tropskaya, Igor Magomedov, Alexander Orekhov, and Kamila Kydralieva. 2021. "Colloidal Stability of Silica-Modified Magnetite Nanoparticles: Comparison of Various Dispersion Techniques" Nanomaterials 11, no. 12: 3295. https://doi.org/10.3390/nano11123295
APA StyleDzhardimalieva, G., Bondarenko, L., Illés, E., Tombácz, E., Tropskaya, N., Magomedov, I., Orekhov, A., & Kydralieva, K. (2021). Colloidal Stability of Silica-Modified Magnetite Nanoparticles: Comparison of Various Dispersion Techniques. Nanomaterials, 11(12), 3295. https://doi.org/10.3390/nano11123295








